Abstract
The mechanism of irritable bowel syndrome (IBS) is still incompletely understood in the world although large amount of investigations have been carried out on it. There are many studies on the pathophysiology of IBS in China, which has huge amount of population suffering from IBS with special ethnicity and culture, including Mainland China, Hong Kong and Taiwan. We collected the literatures to show the results and discuss whether there were any differences in the pathophysiologic findings between China and other countries, whether there were any differences among different subtypes and how the pathophysiology correlated with the manifestations of patients. Gene polymorphism, disturbances of gastrointestinal motility, visceral hypersensitivity, intestinal infection and inflammation, psychological disturbances, food hypersensitivity and intolerance, and altered gut microflora were reviewed in this paper. Some conflicting outcomes between China and other countries were noted although most of them were similar.
Keywords: China, Irritable bowel syndrome, Pathophysiology
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder (FGID) mainly manifested as abdominal pain and correlated changed bowel habits. The chief bowel pattern determines the classification of IBS subtypes, which was set through Rome III criteria in the recent years, including constipation predominant IBS (IBS-C), diarrhea predominant IBS (IBS-D), mixed IBS (IBS-M) and unsubtyped IBS (IBS-U). Since IBS can cause substantial decline in the quality of life for the patients and accounts for a great amount of hospital visits and economic burden for society, many investigations have been done on the mechanisms of pathophysiology for IBS. The commonly stated mechanisms include genetic factors, abnormal gastrointestinal (GI) motility, visceral hypersensitivity, psychological disturbances, intestinal inflammation and so on. However, the results of these investigations have often been conflicting and no specific pathophysiology has been demonstrated to be certain for IBS.
The prevalence of IBS is reported to be 2.9%-15.6% in Asian countries nowadays,1 which is nearly comparable to that in the Western countries. China is a great country with large amount of population, specific ethnicity and custom in Asia. IBS has been found to be common for Chinese in these years and many studies about IBS have been carried out recently. The results of these investigations on pathophysiology and differences in the results between China and other countries have not been comprehensively reported since most of the works in China were published in Chinese journals. To show the results from China and find out the answer, we collected the manuscripts on pathophysiology of IBS by searching for papers in Chinese databases and PubMed to comprise the studies from Mainland China, Hong Kong and Taiwan from 1989 to 2011. In this review, the investigations about pathophysiology in China would be shown from the aspects of genetic factors, disturbances of GI motility, visceral hypersensitivity, intestinal infection and inflammation, psychological disturbances, food hypersensitivity and intolerance, and altered gut microflora, which have been frequently reported. Furthermore, we intended to find out if there were any differences for mechanisms among different subtypes and how the pathophysiology correlated to the manifestations of IBS in China.
Gene Polymorphism
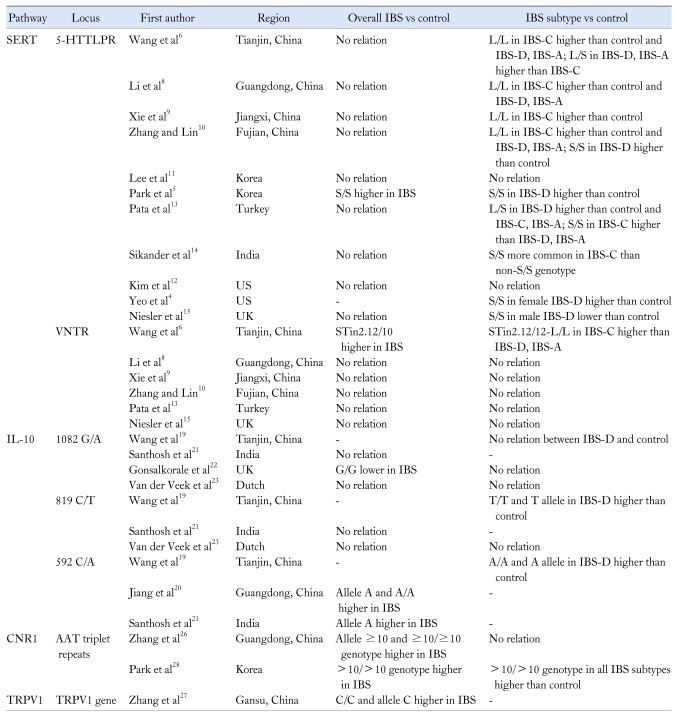
Lots of familial aggregation studies and twin studies have suggested that genetic factors perhaps influenced the susceptibility of IBS although the reports were somewhat conflicting. Up to now, the genes associated with serotonin, inflammation, adrenergic, mucosal barrier, and psychology which may play a role in IBS have been widely examined.2 In China, the investigations about gene polymorphism are extremely limited and most of them are involved in serotonin and inflammation. We tried to find out if there were any differences in the results of gene polymorphism between China and other countries. The main findings of the investigations from China and other countries were shown in Table 1.
Table 1.
Comparisons on Genetic Polymorphism for Irritable Bowel Syndrome in China and Other Countries
IBS, irritable bowel syndrome; SERT, serotonin transporter; 5-HTTLPR, serotonin transporter gene-linked polymorphic region; IBS-C, constipation predominant IBS; IBS-D, diarrhea predominant IBS; IBS-A, alternating IBS; PI-IBS, post-infectious IBS; VNTR, variable number tandem repeat; CNR1, cannabinoid receptor 1; TRPV1, transient receptor potential vanilloid type 1.
Serotonin transporter (SERT) is a protein which reuptakes 5-hydroxytryptamine (5-HT) in synaptic cleft and then reduces the function of 5-HT such as inducing urgency, cramps, diarrhea and vomiting.3,4 The lower expression of SERT will indicate higher level of 5-HT, which may be associated with bowel symptoms in IBS patients. The 2 well investigated polymorphism regions are variable number tandem repeat (VNTR) and serotonin transporter linked polymorphic region (5-HTTLPR).
For the 5-HTTLPR region, there were different associations reported between the genotypes and various subtypes of IBS in different studies. But none of the investigations has found correlations between the genotypes and IBS overall which had not been categorized except for the study by Park.5-15 A meta-analysis comprising studies involving Caucasians or Asians also concluded that there was no association between 5-HTTLPR and IBS overall.16 The transcriptional activity of long (L) allele is apparently greater than short (S) allele in 5-HTTLPR for SERT, then L/L genotype has higher transcriptional efficacy than L/S and S/S genotypes. All of the studies about 5-HTTLPR in China showed that IBS-C patients had significantly higher frequency of L allele or L/L genotype than healthy individuals and patients of other subtypes.6-10 Besides, Zhang and Lin10 also exhibited that IBS-D patients had higher frequency of S allele and S/S genotype than healthy controls and patients of other subtypes. The outcomes of investigations from other countries exhibited some discordance. Lee et al11 found no correlation between the polymorphism of 5-HTTLPR and overall or each subtype of IBS in Korea. While another study by Park et al5 which was also from Korea showed that S/S genotype was more common in patients with IBS than healthy controls, especially in IBS-D subtype. The result of investigation by Kim et al12 from US was the same with Lee et al.11 Yeo et al4 found the frequency of S/S genotype to be higher in female IBS-D patients than healthy controls in US. However, the investigations from Turkey by Pata et al13 and from India by Sikander et al14 both found the contradictory result that S/S genotype was more common in IBS-C than controls. Niesler et al15 also showed that male IBS-D patients had lower frequency of S/S genotype than controls in UK. Therefore, from the various results above, we could not obtain a firm conclusion about the relationship between polymorphism of 5-HTTLPR and IBS subtypes. But the results of investigations from China were almost the same for L/L genotype associated with IBS-C,6-10 which was contradictory to the investigations by Pata and Sikander et al.13,14 In addition, the explanations for the conflicting phenomenon by them were also different. Wang et al6 speculated that the L/L genotype for high expression of SERT caused 5-HT reuptake before the effects of 5-HT were shown and then attenuated the motility and secretion of intestine to cause IBS-C. However, Pata et al13 considered the S/S genotype responding to the low expression of SERT which might have caused the aggregation of 5-HT and then downregulated the 5-HT receptors, therefore led to IBS-C.
