Abstract
Chemoprevention has been acknowledged as an important and practical strategy for the management of skin cancer. Quercetin-3-methyl ether, a naturally occurring compound present in various plants, has potent anticancer-promoting activity. We identified this compound by in silico virtual screening of the Traditional Chinese Medicine Database using extracellular signal-regulated kinase 2 (ERK2) as the target protein. Here, we showed that quercetin-3-methyl ether inhibited proliferation of mouse skin epidermal JB6 P+ cells in a dose- and time-dependent manner by inducing cell cycle G2–M phase accumulation. It also suppressed 12-O-tetradecanoylphorbol-13-acetate-induced neoplastic cell transformation in a dose-dependent manner. Its inhibitory effect was greater than quercetin. The activation of activator protein-1 was dose-dependently suppressed by quercetin-3-methyl ether treatment. Western blot and kinase assay data revealed that quercetin-3-methyl ether inhibited ERKs kinase activity and attenuated phosphorylation of ERKs. Pull-down assays revealed that quercetin-3-methyl ether directly binds with ERKs. Furthermore, a loss-of-function ERK2 mutation inhibited the effectiveness of the quercetin-3-methyl ether. Overall, these results indicated that quercetin-3-methyl ether exerts potent chemopreventive activity by targeting ERKs.
Introduction
Skin cancer is one of the most commonly diagnosed cancers in Americans, and its incidence is rising dramatically (1). Although many environmental and genetic factors contribute to the development of skin cancer, the most important factor is chronic exposure of the skin to ultraviolet (UV) irradiation (2). In particular, UVB is a key contributor because it functions as a complete carcinogen, acting both as a tumor initiator and promoter by induction of oxidative stress, DNA damage and immunosuppression (1,2). UVB is a potent inducer of the mitogen-activated protein kinase (MAPK) cascades (3). MAPKs are serine–threonine kinases that play an important role in cell proliferation, differentiation, development, transformation and apoptosis (4) and are commonly up-regulated in various cancer cells (5). Therefore, targeting UVB-induced MAPKs signaling pathways might be an effective strategy for preventing skin tumorigenesis.
Activator protein-1 (AP-1) acts as a pivotal transcription factor involved in neoplastic transformation and development of cancer (6–9) and is regulated by upstream kinases, such as the MAPKs signaling pathways. The mammalian MAPK family consists of extracellular signal-regulated kinases (ERKs), c-jun N-terminal kinases (JNKs; also known as stress-activated protein kinases or SAPKs) and p38 (10). Among the MAPKs families, the ERKs cascade has been a focus of cancer chemoprevention because of its relevance in carcinogenesis. A diverse range of tumor promoters, including 12-O-tetradecanoylphorbol-13-acetate (TPA), induce neoplastic transformation through activation of the ERKs pathway in various cells (6,11). Aberrant activation of ERKs has been reported in various tumors (12,13), and thus, the direct targeting of ERKs might be an effective method for intervening in skin carcinogenesis.
Chemoprevention has been acknowledged as an important and practical strategy for the management of cancer. Flavonoids are naturally occurring compounds that are believed to exhibit preventive effects in various diseases, including cancer. However, the search for novel natural agents and the determination of novel targets for chemoprevention is challenging. With the development of computational biology, supercomputing technology could help solve these problems. Molecular docking-based virtual screening is an important tool in identifying potential inhibitors of protein kinase activity. We used the software program, Glide, for virtual screening of various chemical libraries and identified a compound, quercetin-3-methyl ether, from the Traditional Chinese Medicine Database as a potential candidate for inhibiting ERK2 activity. Quercetin-3-methyl ether (Figure 1A) is a flavonoid found in various plants, including Allagopappus viscosissimus (14), Opuntia ficus-indica var. saboten (15), Lychnophora staavioides (16) and Rhamnus species (17). Research data have shown that querectin-3-methyl ether exerts antioxidant (18), anti-inflammatory (17), antitrypanocidal, antimutagenic, tracheal relaxant, radical scavenging and xanthine oxidase inhibitory activities (16). Based on the supercomputing results and previous studies (14–18), quercetin-3-methyl ether could be a promising compound as a chemopreventive agent against skin cancer.
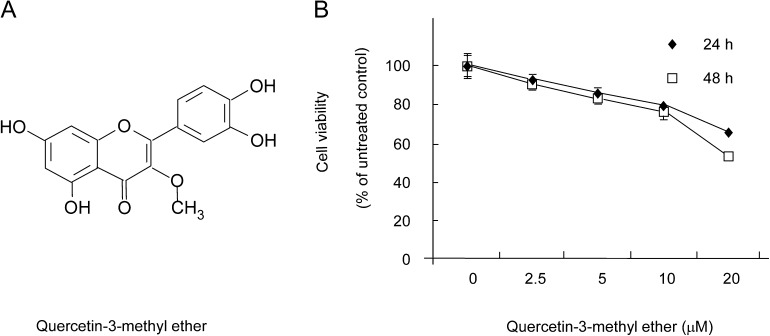
Fig. 1.
Quercetin-3-methyl ether at 20 μM is cytotoxic to JB6 P+ cells. (A) Chemical structure of quercetin-3-methyl ether. (B) Cells were treated with quercetin-3-methyl ether (0–20 μM) or its vehicle, dimethyl sulfoxide, as a negative control in 5% FBS/EMEM for 24 or 48 h. Cell viability was determined by (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Data are represented as means ± SE.
TPA is known to promote two-stage skin carcinogenesis and UVB is a tumor initiator and promoter in skin cancer (2,19). The JB6 mouse skin epidermal cell system, including promotion sensitive (P+) and promotion resistant (P−) components, allows the study of tumor promoter-induced carcinogenic processes at the molecular level. TPA induces large, tumorigenic and anchorage-independent colonies in soft agar (19). In this study, we examined the novel quercetin-3-methyl ether as a natural chemopreventive agent against skin cancer and its mechanism of antitumorigenic effects, using TPA and UVB as tumor promoters in the JB6 P+ mouse epidermal skin cell model. We report that quercetin-3-methyl ether is an inhibitor of ERKs kinase activity and this inhibition suppresses activation of AP-1, which subsequently inhibits cell proliferation and transformation.
Materials and methods
Chemicals
Quercetin-3-methyl ether was obtained from Analyticon Discovery (Potsdam, Germany). Eagle'’s minimum essential medium (EMEM), basal medium Eagle, gentamicin and l-glutamine were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Calabasa, CA). Quercetin and TPA were obtained from Sigma Chemical (St Louis, MO). The antibodies against phosphorylated ERKs (Tyr-202/Tyr-204), total ERKs, phosphorylated JNKs (Thr-183/Tyr-185), total JNKs, phosphorylated p38 (Thr-180/Tyr-182) and total p38 were purchased from Cell Signal Biotechnology (Beverly, MA). The antibody against phosphorylated mitogen-and stress activated protein kinase (Ser-376/Ser-360) was purchased from R&D Systems (Minneapolis, MN) and the antibody against total mitogen-and stress activated protein kinase1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CNBr-Sepharose 4B and [γ-32P] ATP were purchased from Amersham Biosciences (Piscataway, NJ) and the protein assay kit was from Bio-Rad (Hercules, CA). The histone H1 protein, active Cdk1/cyclin B, ERK1 and ERK2 kinases were obtained from Upstate Biotechnology (Lake Placid, NY) and the CellTiter96 Aqueous One Solution Cell Proliferation Assay Kit and the luciferase assay substrate were from Promega (Madison, WI).
Cell culture
The JB6 P+ cell line and JB6 cells stably transfected with an AP-1 luciferase reporter plasmid were cultured in monolayers at 37°C in a 5% CO2 incubator in 5% FBS/EMEM supplemented with penicillin/streptomycin (100 units/ml; Invitrogen).
Cytotoxicity assay
To estimate cytotoxicity, JB6 P+ cells were seeded (2 × 104 cells per well) in 96-well plates with 5% FBS/EMEM at 37°C in a 5% CO2 incubator, and after 4 h, fed with fresh medium and treated with quercetin-3-methyl ether at various concentrations (0, 2.5, 5, 10 or 20 μM). After culturing for various times, 20 μl of Cell Titer 96 Aqueous One Solution were added to each well, and the cells were then incubated for 1 h at 37°C in a 5% CO2 incubator. Absorbance was finally measured at 490 and 690 nm.