Racial difference might be one of the reasons for the variations of these results since the distribution of 5-HTTLPR gene polymorphism varied among different races and regions. Homozygous for the S allele in Asians (64% of patients) was reported to be markedly higher than that in Caucasians (22% of patients) in a meta-analysis.16 Xie J et al17 investigated the polymorphism regions of 5-HTTLPR for healthy individuals of Han Chinese and found the frequencies of S allele and S/S genotype to be predominantly higher than the frequencies of L allele and L/L genotype. The result was similar to that in Korean and Japanese with this predominance, while different from that in Turkish, European and American without this predominance.17 Therefore the racial difference may play a role in the gene polymorphism of IBS, although many other factors such as environment, habits and study designs could also influence the results. In addition, the gender difference probably also influenced the gene polymorphism, since S/S genotype was found to be higher in female IBS-D patients4 and lower in male IBS-D patients15 than controls. The investigation by Sikander et al14 which showed S/S genotype was more common in IBS-C patients included more male patients, while the investigations in China which showed higher level of L/L genotype in IBS-C patients included more female patients.6-10
Particularly, the polymorphism has been recently shown to be associated with the treatment response to the drug related to 5-HT in patients with IBS. Smeraldi et al18 found that the effect of selective serotonin reuptake inhibitors (SSRIs) fluvoxamine was better for patients with L/L and L/S genotype than S/S genotype. Camilleri et al3 reported that 5-HT3 receptor antagonist alosetron was better for L/L genotype in IBS-D patients. Li et al8 showed that the effect of 5-HT4 agonist tegaserod was worst for L/L genotype. These different pharmacologic responses might indicate that genetics influenced the effect of drugs on patients with IBS although the underlying reasons were unclear.
For VNTR region, most of the investigations in China8-10 and all of the investigations in other countries13,15 found no significant difference between IBS patients and healthy adults, also with no difference among the subtypes. But Wang et al6 found that STin2.12/10 genotype was more common in patients with IBS than healthy controls, and patients with IBS-C showed increased frequency of STin2 VNTR 12/12 and 5-HTTLPR L/L genotype (12/12-L/L) than controls and other subtypes. Chen et al7 showed similar result for post-infectious IBS (PI-IBS) patients. These 2 particular results may need further confirmation by more meticulous investigations with larger sample size.
IL-10 is an important anti-inflammatory cytokine in body. Therefore the genotype encoding low producer of IL-10 might be more prevalent in IBS patients since the infection and inflammation are always thought to be associated with IBS. Three well investigated regions for IL-10 were 1082, 819 and 592 loci in promoter. As mentioned in reports, 1082A, 819T and 592A were the lower producer alleles for IL-10 compared to 1082G, 819C and 592C respectively, so they might predict higher prevalence of IBS.19 Most studies showed IBS patients had higher frequency of low producer genotypes in these regions, which would lead to lower level of IL-10. Wang et al19 reported that IBS-D patients had higher frequency of low producer genotypes 819 T/T and 592 A/A than healthy individuals. Higher frequency of low producer genotype 592A/A in patients with IBS overall was found in report of Jiang et al.20 A recent investigation in India reported patients with IBS overall had higher frequency of A allele in 592 locus, but no significant difference was found for alleles in 819 and 1082 loci and genotypes in these 3 regions.21 Different results also presented in the studies from the West. One study showed that the high producer 1082 G/G genotype was significantly lower in patients with IBS overall than controls.22 While another study found no relationship between overall or each subtype of IBS and the genotypes in 1082 and 819 loci.23 These results suggested that the genetic alteration might contribute to the alteration of inflammatory cytokine level in some IBS patients, and thus affected the inflammatory state in these patients. In addition, there were several investigations reporting variation in genotypes regarding IL-10 according to ethnicity, with increased frequency of low producer genotype A/A or A allele in Chinese group than other population.24,25
Other 2 less investigated genes for IBS in China were associated with cannabinoid receptor 1 (CNR1)26 and capsaicin receptor/transient receptor potential vanilloid type 1 (TRPV1).27 Since the cannabinoid could affect GI function, genetic variants for the CNR1 were hypothesized to be associated with pathogenesis of IBS. Zhang et al26 found that allele ≥ 10 and ≥ 10/ ≥ 10 genotype for AAT triplet repeats in the 3-flanking region of CNR1 gene were significantly higher in patients with IBS than controls in Han population in Guangdong. Another investigation involving Korean subjects also showed the similar result and found that the patients with CNR1 > 10/ > 10 genotype had more severe abdominal symptoms.28 The capsaicin receptor TRPV1 was estimated to be related with visceral pain and hypersensitivity states in IBS. The frequency of C/C genotype and C allele for TRPV1 gene were both reported to be higher in patients with IBS than healthy individuals.27 These gene polymorphisms for CNR1 and TRPV1 may also be related to the susceptibility to IBS, which have not been reported in the West.
In conclusion, although the investigations of the gene polymorphism for IBS in China are limited, some of the results are different from those in other countries. The reason may be complex, and ethnicity and gender differences probably may somewhat correlate with it. More efforts are demanded to provide special data in China about candidate genes, which may contribute to future new therapy.
Disturbances of Gastrointestinal Motility
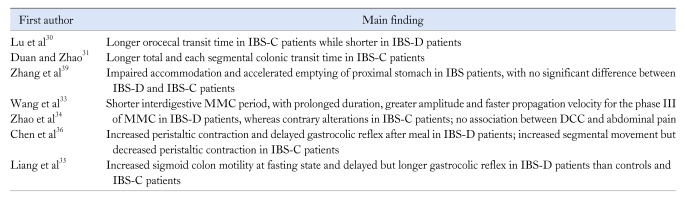
Different results of GI motility measurements between IBS patients and healthy controls have been commonly reported in China (Table 2), which indicate that altered GI motility may contribute to the pathogenesis of IBS. The dysmotility of GI tract accounting for the symptoms of IBS is still in dispute.29 However the clinical investigations show the association between GI motility disturbances and bowel habits of different IBS subtypes. In general, the findings of parameters about gut motility suggested that the motility increased in IBS-D patients while decreased in IBS-C patients. From the aspect of GI transit time, all related studies in China showed that either the orocecal transit time or the total and each segmental colonic transit time was shorter in IBS-D patients and longer in IBS-C patients than healthy controls.30-32 The results were in agreement with those in many studies of other countries, although this correlation was not always consistent.
Table 2.
Studies on Gastrointestinal Motility Disturbances for Irritable Bowel Syndrome in China
IBS, irritable bowel syndrome; IBS-C, constipation predominant IBS; IBS-D, diarrhea predominant IBS; MMC, migrating motor complex; DCC, discrete clustered contraction.
Disturbances of small bowel motility were widely reported in patients with IBS from various aspects in the world. In China, only the studies about discrete cluster contractions (DCC) and migrating motor complex (MMC) has been reported. The work by Wang and Zhao et al33,34 showed that patients with IBS-D exhibited shorter period for MMC, with prolonged duration proportion, greater amplitude, higher motility index and faster propagation velocity for the phase III of MMC than healthy controls, whereas patients with IBS-C exhibited the contrary alterations. Since phase III was important to the propagating movement of the intestine, the longer duration and greater amplitude of this phase was closely related to the symptom of diarrhea with quicker bowel movement. The duration of DCC in phase II was reported to be longer in patients with IBS than healthy controls, but no significant relationship between DCC and the episode of abdominal pain was found in Wang et al,33 while investigations in the West showed that the increased frequency and duration of DCC were associated with abdominal pain.29 Perhaps larger sample size was needed to clarify this result in China. Zhao et al34 also observed that the plasma motilin and 5-HT level were higher in IBS patients and fluctuated with the phase of MMC cycle, indicating the association of GI hormone and motility.