Cell proliferation assay
JB6 P+ cells were seeded (8 × 104 cells per well) in six-well plates with 5% FBS/EMEM at 37°C in a 5% CO2 incubator overnight and then starved in serum-free medium for 24 h. Cells were then fed with fresh medium and treated with different doses of quercetin-3-methyl ether (0, 2.5, 5 or 10 μM). After 24 or 48 h of treatment, total cells were collected by brief trypsinization and washed with phosphate-buffered saline (PBS). Total cell number was determined by counting each sample in duplicate using a hemocytometer under an inverted microscope. The data are presented as means ± SD of three independent experiments. JB6 P+ cells were also seeded (2 × 103 cells per well) in 96-well plates with 5% FBS/EMEM and incubated at 37°C in a 5% CO2 incubator overnight. Cells were then fed with fresh medium and treated with 10 μM quercetin-3-methyl ether or quercetin. After culturing for various times, 20 μl of Cell Titer 96 Aqueous One Solution were added to each well, and the cells were then incubated for 1 h at 37°C in a 5% CO2 incubator. Absorbance was measured at 490 and 690 nm.
Cell cycle assay
JB6 P+ cells were seeded (2 × 105 cells per well) in 60 mm dishes with 5% FBS/EMEM and incubated at 37°C in a 5% CO2 incubator overnight. Cells were then starved in serum-free medium for 24 h followed by treatment for 48 h with quercetin-3-methyl ether (0, 2.5, 5 or 10 μM) in EMEM containing 5% FBS. The cells were trypsinized and then washed twice with cold PBS and fixed with ice-cold 70% ethanol at −20°C overnight. Cells were then washed twice with PBS, incubated with 20 mg/ml RNase A and 200 mg/ml propidium iodide in PBS at room temperature for 30 min in the dark and subjected to flow cytometry using the FACSCalibur flow cytometer. Data were analyzed using ModFit LT (Verity Software House, Topsham, ME).
In vitro kinase assay
Histone H1 proteins (2 μg) were used for an in vitro kinase assay with 100 ng of active Cdk1/cyclin B kinase. Reactions were carried out in 1× kinase buffer [25 mM Tris–HCl, pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4 and 10 mM MgCl2] containing 50 μM unlabeled adenosine triphosphate with or without 10 μCi of [γ-32P] ATP at 30°C for 30 min. Reactions were stopped and then proteins resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and visualized by autoradiography. As for the ERK1 and ERK2 kinase assay, the plasmids for the glutathione S-transferase-tagged ribosomal S6 kinase 2 fusion protein were transformed into BL21 cells and induced with 0.5 mM isopropyl-l-thio-h-d-galactopyranoside at 30°C for 5 h. The soluble glutathione S-transferase-fusion protein was purified using glutathione-Sepharose 4B beads and eluted with 10 mM reduced glutathione in 50 mM Tris–HCl (pH 8.0) buffer. Purified fusion proteins (900 ng) were used for an in vitro kinase assay with 50 ng of active ERK1 or 20 ng of active ERK2.
Anchorage-independent cell transformation assay
JB6 P+ cells (8 × 103/ml) were exposed to TPA (20 ng/ml) with or without quercetin-3-methyl ether or quercetin in 1 ml of 0.33% basal medium Eagle agar containing 10% FBS or in 3 ml of 0.5% basal medium Eagle agar containing 10% FBS. The cultures were maintained at 37°C in a 5% CO2 incubator for 14 days, after which time the cell colonies were counted under a microscope with the aid of the Image-Pro Plus software program (v.4, Media Cybernetics, Silver Spring, MD) (20).
UVB irradiation
A UVB irradiation system was used to stimulate cells in serum-free media. The spectral peak from the UVB source (Bio-Link Crosslinker, Vilber Lourmat, Cedex 1, France) was at 312 nm.