For colon motility, Liang et al35 reported that patients with IBS-D had stronger sigmoid colon motility, which represented as elevated wave amplitude and longer duration of high amplitude propagative bursts and higher MI at fasting state. Chen et al36 observed the motility of sigmoid colon by ultrasonography, and found that patients with IBS-D had more, while patients with IBS-C had less peristaltic contraction, whether at fasting or after meal. These 2 studies both reported that the patients with IBS-D had delayed but longer gastrocolic reflex after meal, which was not found in controls and patients with IBS-C.35,36 Most studies in other countries also showed the concordant results that there were more colonic motility in IBS-D patients while fewer in IBS-C patients.37,38
Upper GI symptoms were commonly seen in patients with IBS, and maybe these symptoms are somewhat correlated with the abnormal gastric motility. Our group investigated the accommodation and emptying of proximal stomach by real-time ultrasonography, and found that both the initial and maximal volumes of proximal stomach in IBS-D and IBS-C groups were lower than those in healthy group. This result indicated impaired accommodation of proximal stomach, which might induce higher pressure in stomach contributing to the dyspepsia symptoms such as early satiety and abdominal distention.39 The emptying of proximal stomach in IBS patients was recorded to be accelerated, which may also be correlated with the higher pressure of stomach in IBS patients.39 However, delayed gastric emptying in IBS patients have been shown in many studies of other countries,40-42 especially in patients with dyspepsia symptoms40 or IBS-C patients.42 The variation of results between China and other countries may be caused by the different test methods and trial designs.
To summarize, various disturbances of GI motility have been found in different subgroups of IBS patients, which are likely to play an important role in bowel habits of IBS. There are still a few conflicting results found between China and other countries, and further confirmatory work is needed in China to get the truth.
Visceral Hypersensitivity
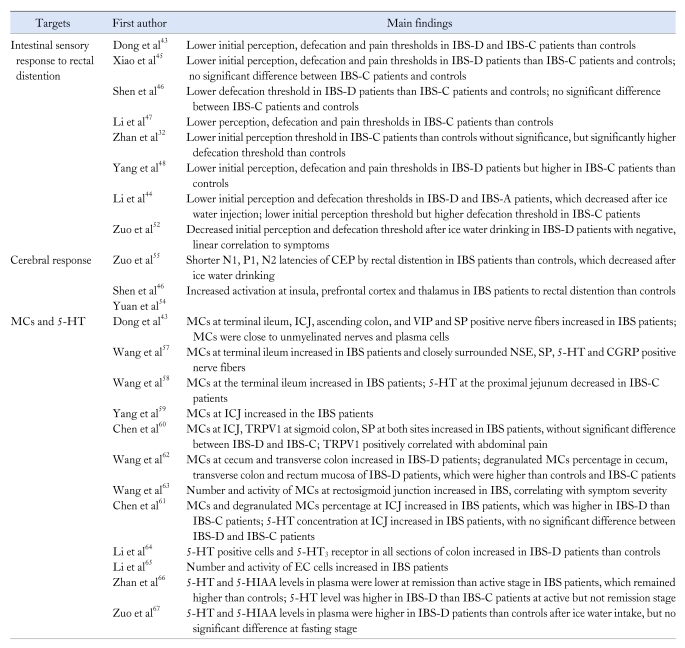
Visceral hypersensitivity has been commonly reported as a pathophysiologic factor for IBS in recent years, which may play a role in the symptoms of IBS especially abdominal pain. Many studies have shown higher sensitivity to rectal balloon distention in IBS patients in China (Table 3). Most of the studies reported that the initial perception thresholds, defecation thresholds and pain thresholds were much lower in IBS-D patients than those in healthy volunteers.43-46 But different from the consistent result in patients with IBS-D, there was still controversy for patients with IBS-C. Dong and Li et al43,47 reported that patients with IBS-C had lower initial perception, defecation and pain thresholds than healthy controls; Xiao and Shen et al45,46 reported no significant difference compared with controls; Li's group and Zhan et al32,44 reported lower initial perception thresholds in IBS-C patients than controls without significance, but significantly higher defecation thresholds than controls; Yang et al48 even reported higher perception, defecation and pain thresholds than controls. Chen et al49 showed that the perception thresholds of IBS patients were lower than controls with quick dilation test but no significant difference was noted on the slow dilation test. In conclusion, most of these researches above suggested higher sensation feeling in IBS-D patients and related lower sensation in IBS-C patients, and the method of dilatation might have influenced the results.
Table 3.
Studies on Visceral Hypersensitivity for Irritable Bowel Syndrome in China
IBS, irritable bowel syndrome; IBS-D, diarrhea predominant IBS; IBS-C, constipation predominant IBS; IBS-A, alternating IBS; CEP, cerebral evoked potentials; MCs, mast cells; 5-HT, 5-hydroxytryptamine; ICJ, ileocecal junction; VIP, vasoactive intestinal polypeptide; SP, substance P; NSE, neuron-specific enolase; CGRP, calcitonin gene-related peptide; TRPV1, transient receptor potential vanilloid type 1; EC, enterochromaffin; 5-HIAA, 5-hydroxyindoleacetic acid.
Similar to the results in China, many studies in other countries also reported decreased sensory thresholds to rectal distension in IBS patients. Moreover, enhanced perceptions to visceral stimulation were also reported in other regions of the GI tract in other countries. However, not all of the investigations showed identical results. The results for sensory thresholds were also found to be different among the investigations measured by different distension methods in the West, with lower thresholds for rapid phasic distensions than slow ramp distensions.50 In addition, IBS-D patients were reported to be more sensitive than IBS-C patients and the different results for IBS-C patients also existed in the studies from other countries. In these studies, less, equal or higher sensitivities corresponding to rectal distension in IBS-C patients compared to healthy subjects have been reported.51 The different results for IBS-C patients may be related to the long-term high pressure of rectum for some patients with constipation and the variability among these patients such as dietary fiber intakes and other managements.
The sensation and related symptoms of IBS patients in respond to other stimulations aside from pressure stimulus have also been reported in China. Li's group44 found that the initial and defecation thresholds responding to rectal distension in IBS patients decreased significantly after ice-water injected into balloon, especially in IBS-D patients, while cold stimulation on abdomen did not affect the thresholds although it could have induced the symptoms of IBS. This investigation indicated that hypersensitivity was also related to thermal stimulation and might have been limited to the viscera.44 Another study by them reported that the initial perception and defecation thresholds decreased after ice water drinking in IBS-D patients, which were negatively related to the symptoms.52 These results above suggested visceral hypersensitivity to mechanical and temperature stimulation might exist in IBS patients, and the altered sensitivity correlated with abdominal symptoms. Although it remains controversial whether somatic sensory dysfunction exists in IBS for different results in various studies worldwide, most studies tend to report no significant relationship between somatic sensitivity and IBS patients.51,53 Lots of investigations in other countries have also displayed the altered visceral sensitivity correlating with GI symptom severity of IBS, despite some conflicting results.
The altered cerebral response to rectal balloon distension has been shown in patients with IBS assessed by various techniques in most studies worldwide. In China, the cerebral responses assessed by methods of functional magnetic resonance imaging (fMRI) and cerebral evoked potentials (CEP) have been reported (Table 3). Yuan et al54 showed both IBS patients and controls to present exaggerated activity at the regions of anterior cingulate cortex, insula, prefrontal cortex and thalamus by fMRI during rectal distension, while IBS patients showed enhanced activation in insula, prefrontal cortex and thalamus than controls. They also found that IBS patients had more severe pain responding to rectal distention than controls, which supported the association between the altered cerebral response and pain perception in IBS patients.54 Zuo et al55 recorded the CEP to rectal balloon distension and then found specific latencies of CEP to be decreased in IBS patients with shorter CEP after ice water intake. The result suggested the role of visceral hypersensitivity and defects in visceral afferent pathway.55 Despite contradictory results presented among different studies worldwide, most of them suggested differences existed between IBS patients and healthy controls in the response of brain during gut stimulation. Although the activated cerebral regions in respond to rectal distension varied in different reports, a meta-analysis showed IBS patients had consistently greater activations in the regions related to pain modulation and emotional arousal than controls by collecting researches with fMRI or positron emission tomography techniques. It was supposed that disturbances in central nervous system processing might have been involved in the pathophysiology of IBS.56
Neurohumoral abnormalities have been identified in IBS patients with visceral hypersensitivity, of which mast cells (MCs) and 5-HT were most commonly reported. The MCs in gut including terminal ileum,43,57,58 ileocecal junction,43,59-61 cecum,60 ascending colon,43 transverse colon62 and rectosigmoid junction63 have been reported to increase in IBS patients compared with health controls (Table 3). Dong et al43 also reported apparent variation of MCs existing in IBS patients. The expression of certain transmitters and neuropeptides such as substance P, neuron-specific enolase, calcitonin gene-related peptide and 5-HT were reported to be elevated in IBS patients. The nerve fibers with the positive expression of these substances were found to surround MCs closely, which indicated that MCs might play an important role in pathophysiology of IBS through the connection of nerve and immune systems.43,57 Furthermore, Chen et al61 and our group62 both reported the percentage of degranulated MCs in IBS patients which was higher than controls, and this percentage was significantly higher in IBS-D patients than that in IBS-C patients. The concentration of 5-HT and the number and activity of EC cells in colonic mucosa were always found to be elevated in IBS patients.61,64,65 But Wang et al58 showed variant levels of 5-HT in different parts of small intestine, with significantly lower level of 5-HT in the proximal jejunum. For 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) level in plasma, Zhan66 reported significantly higher level in patients with IBS than controls whether at the active or remission stage, while Zuo et al67 reported higher level in IBS-D patients than controls after ice water drinking but not at fasting state. However, whether difference existed in the expression of MCs and 5-HT in IBS-D and IBS-C patients was controversial. Some studies reported higher level in IBS-D than IBS-C patients, while others reported no significant difference between them.60-64 In addition, the activation percentage of MCs and 5-HT level were reported to be positively correlated with severity of symptoms.63,67 TRPV1 was also shown to be positively related to the score of abdominal pain.60 These studies about relationship between symptoms of IBS and immunity materials associated with sensitivity may further support the mechanism of visceral hypersensitivity in IBS.
In general, visceral hypersensitivity is involved in both the gut and brain in IBS patients and probably contributes to the symptoms of these patients. The underlying mechanisms of hypersensitivity have not been clear yet, but MCs and 5-HT are probably involved.
Intestinal Infection and Inflammation
Intestinal infection has been considered as an important pathogenic factor in IBS, which may cause related bowel symptoms in a short period defined as PI-IBS. Many epidemiologic studies in China exhibited that the infection of dysentery was an independent risk factor for IBS.68,69 A cohort study in Beijing followed up patients after intestinal infection and healthy persons who had no previous history of functional bowel disorder. About 8.1% of individuals with intestinal infection and 10.2% of those with Shigella infection were found to develop IBS in the 2-year-period compared to only 0.8% in controls.70 The incidence of PI-IBS has been reported to vary from 4% to 32% worldwide in a meta-analysis, with most about 10%, which was significantly higher than the IBS incidence in control group without infection.71 Although intestinal infection was not specific for an area or a race, the types of microorganism infected on IBS patients were somewhat regional specific. Infections of Salmonella and Campylobacter were always reported in the West,71 while Shigella seemed to be a more usual bacterium in China and Korea from Asia.70,72 In Pakistan, Blastocystis hominis has been shown to be more common in IBS patients than healthy controls.73 Although infections of parasites such as Giardia lamblia were common in some areas of Asia like India, there were few studies reporting their association with IBS in Asia.74 The different types of infected microorganism may be associated with the different resident environment and diet habits.
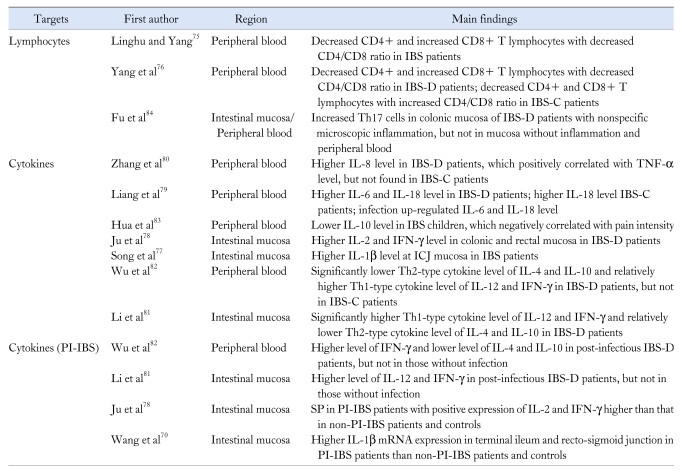
There were a lot of investigations suggesting the role of inflammation and immunity activation for IBS in China, most presenting as the alteration of lymphocytes and cytokines (Table 4). Although conflicting results existed, decreased CD4/CD8 lymphocytes ratio was reported in IBS patients compared with controls.75,76 Most investigations showed the immunity alteration in IBS patients with significantly higher level of proinflammatory cytokines (IL-1β,77 IL-2,78 IL-6,79 IL-8,80 IL-12,81 IL-18,79 TNF-α,80 IFN-γ78,81) and lower level of anti-inflammatory cytokines (IL-4,82 IL-1082,83), whether in peripheral blood or in intestinal mucosa. A study from Taiwan involving children suggested anti-inflammatory cytokine IL-10 to negatively correlate with abdominal pain intensity,83 implying the correlation between immune alteration and symptoms. Wu and Li et al81,82 reported IBS-D patients had higher Th1-type cytokine level and lower Th2-type cytokine level in peripheral blood and intestinal mucosa.2 Our group measured the Th1/Th2/Th17 level in peripheral blood and colonic mucosa for IBS-D patients, and showed Th17 proportion to be increased only in colonic mucosa with nonspecific microscopic inflammation, but not in the mucosa without inflammation and peripheral blood. There was no significant change for the Th1, Th2 and associated cytokines between IBS patients and controls in either colonic mucosa or peripheral blood.84 Moreover, PI-IBS patients were always found to have more severe inflammation than non-PI-IBS patients and healthy controls in China (Table 4).70,78,81,82 It indicated that the role of intestinal infection for the pathogenesis of IBS probably acted through some immunological changes.
Table 4.
Studies on Inflammation for Irritable Bowel Syndrome in China
IBS, irritable bowel syndrome; IBS-C, constipation predominant IBS; IBS-D, diarrhea predominant IBS; ICJ, ileocecal junction; PI-IBS, post-infectious IBS; SP, substance P.
The underlying mechanisms of PI-IBS have not been clearly identified worldwide. Persistent mucosal inflammation and immune activation, manifested as increased lymphocytes, MCs, EC cells and inflammatory cytokines have been commonly reported in the West.85 Similar to the results in China, there is also a trend toward higher level of proinflammatory cytokines and lower level of anti-inflammatory cytokines found in the investigations of other countries, although some conflicting results exist. Whether Th1-type or Th2-type profile is presented in IBS patients is still controversial with the contradictory results from different studies in the West.86 Different from the results on lymphocytes in China, increased level of CD4+ and CD8+ T lymphocytes has always been reported in intestinal mucosa of IBS patients, while regular level was reported in peripheral blood.87 The reasons for these different results are unclear, therefore additional investigations are needed. Anyway, all these studies above indicated that intestinal infection and inflammation might take part in the pathophysiology of IBS through immunological mechanism.