Luciferase assay to determine AP-1 transactivation
Confluent monolayers of JB6 P+ cells stably transfected with an AP-1 luciferase reporter plasmid were trypsinized, and 4 × 104 viable cells suspended in 1 ml of 5% FBS/EMEM were added to each well of a 24-well plate. Plates were incubated overnight at 37°C in a humidified atmosphere of 5% CO2. Cells were starved in serum-free medium for another 24 h. The cells were then treated for 2 h with quercetin-3-methyl ether (0–10 μM) and then exposed to 4 kJ/m2 UVB or TPA (20 ng/ml) and then harvested after 3 h or 12 h. After treatment, cells were disrupted with 100 μl of lysis buffer [0.1 M potassium phosphate buffer (pH 7.8), 1% Triton X-100, 1 mM DTT and 2 mM ethylenediaminetetraacetic acid], and the luciferase activity was measured using a luminometer (Luminoskan Ascent, Thermo Electro, Helsinki, Finland).
Western blotting
After cells (1 × 106) were cultured in a 10 cm dish overnight, they were starved in serum-free medium for another 24 h to eliminate the influence of FBS on the activation of MAPKs. The cells were then treated with quercetin-3-methyl ether (0–10 μM) for 2 h before they were exposed to 4 kJ/0.5 m2 UVB or the cells were then treated with quercetin-3-methyl ether (0–5 μM) for 6 h before they were exposed to 20 ng/ml TPA for 30 min. The harvested cells were disrupted, and the supernatant fractions were boiled for 5 min. The protein concentration was determined using a dye-binding protein assay kit (Bio-Rad) as described in the manufacturer's manual. Lysate protein (50 μg) was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred to a polyvinylidene difluoride membrane. After blotting, the membrane was incubated with a specific primary antibody at 4°C overnight. Protein bands were visualized by a chemiluminescence detection kit after hybridization with an AP-linked secondary antibody.
Preparation of quercetin-3-methyl ether-Sepharose 4B beads
Sepharose 4B powder (0.3 g) was suspended in 1 mM HCl and the coupling solution [0.1 M NaHCO3 (pH 8.3) and 0.5 M NaCl] containing quercetin-3-methyl ether was mixed and rotated at 4°C overnight. The medium was transferred to 0.1 M Tris–HCl buffer (pH 8.0) and again rotated end-over-end at 4°C overnight. The medium was washed 3× with 0.1 M acetate buffer (pH 4.0) containing 0.5 M NaCl followed by a wash with 0.1 M Tris–HCl (pH 8.0) containing 0.5 M NaCl.
In vitro pull-down assay
Active ERK1 and ERK2 proteins (0.2 μg) were separately incubated with the quercetin-3-methyl ether-Sepharose 4B (or Sepharose 4B only as a control) beads (100 μl, 50% slurry) in a reaction buffer [50 mM Tris (pH 7.5), 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mM phenylmethylsulfonyl fluoride and 1× protease inhibitor mixture]. After incubation with gentle rocking overnight at 4°C, the beads were washed five times with buffer [50 mM Tris (pH 7.5), 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40 and 0.02 mM phenylmethylsulfonyl fluoride), and proteins bound to the beads were analyzed by western blotting.
Statistical analysis
As necessary, data are expressed as means ± SE and significant differences were determined using one-way analysis of variance. A probability value of P < 0.05 was used as the criterion for statistical significance.
Results
Quercetin-3-methyl ether inhibits growth of JB6 P+ cells by inducing cell cycle G2–M phase accumulation
We first examined the cytotoxicity of quercetin-3-methyl ether in JB6 P+ cells using the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. Quercetin-3-methyl ether (0–10 μM) and the time of exposure had minimal effect on the viability of cells (Figure 1B). However, doses of 20 μM were cytotoxic, and therefore, we chose 10 μM as the highest dose to be used in additional experiments.
We next assessed the dose- and time-dependent effects of quercetin-3-methyl ether on JB6 P+ cell proliferation. Cells were treated with different concentrations of the agent (0, 2.5, 5 or 10 μM, final concentration) dissolved in dimethyl sulfoxide (vehicle) for 24 and 48 h. At the end of each treatment time, determination of total cell number showed that quercetin-3-methyl ether inhibits cell growth in a dose- as well as a time-dependent manner (Figure 2A). The middle dose of quercetin-3-methyl ether (5 μM) caused a decrease to 71 and 54% (P < 0.05) of control after 24 and 48 h of treatment, respectively (Figure 2A). The highest dose (10 μM) decreased cell number to 51 and 34% of control (P < 0.05) after 24 and 48 h of treatment, respectively (Figure 2A).