Food Hypersensitivity and Intolerance
Many patients with IBS always complained of their bowel symptoms relating to meal, implying some association between IBS and food. Although diet ingestion could affect symptoms through various pathways,88 food hypersensitivity and intolerance were still considered as possible pathophysiologic factors for IBS. There were many studies reporting the levels of food-specific IgE and IgG antibodies in serum and the effectiveness of food elimination in China. Most studies reported that the levels of food-specific IgG antibody were higher in IBS patients than healthy controls.89-92 Zuo et al90 reported the serum IgG antibody titers of specific foods including shrimp, crab, soybean, egg and wheat, which were higher in patients with IBS, but there was no obvious association found between the IgG antibody titers and symptom severity. Zhang et al89 showed that the frequency and severity of symptoms of IBS were obviously decreased in patients after eliminating intolerant foods based on IgG antibody for 8 weeks. In the West, many studies have also displayed that serum IgG antibody levels were elevated in IBS patients,93 and food elimination diets for the patients with increased IgG antibody to specific food antigens could help to reduce symptoms.94 A systematic review of 7 clinical trials showed a 15%-71% response rate to diet exclusion, and the most commonly incriminated foods included milk, wheat and eggs.95
Different from the IgG antibody, there is controversy for the results of food-specific IgE antibody in China. Xu and Zhu91 reported higher positive rate of food-specific IgE antibody in IBS patients than healthy individuals. Yang and Li92 showed higher positive rate in IBS-D patients than controls, but not in IBS-C patients. No significant difference was found for food-specific and total IgE titers between IBS patients and healthy controls in another study by Zuo et al.90 In the West, food-specific IgE level had been evaluated as one of the markers for food hypersensitivity,88 and there was little evidence for the role of IgE mediated immediate phase reaction in IBS.96
Therefore most studies supported the role of food hypersensitivity and intolerance in IBS, and diet exclusion based on IgG might be effective for treatment of IBS patients with elevated IgG level. However further investigations are needed to clarify the mechanisms underlying food hypersensitivity and intolerance, and find more effective treatments for the patients with these problems.
Psychological Disturbances
Psychological disturbances occur commonly in IBS patients from both the Western or Eastern studies. Although psychological factor might not be the cause of IBS, it always correlated with the aggravation of symptoms. In China, many epidemiologic investigations showed that the IBS patients had more psychological problems.61,97-101 A hospital-based investigation conducted in 3 metropolises in China reported one quarter of patients with IBS had depressive or anxiety symptoms.97 A recent large-scale study for undergraduates in Shandong province reported the score of depression and anxiety by hospital anxiety and depression scale was higher in undergraduates diagnosed as IBS than healthy controls.98 Our group also got the similar result for undergraduates in Wuhan.99 Lee et al100 showed IBS was closely related to generalized anxiety disorder in a community study of Hong Kong. Chen et al61 found IBS patients who had more psychological disturbance showed the increased number and degranulation of MCs in mucosa, implying the association between psychology and mucosal immunity alteration. Wang's group101 reported that IBS patients paid more attention to GI symptoms and up-regulated visceral sensation under the stimulus of uncomfortable pictures about digestive diseases, which were relieved by diverting attention. Then this research not only showed the psychological disturbances in IBS patients but also displayed the effect of psychological factor on visceral hypersensitivity. In other studies, they showed the IBS patients had more negative life events and inappropriate coping styles,102 and proposed the effectiveness of cognitive therapy for the patients with refractory IBS.103 There were also many other studies reporting that psychotherapy was effective in the treatment of IBS,104 which further supported that psychological factors played a role in pathogenesis of IBS. The association between IBS and psychological disturbances has also been commonly reported in other countries. On the whole, psychological disturbances have been shown to be associated with IBS, but whether it is a pathophysiologic factor for IBS is still not certain. Hence much effort should be done to clarify the true relation between them and find out the underlying mechanisms.
Altered Gut Microflora
It has been noticed that intestinal infection was related to IBS and antibiotics could affect digestive symptoms, which may indicate the role of gut microflora in IBS. There were many studies reporting the changes in intestinal microflora in IBS patients and the effectiveness of probiotics for them in China. An early study of our group examined 9 kinds of common microflora in feces, and showed that the number of Bacteroides, Bifidobacterium and Enterobacteria decreased in patients with IBS-D compared with that in healthy controls.105 Si et al106 found IBS patients had decreased number of Bifidobacterium and increased number of Enterobacteriaceae. The microbial colonization resistance, calculated as the ratio of Bifidobacterium to Enterobacteriaceae (B/E ratio), was significantly lower in IBS patients (< 1) than that in controls (> 1), indicating impairment of colonization resistance and increased susceptibility to pathogenic bacteria overgrowth in IBS patients.106 Chen et al107 got the similar result for IBS-D patients, and they also reported decreased number of Lactobacillus and increased number of Saccharomycete. Zhang108 found significantly decreased number of anaerobic flora and increased number of aerobic flora in IBS patients and the dysbacteria state was different in distinct subtypes. However, the number of Bifidobacterium and Lactobacillus was always decreased in all subtypes of IBS to varying degrees.108
Although the results were different in various studies, most of the studies from China reported decreased level of Bifidobacterium and Lactobacillus in IBS patients. Therefore targeted supplement of probiotics containing these floras might be an effective therapeutic pathway. The benefits of probiotics to the improvement of IBS symptoms have been commonly reported in numerous studies in China.109-112 A multi-center, double-blind, randomized controlled trial exhibited the effect of Bifidobacterium for symptom improvement in IBS-D patients which was better than placebo group in all stages of the treatment.109 Zhu et al110 compared the total and each symptom scores for IBS-D patients before and after the treatment with Medilac-S (Bacillus subtilis and Enterococcus faecium) and BIFICO (Bifidobacterium longum, Lactobacillus acidophilus and Enterococcus), and showed the benefits of them. An open-label trial by Fan et al111 reported that treatment with probiotics comprising Bifidobacterium, Lactobacillus and Enterococcus could regulate the balance of microorganism in intestine and relieved the bowel symptoms of IBS. Zeng et al112 showed treatment with active lactic acid bacteria, which decreased the mucosal permeability of small bowel and achieved symptom improvement in IBS-D patients. The result implied that microflora imbalance contributed to bowel symptoms of IBS through a pathway of changing the mucosal permeability.112 To conclude, all these clinical studies in China showed that there was gut microflora alteration in patients with IBS and the treatment with probiotics probably improved the IBS symptoms by modulating the microorganism environments.
In the West, there were also a large amount of investigations exploring the microorganism states in IBS patients and the effects of different kinds of probiotics for patients. These researches didn't exhibit concordant alterations of microflora among all IBS patients, while different microflora composition was found in different subtypes.113 The underlying mechanisms for the role of microflora imbalance in IBS have not been clearly identified. Perhaps higher level of organic acid and excessive gas caused by altered intestinal flora, which was found to correlate with GI symptoms, would play a role in the pathophysiology of IBS.113 The therapeutic effect of probiotics varied in different studies for different strains chosen and trial designs. But a meta-analysis of these studies confirmed the benefit of probiotics in the treatment of IBS.114 However, probiotics cannot be effective for all IBS patients, so further work is deserved to identify the patient groups which can get most benefit from it and to choose the most appropriate and effective strains.
Conclusion
The factors discussed above have been displayed to be associated with the pathophysiology of IBS. Although most of the results of related investigations in China were similar to the Western and other Eastern countries, it was needed to pay more attention that some conflicting outcomes existed in China. Since IBS is a heterogeneous disorder, it is probable that more than one mechanism to be presented in a specific patient and these particular mechanisms are involved in different patients to various extents. IBS still remains a complicated and inadequately understood disease. So much effort is needed in future to get a better understanding about the pathophysiology for symptom generation and to develop more effective and appropriate therapy for IBS.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 2.Saito YA. The role of genetics in IBS. Gastroenterol Clin North Am. 2011;40:45–67. doi: 10.1016/j.gtc.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 4.Yeo A, Boyd P, Lumsden S, et al. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53:1452–1458. doi: 10.1136/gut.2003.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JM, Choi MG, Park JA, et al. Serotonin transporter gene polymorphism and irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:995–1000. doi: 10.1111/j.1365-2982.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang BM, Wang YM, Zhang WM, et al. Serotonin transporter gene polymorphism in irritable bowel syndrome. Zhonghua Nei Ke Za Zhi. 2004;43:439–441. [PubMed] [Google Scholar]
- 7.Chen YB, Zhang BL, Zhang XY, Liu FT, Wu AC. Relationship of IBS and SERT polymorphism after intestinal infection. Modern Hospital. 2010;10:11–13. [Google Scholar]
- 8.Li YY, Nie YQ, Xie J, Tan HZ, Zhou YJ, Wang H. Serotonin transporter gene polymorphisms in irritable bowel syndrome and their impact on tegaserod treatment. Zhonghua Nei Ke Za Zhi. 2006;45:552–555. [PubMed] [Google Scholar]
- 9.Xie J, Zhang ZX, Huang CB, Shu T, Wu XJ. Serotonin transporter gene polymorphism in constipation-predominant irritable bowel syndrome. J Gannan Med Univ. 2008;28:484–486. [Google Scholar]
- 10.Zhang XM, Lin ZH. Relationship between serotonin transporter gene polymorphisms and irritable bowel syndrome. World J Gastroenterol. 2006;14:1790–1794. [Google Scholar]
- 11.Lee DY, Park H, Kim WH, Lee SI, Seo YJ, Choi YC. Serotonin transporter gene polymorphism in healthy adults and patients with irritable bowel syndrome. Korean J Gastroenterol. 2004;43:18–22. [PubMed] [Google Scholar]
- 12.Kim HJ, Camilleri M, Carlson PJ, et al. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pata C, Erdal ME, Derici E, Yazar A, Kanik A, Ulu O. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97:1780–1784. doi: 10.1111/j.1572-0241.2002.05841.x. [DOI] [PubMed] [Google Scholar]
- 14.Sikander A, Rana SV, Sinha SK, et al. Serotonin transporter promoter variant: analysis in Indian IBS patients and control population. J Clin Gastroenterol. 2009;43:957–961. doi: 10.1097/MCG.0b013e3181b37e8c. [DOI] [PubMed] [Google Scholar]
- 15.Niesler B, Kapeller J, Fell C, et al. 5-HTTLPR and STin2 polymorphisms in the serotonin transporter gene and irritable bowel syndrome: effect of bowel habit and sex. Eur J Gastroenterol Hepatol. 2010;22:856–861. doi: 10.1097/MEG.0b013e32832e9d6b. [DOI] [PubMed] [Google Scholar]
- 16.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 17.Xie J, Li YY, Nie YQ, Liang PZ, Zhang L. Serotonin transporter gene polymorphism in healthy adults and patients with irritable bowel syndrome. Acad J Guangdong Med College. 2005;33:1–4. [Google Scholar]
- 18.Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- 19.Wang BM, Jiang XZ, Yang YL, Liu WT, Cao XC, Zhao XZ. A study of interleukin-10 gene polymorphism in irritable bowel syndrome. Zhonghua Nei Ke Za Zhi. 2006;45:289–292. [PubMed] [Google Scholar]
- 20.Jiang YJ, Nie YQ, Lai XB. IL-10 gene promoter-592 polymorphism and irritable bowel syndrome. Modern Hospital. 2010;10:8–10. [Google Scholar]
- 21.Santhosh S, Dutta AK, Samuel P, Joseph AJ, Ashok Kumar J, Kurian G. Cytokine gene polymorphisms in irritable bowel syndrome in Indian population - a pilot case control study. Trop Gastroenterol. 2010;31:30–33. [PubMed] [Google Scholar]
- 22.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91–93. doi: 10.1136/gut.52.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Veek PP, van den Berg M, de Kroon YE, Verspaget HW, Masclee AA. Role of tumor necrosis factor-alpha and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510–2516. doi: 10.1111/j.1572-0241.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 24.Meenagh A, Williams F, Ross OA, et al. Frequency of cytokine polymorphisms in populations from western Europe, Africa, Asia, the Middle East and South America. Hum Immunol. 2002;63:1055–1061. doi: 10.1016/s0198-8859(02)00440-8. [DOI] [PubMed] [Google Scholar]
- 25.Padyukov L, Hahn-Zoric M, Lau YL, Hanson LA. Different allelic frequencies of several cytokine genes in Hong Kong Chinese and Swedish Caucasians. Genes Immun. 2001;2:280–283. doi: 10.1038/sj.gene.6363771. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Nie YY, Jiang YY, Li YY, Lin Y. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome. J Gannan Med Univ. 2010;30:363–367. [Google Scholar]
- 27.Zhang ZQ, Wang Q, Wang BJ. Relationship between TRPV1 gene polymorphism and irritable bowel syndrome. World J Gastroenterol. 2009;17:3514–3518. [Google Scholar]
- 28.Park JM, Choi MG, Cho YK, et al. Cannabinoid receptor 1 gene polymorphism and irritable bowel syndrome in the Korean population: a hypothesis-generating study. J Clin Gastroenterol. 2011;45:45–49. doi: 10.1097/MCG.0b013e3181dd1573. [DOI] [PubMed] [Google Scholar]
- 29.Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (Lond) 1998;95:165–169. [PubMed] [Google Scholar]
- 31.Duan JH, Zhao HC. A clinical study on colonic motility and anorectal manometry in constipation predominant irritable bowel syndrome. Chin J Gastroenterol Hepatol. 2006;15:191–193. [Google Scholar]
- 32.Zhan LX, Zou DW, Xu GM, Li ZS, Yin N, Zhang MQ. A study on colonic transit test and ano-manometry in functional constipation and constipation-predominant irritable bowel syndrome. Chin J Dig. 2002;22:19–21. [Google Scholar]
- 33.Wang SH, Dong L, Luo JY, et al. A research of migrating motor complex in patients with irritable bowel syndrome. Zhonghua Nei Ke Za Zhi. 2009;48:106–110. [PubMed] [Google Scholar]
- 34.Zhao JH, Dong L, Hao XQ. Small intestine motility and gastrointestinal hormone levels in irritable bowel syndrome. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1492–1495. [PubMed] [Google Scholar]
- 35.Liang RX, Zhang ZX, Cai LY, Zheng QF, Zhang FC. The study of sigmoid colon motility in patients with irritable bowel syndrome. Guangxi Medical Journal. 2004;26:30–32. [Google Scholar]
- 36.Chen YM, Ke MY, Zhang SQ, Jiang YX. Sigmoid colon motility and gastrocolic reflex in IBS patients under ultrasonography. Chin J Dig. 1999;19:272–273. [Google Scholar]