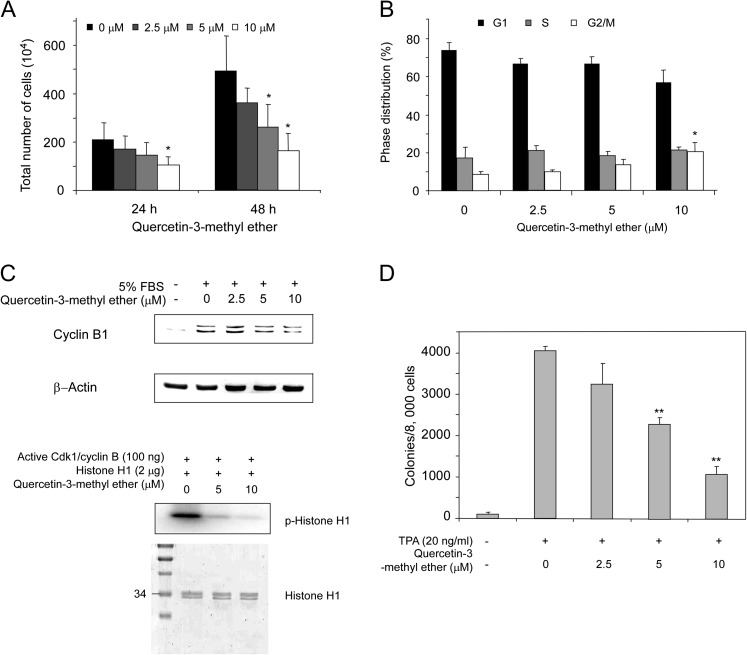
Fig. 2.
Quercetin-3-methyl ether inhibits cell growth by inducing G2–M accumulation and suppresses transformation of JB6 P+ cells. (A) Quercetin-3-methyl ether inhibits cell growth. Cells were starved in serum-free medium for 24 h and then treated with quercetin-3-methyl ether (0–10 μM) or its vehicle dimethyl sulfoxide (control) in 5% FBS/EMEM for 24 or 48 h. At the end of each treatment time, cells were collected and processed for determination of total cell number. Data are shown as means ± SE. The asterisk (*) indicates a significant difference (P < 0.05) between groups treated with quercetin-3-methyl ether and the group treated with dimethyl sulfoxide. (B) Quercetin-3-methyl ether induces G2/M accumulation and suppresses cyclin B1 protein expression in JB6 P+ cells. Cells were starved in serum-free medium for 24 h and then treated with quercetin-3-methyl ether (0–10 μM) or dimethyl sulfoxide (control) for 48 h. Cell cycle analysis was performed by flow cytometry. Data are shown as means ± SE. The asterisk (*) indicates a significant difference (P < 0.05) between groups treated with quercetin-3-methyl ether and the group treated with dimethyl sulfoxide. (C) For western blot analysis, cells were treated with quercetin-3-methyl ether at the indicated concentrations for 48 h. In vitro Cdk1/cyclin B kinase assay was performed as described in Materials and methods. Coomassie blue staining (lower) shows the histone H1 protein as a loading control. Lane 1, control, which indicates that active Cdk1/cyclin B phosphorylates the histone H1 protein; lanes 2 and 3, increasing amounts of quercetin-3-methyl ether suppress Cdk1/cyclin B kinase activity. (D) Quercetin-3-methyl ether inhibits TPA-induced transformation of JB6 P+ cells. Cells were treated as described under Materials and methods and colonies were counted under a microscope with the aid of Image-Pro Plus software (v.4). Data are shown as means ± SE. The asterisks (**) indicate a significant difference (P < 0.001) between groups treated with TPA and quercetin-3-methyl ether compared with the group treated with TPA alone.