- 37.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–1506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead WE, Engel BT, Schuster MM. Irritable bowel syndrome: physiological and psychological differences between diarrhea-predominant and constipation-predominant patients. Dig Dis Sci. 1980;25:404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K, Sun SB, Xie XP, Huang XQ, Hou XH. Study on accommodation and empting of proximal stomach in patients with irritable bowel syndrome. Chin J Gastroenterol Hepatol. 2007;12:298–300. [Google Scholar]
- 40.Stanghellini V, Tosetti C, Barbara G, et al. Dyspeptic symptoms and gastric emptying in the irritable bowel syndrome. Am J Gastroenterol. 2002;97:2738–2743. doi: 10.1111/j.1572-0241.2002.07062.x. [DOI] [PubMed] [Google Scholar]
- 41.Van Wijk HJ, Smout AJ, Akkermans LM, Roelofs JM, ten Thije OJ. Gastric emptying and dyspeptic symptoms in the irritable bowel syndrome. Scand J Gastroenterol. 1992;27:99–102. doi: 10.3109/00365529209165425. [DOI] [PubMed] [Google Scholar]
- 42.Caballero-Plasencia AM, Valenzuela-Barranco M, Herrerías-Gutiérrez JM, Esteban-Carretero JM. Altered gastric emptying in patients with irritable bowel syndrome. Eur J Nucl Med. 1999;26:404–409. doi: 10.1007/s002590050404. [DOI] [PubMed] [Google Scholar]
- 43.Dong WZ, Zou DW, Li ZS, et al. Mechanism of hypersensitivity in the patients with irritable bowel syndrome. Chin J Dig. 2004;24:18–22. doi: 10.1111/j.1443-9573.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 44.Li YQ, Wang YM, Lv GP, Gu XM, Zuo XL, Zhang HY. Effects of rectal thermal- and pressure-stimuli on visceral perception thresholds in patients with irritable bowel syndrom. Chin J Dig. 2003;23:659–661. [Google Scholar]
- 45.Xiao WB, Liu YL, Zhao LL. The differences of rectal perception among dirrhea-dominant, constipation-dominant irritable bowel syndrome and functional constipation. World Chin J Digestol. 2002;10:1291–1294. [Google Scholar]
- 46.Shen J, Zhu Q, Yuan RZ, Zhang ZW, Chen KM. Alterations in focal encephalic function in patients of irritable bowel syndrome. Chin J Gastroenterol Hepatol. 2005;14:167–170. [Google Scholar]
- 47.Li QX, Yin HK, Chen LJ. Effect of tegaserod on rectal sensation thresholds in visceral hypersensitivity patients with constipation predominant irritable bowel syndrome. Chin J Postgrad Med. 2007;30:9–14. [Google Scholar]
- 48.Yang CM, Liu JY, Wang YQ, Zhang AZ, Ye YH. Dynamic study of anorectum in patients with irritable bowel syndrome. J Shandong Univ (Healthy Science) 2002;40:141–142. [Google Scholar]
- 49.Chen Q, Chen MH, Lin JK, Hu PJ. Investigation on sensation and motility in IBS patients. Chin J Dig. 2003;23:241–242. [Google Scholar]
- 50.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 51.Stacher G, Christensen J. Visceral hypersensitivity in irritable bowel syndrome: a summary review. Dig Dis Sci. 2006;51:440–445. doi: 10.1007/s10620-006-3152-9. [DOI] [PubMed] [Google Scholar]
- 52.Zuo XL, Li YQ, Shi L, et al. Visceral hypersensitivity following cold water intake in subjects with irritable bowel syndrome. J Gastroenterol. 2006;41:311–317. doi: 10.1007/s00535-005-1766-x. [DOI] [PubMed] [Google Scholar]
- 53.Hasler WL. Traditional thoughts on the pathophysiology of irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:21–43. doi: 10.1016/j.gtc.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Yuan YZ, Tao RJ, Xu B, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo XL, Li YQ, Huang KM, et al. Alterations in cerebral potentials evoked by rectal distention and drinking ice water in patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:1844–1849. doi: 10.1111/j.1440-1746.2006.04176.x. [DOI] [PubMed] [Google Scholar]
- 56.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2010;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang LH, Fang XC, Pan GZ. Relationship between mast cells and nerve fibers in the intestinal mucosa in patients with irritable bowel syndrome. Chin J Dig. 2003;23:332–335. [Google Scholar]
- 58.Wang SH, Dong L, Luo JY, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YS, Feng FC, Pan DS, Zhou DY, Zhang WD, Zhang ZS. Mast cells in ilececal junction and gastrointestinal hormones in colonic mucosa in irritable bowel syndrome patients. Chin J Dig Endosc. 1997;14:149–152. [Google Scholar]
- 60.Chen XM, Luo Y, Wu YL, Jiang M. Alterations of capsaicin receptor, substance P and mast cells in colonic mucosa of patients with irritable bowel syndrome. Chin J Gastroenterol Hepatol. 2010;15:672–675. [Google Scholar]
- 61.Chen WK, Zou YY, Li FJ, Luo D. Changes of psychosocial factors, enteral mucosal mast cells and 5-hydroxytryptamine in irritable bowel syndrome. World Chin J Digestol. 2007;15:46–50. [Google Scholar]
- 62.Wang J, Liang LX, Zhang ZX, Li GH, Qian W, Hou XH. Alterations of enteric mucosal mast cells in patients with irritable bowel syndrome. World Chin J Digestol. 2002;22:19–21. [Google Scholar]
- 63.Wang WA, Qian JM, Pan GZ. The role of activation of colonic mucosal mast cells in the pathophysiology of irritable bowel syndrome. Chin J Dig. 2003;23:263–266. [Google Scholar]
- 64.Li YH, Zhu XL, Xu ZM. Changes of colonic 5-hydroxytryptamine and 5-hydroxytryptamine3 receptor in diarrhea-predominant irritable symptom. Chin J Gastroenterol Hepatol. 2006;11:477–480. [Google Scholar]
- 65.Li ZS, Zhan LX, Zhou DW, Xu GM, Man XH, Ye XT. Morphological and functional alteration of serotonin-producing intestinal enterochromaffin cells in patients with irritable bowel syndrome. Chin J Dig. 2004;24:94–97. [Google Scholar]
- 66.Zhan LX, Xu GM, Li ZS, Zou DW, Jin ZD, Tu ZX. Plasma 5-HT, 5-HIAA elevated in patients with irritable bowel syndrome at active stage and remission stage. Acad J Mil Med Univ. 2003;24:152–154. [Google Scholar]
- 67.Zuo XL, Li YQ, Guo M, et al. Relationship between the symptomatology and plasma 5-hydroxytryptamine concentrations after cold water intake in patients with diarrhea predominant irritable bowel syndrome. Chin J Dig. 2007;27:516–519. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 68.Pan G, Lu S, Ke M, Han S, Guo H, Fang X. An epidemiologic study of irritable bowel syndrome in Beijing-A stratified randomized study by clustering sampling. Zhonghua Liu Xing Bing Xue Za Zhi. 2000;21:26–29. [PubMed] [Google Scholar]
- 69.Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–1224. doi: 10.1111/j.1365-2036.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 70.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 72.Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005;20:381–386. doi: 10.1111/j.1440-1746.2005.03574.x. [DOI] [PubMed] [Google Scholar]
- 73.Yakoob J, Jafri W, Jafri N, et al. Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:383–385. [PubMed] [Google Scholar]
- 74.Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: The good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244–251. doi: 10.1111/j.1440-1746.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- 75.Linghu EQ, Yang YS. Analysis of peripheral blood lymphocytes subgroup in irritable bowel syndrome. Chin J Dig. 2002;22:423–425. [Google Scholar]
- 76.Yang TX, Zhang ZK, Zeng HL, et al. T-lymphocytes subgroups of peripheral blood and enteromucosal mast cells in patients with irritable bowel syndrome. Qingdao Med J. 2008;40:85–87. [Google Scholar]