To determine whether the inhibitory effect on cell proliferation was caused by modulation of cell cycle phase, we investigated the effect of quercetin-3-methyl ether on cell cycle progression. After treatment with various doses of quercetin-3-methyl ether, cells were stained with propidium iodide and analyzed by flow cytometry. Cell cycle distribution analysis showed that the high dose of quercetin-3-methyl ether treatment for 48 h results in an increased accumulation of cells in G2–M phase (Figure 2B). The G2–M phase of the cell cycle is reportedly controlled primarily by cyclin B1 and its associated catalytically active partner Cdk1 (21). Therefore, we tested the effect of quercetin-3-methyl ether on cyclin B1 expression and western blot data showed decreases in cyclin B1 protein level after treatment for 48 h (Figure 2C). Furthermore, an in vitro kinase assay showed that quercetin-3-methyl ether strongly inhibited Cdk1/cyclin B activity (Figure 2C, lower panel). These results suggested that quercetin-3-methyl ether inhibits cell growth by inducing cell cycle G2–M accumulation.
Quercetin-3-methyl ether inhibits TPA-induced neoplastic transformation of JB6 P+ cells
We next examined the effect of quercetin-3-methyl ether on TPA-induced neoplastic transformation of JB6 P+ cells. Based on the numbers of transformed cell colonies, treatment with quercetin-3-methyl ether significantly inhibited TPA-promoted neoplastic transformation in a dose-dependent manner (Figure 2D). Quercetin-3-methyl ether at 5 or 10 μM caused a decrease to 56 or 26% of TPA control (Figure 2D). The inhibition of colony formation by quercetin-3-methyl ether was not caused by cytotoxicity because the effective concentration range for inhibiting cell transformation did not affect JB6 P+ cell viability.
Quercetin-3-methyl ether is more effective than quercetin to inhibit cell growth and neoplastic transformation in JB6 P+ cells
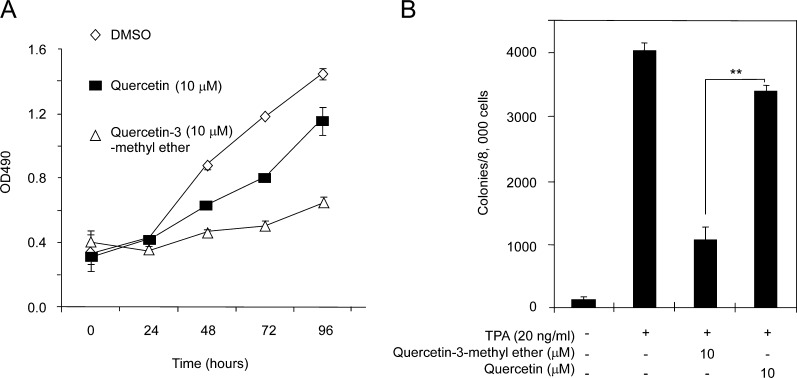
To determine whether quercetin-3-methyl ether is comparable with quercetin in suppressing cell proliferation and transformation, we examined the effects of both compounds on proliferation and TPA-induced neoplastic transformation of JB6 P+ cells. Results showed that both compounds effectively inhibited cell proliferation and transformation (Figure 3A and B). However, quercetin-3-methyl ether was more effective. These data indicated that quercetin-3-methyl ether is more potent than quercetin at suppressing cell proliferation and transformation.
Fig. 3.
Quercetin-3-methyl ether is more potent than quercetin in suppressing growth and transformation of JB6 P+ cells. (A) Quercetin-3-methyl ether inhibits cell growth more strongly compared with quercetin. Cells were treated with 10 μM quercetin-3-methyl ether or quercetin in 5% FBS/EMEM and cell growth was measured by (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay at the indicated times. (B) Compared with quercetin, quercetin-3-methyl ether more strongly inhibits TPA-induced transformation of JB6 P+ cells. Cells were treated as described in Materials and methods and colonies were counted 14 days later under a microscope with the aid of Image-Pro Plus software (v.4). Data are shown as means ± SE. The asterisk (**) indicates a significant difference (P < 0.001) between groups treated with TPA and quercetin-3-methyl ether compared with the group treated with TPA and quercetin.
Quercetin-3-methyl ether attenuates UVB- or TPA-induced transactivation of AP-1 and phosphorylation of ERKs in JB6 P+ cells
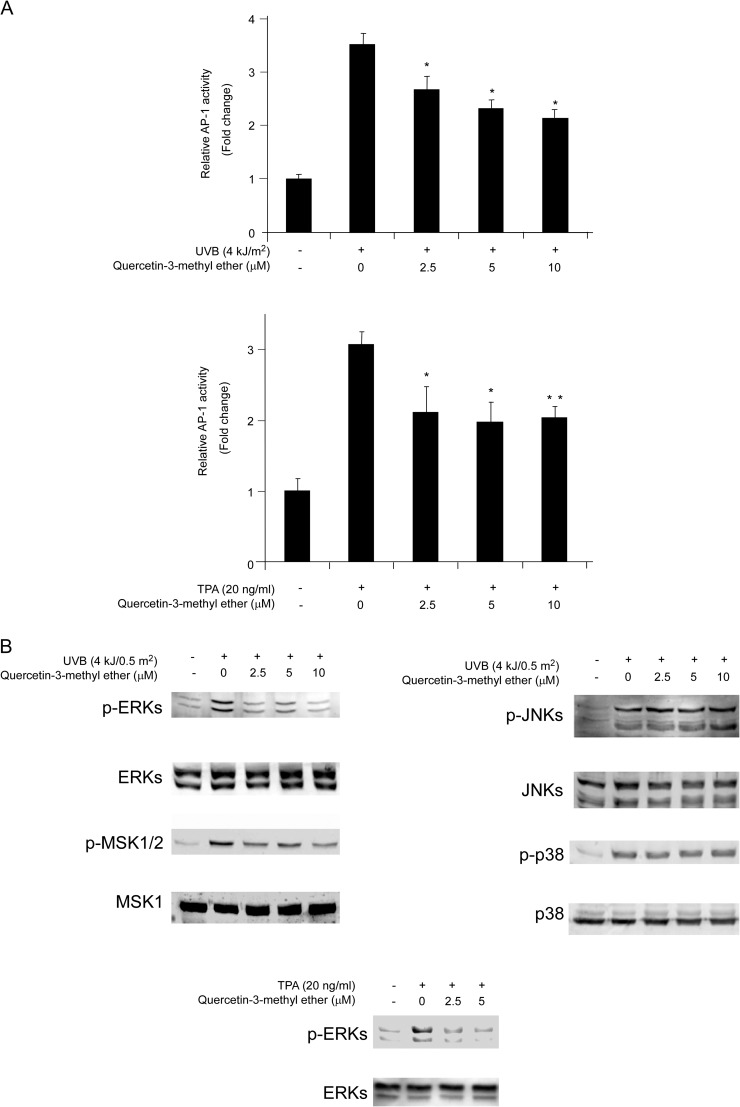
To determine the molecular mechanism of quercetin-3-methyl ether's cancer chemopreventive activity, we measured the effect of quercetin-3-methyl ether on transactivation of AP-1 using JB6 P+ cells stably transfected with an AP-1 luciferase reporter plasmid. Quercetin-3-methyl ether attenuated UVB- or TPA-induced transactivation of AP-1 (Figure 4A) in a dose-dependent manner, which may contribute to its antitumor-promoting activities. AP-1 activation is regulated by upstream kinases, such as the MAPKs. Hence, we examined the effect of quercetin-3-methyl ether on MAPKs activation. We found that quercetin-3-methyl ether strongly suppressed UVB-induced phosphorylation of ERKs but did not affect JNKs or p38 (Figure 4B). Quercetin-3-methyl ether (2.5 μM) also substantially inhibited TPA-induced phosphorylation of ERKs (Figure 4B). These results suggested that the inhibition of the ERKs pathway by quercetin-3-methyl ether leads to suppression of AP-1 transactivation, resulting in antitumor effects.
Fig. 4.