- 77.Song JZ, Wang QM, Ding XP, Yu Y. Change of mucous neuropeptide and IL-1β in IBS. Chin J Gastroenterol Hepatol. 2007;16:219–222. [Google Scholar]
- 78.Ju H, Zhang XF, Liu XS, Wei LZ. Correlations of substance P with interleukin-2 and interferon-γ expression in colonic mucosa of patients with post- and non-post-infectious irritable bowel syndrome. World Chin J Digestol. 2006;14:3116–3120. [Google Scholar]
- 79.Liang HQ, Wang SH, Li YQ, Wang FS. Analysis on expression imbalance of peripheral blood inflammatory cytokines in patients with irritable bowel symptom. Chin J Gastroenterol. 2008;13:111–113. [Google Scholar]
- 80.Zhang R, Zhang XH, Sun MY, Zhang HT, Yang AJ, Wang FX. Alternation of IL-8 and TNF in irritable bowel syndrome. Chin J Celiopath. 2004;4:7–10. [Google Scholar]
- 81.Li YQ, Zhang HY, Zuo XL, Yuan HP, Lu XF, Li JM. Study on the shifting of Th1/Th2 balance of large intestinal mucosa in patients with irritable bowel syndrome. Chin J Dig. 2004;24:728–731. [Google Scholar]
- 82.Wu P, Zhang HY, Zhang B, Lu B, Wu MB, Lv YL. Change of cytokine in irritable bowel syndrome and the clinical significance. J Chin Physci. 2006;8:1406–1407. [Google Scholar]
- 83.Hua MC, Lai MW, Kuo ML, Yao TC, Huang JL, Chen SM. Decreased interleukin-10 secretion by peripheral blood mononuclear cells in children with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2011;52:376–381. doi: 10.1097/MPG.0b013e3181fd9816. [DOI] [PubMed] [Google Scholar]
- 84.Fu Y, Tong JJ, Pan Q, et al. Phenotypic analysis of Th cells in colon and peripheral blood in patients with irritable bowel syndrome. Zhonghua Yi Xue Za Zhi. 2009;89:2120–2123. [PubMed] [Google Scholar]
- 85.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 86.Ortiz-Lucas M, Saz-Peiró P, Sebastián-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part two: the role of cytokines. Rev Esp Enferm Dig. 2010;102:711–717. doi: 10.4321/s1130-01082010001200006. [DOI] [PubMed] [Google Scholar]
- 87.Ortiz-Lucas M, Saz-Peiró P, Sebastián-Domingo JJ. Irritable bowel syndrome immune hypothesis. Part one: the role of lymphocytes and mast cells. Rev Esp Enferm Dig. 2010;102:637–647. doi: 10.4321/s1130-01082010001100004. [DOI] [PubMed] [Google Scholar]
- 88.Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:141–162. doi: 10.1016/j.gtc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Zhang XD, Deng M, Li M, Wan XL. Food intolerance and diarrhea-dominant irritable bowel syndrome. World Chin J Digestol. 2007;15:3877–3879. [Google Scholar]
- 90.Zuo XL, Li YQ, Li WJ, et al. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin Exp Allergy. 2007;37:823–830. doi: 10.1111/j.1365-2222.2007.02727.x. [DOI] [PubMed] [Google Scholar]
- 91.Xu WB, Zhu JQ. The effect of the specific food IgG and IgE on the pathogenesis of irritable bowel syndrome. Acad Jiangxi. 2008;48:68–70. [Google Scholar]
- 92.Yang CM, Li YQ. The examination of the food-specific antibodies IgG and IgE in patients with irritable bowel syndrome. Chin J Dig Endosc. 2007;24:346–349. [Google Scholar]
- 93.Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil. 2006;18:595–607. doi: 10.1111/j.1365-2982.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 94.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459–1464. doi: 10.1136/gut.2003.037697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niec AM, Frankum B, Talley NJ. Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol. 1998;93:2184–2190. doi: 10.1111/j.1572-0241.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 96.Zar S, Kumar D, Benson MJ. Food hypersensitivity and irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:439–449. doi: 10.1046/j.1365-2036.2001.00951.x. [DOI] [PubMed] [Google Scholar]
- 97.Fu CW, Xu B, Chen WQ, Luan RS, Zhan SY. Cross-sectional study on the prevalence of depressive, anxiety disorder in outpatients with irritable bowel syndrome and functional dyspepsia in urban China. Chin J Dig. 2006;26:151–154. [Google Scholar]
- 98.Dong YY, Zuo XL, Li CQ, Yu YB, Zhao QJ, Li YQ. Prevalence of irritable bowel syndrome in Chinese college and university students assessed using Rome III criteria. World J Gastroenterol. 2010;16:4221–4226. doi: 10.3748/wjg.v16.i33.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen L, Kong H, Hou X. Prevalence of irritable bowel syndrome and its relationship with psychological stress status in Chinese university students. J Gastroenterol Hepatol. 2009;24:1885–1890. doi: 10.1111/j.1440-1746.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- 100.Lee S, Wu J, Ma YL, Tsang A, Guo WJ, Sung J. Irritable bowel syndrome is strongly associated with generalized anxiety disorder: a community study. Aliment Pharmacol Ther. 2009;30:643–651. doi: 10.1111/j.1365-2036.2009.04074.x. [DOI] [PubMed] [Google Scholar]
- 101.Wang WA, Pan GZ, Qian JM. Effect of psychological factors on visceral sensation of patients with irritable bowel syndrome. Zhonghua Yi Xue Za Zhi. 2002;82:308–311. [PubMed] [Google Scholar]
- 102.He JQ, Wang WA, Hu PJ. Coping characters of patients with irritable bowel syndrome. Chin J Dig. 2003;23:527–529. [Google Scholar]
- 103.Wang W, Pan G, Qian J. Cognitive therapy for patients with refractory irritable bowel syndrome. Zhonghua Nei Ke Za Zhi. 2002;41:156–159. [PubMed] [Google Scholar]
- 104.Song DL, Wang MX, Zeng J, Li YQ. Effect of psychological treatment for patients with irritable bowel syndrome. J Shandong Univ (Healthy Science) 2007;45:1068–1071. [Google Scholar]
- 105.Hou XH, Zhang JK, Yi CQ, et al. The changes of fecal microflora in irritable bowel syndrome with diarrhea. Chin J Dig. 1990;2:72–74. [Google Scholar]
- 106.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen ST, Huang HY, Zhang SZ, Chen RL. Observation and analysis of intestinal microecology in patients with diarrhea-predominant irritable bowel syndrome. China Modern Doctor. 2009;47:94–97. [Google Scholar]
- 108.Zhang L. Significance of intestinal tract normal bacteria flora quantitative analysis of IBS sufferers. China Medical Herald. 2008;5:94–95. [Google Scholar]
- 109.Lin JK, Hu PJ, Li YY, Nie YQ, Zhao YH, Lin MP. A multi-center double-blind randomized controlled trial in the treatment of irritable bowel syndrome with bifidobacteria. Chin J Dig. 2003;23:755–756. [Google Scholar]
- 110.Zhu LM, Ke MY, Zhou LY, et al. A clinical trail of probiotics in the treatment of IBS-D. Basic and Clinical Medicine. 2008;28:1070–1074. [Google Scholar]
- 111.Fan YJ, Chen SJ, Yu YC, Si JM, Liu B. A probiotic treatment containing Lactobacillus, Bifidobacterium and Enterococcus improves IBS symptoms in an open label trial. J Zhejiang Univ Sci B. 2006;7:987–991. doi: 10.1631/jzus.2006.B0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 113.Salonen A, de Vos WM, Palva A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology. 2010;156(Pt 11):3205–3215. doi: 10.1099/mic.0.043257-0. [DOI] [PubMed] [Google Scholar]
- 114.Parkes GC, Sanderson JD, Whelan K. Treating irritable bowel syndrome with probiotics: the evidence. Proc Nutr Soc. 2010;69:187–194. doi: 10.1017/S002966511000011X. [DOI] [PubMed] [Google Scholar]