Quercetin-3-methyl ether blocks UVB/TPA-induced AP-1 transactivation in JB6 cells by attenuating ERKs signaling. (A) For the luciferase assay, JB6 cells stably transfected with an AP-1 luciferase reporter plasmid were cultured in 5% FBS/EMEM. Cells were starved in serum-free medium for 24 h and then treated with quercetin-3-methyl ether (0–10 μM) or its vehicle dimethyl sulfoxide (control) in 5% FBS/EMEM for 2 h. Cells were then exposed to 4 kJ/m2 UVB (upper panel) or 20 ng/ml TPA (lower panel) and harvested at 3 or 12 h, respectively. Luciferase activity was assessed and AP-1 activity is expressed relative to control cells without UVB or TPA treatment. Data are shown as means ± SE. The asterisk (*) indicates a significant difference (*P < 0.05; **P < 0.001) between groups treated with UVB/TPA and quercetin-3-methyl ether compared with the group treated with UVB/TPA alone. (B) Quercetin-3-methyl ether inhibits UVB/TPA-induced phosphorylation of ERKs but not JNKs or p38. Cells were treated with quercetin-3-methyl ether at the indicated concentrations (0–10 μM) for 2 or 6 h and then exposed to 4 kJ/0.5 m2 UVB or 20 ng/ml TPA and harvested 30 min later. The levels of phosphorylated and total ERKs, mitogen-and stress activated protein kinase. JNKs and p38 proteins were determined by western blot analysis.
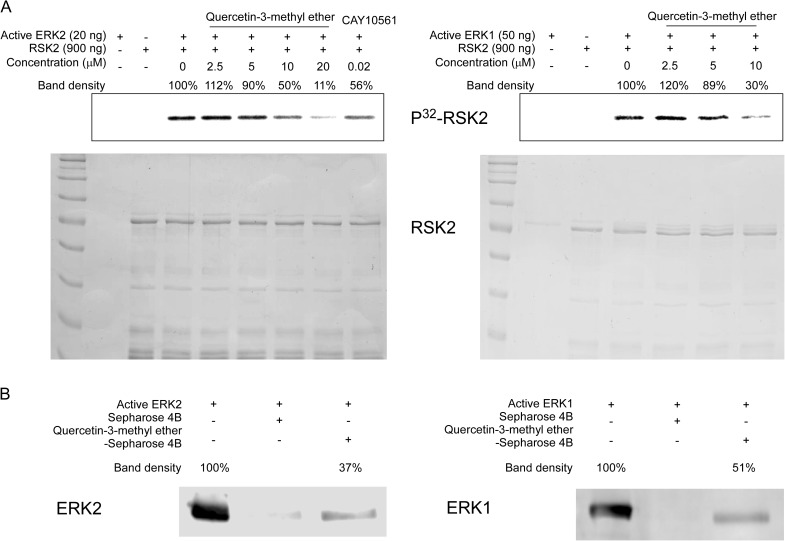
Quercetin-3-methyl ether inhibits ERKs activity and binds directly to ERK1 or ERK2 in vitro
We investigated the effects of quercetin-3-methyl ether on the kinase activity of ERK1 and 2. Kinase assay data revealed that quercetin-3-methyl ether (10 μM) suppressed ERK1 or ERK2 activity in vitro (Figure 5A). We next determined whether quercetin-3-methyl ether interacts directly with ERK1 or ERK2 and direct binding of quercetin-3-methyl ether to ERK1 or ERK2 was demonstrated by an in vitro pull-down assay (Figure 5B). These results indicated that ERKs are important molecular targets of quercetin-3-methyl ether for its antitumor-promoting activities.
Fig. 5.
Quercetin-3-methyl ether inhibits ERK1 or ERK2 kinase activity and directly binds with ERK1 or ERK2. (A) Quercetin-3-methyl ether inhibits ERKs kinase activity. An in vitro ERK1 or ERK2 kinase assay was performed as described in Materials and methods. A glutathione S-transferase–ribosomal S6 kinase 2 fusion protein was used in the in vitro kinase assay with active ERK1 or ERK2 and results were visualized by autoradiography. Coomassie blue staining shows the glutathione S-transferase fusion protein as a loading control. Left: lane 1, ERK2 control; lane 2, ribosomal S6 kinase 2 substrate control; lane 3, positive control, which indicates that active ERK2 phosphorylates the glutathione S-transferase–ribosomal S6 kinase 2 fusion protein; lanes 4, 5, 6 and 7, increasing amounts of quercetin-3-methyl ether suppresses ERK2 kinase activity; lane 8, negative control, which indicates that CAY10561 (commercial ERK2 inhibitor) suppresses ERK2 kinase activity. Right: lane 1, ERK1 control; lane 2, substrate control; lane 3, positive control, which indicates that active ERK1 phosphorylates the glutathione S-transferase–ribosomal S6 kinase 2 fusion protein; lanes 4, 5 and 6, increasing amounts of quercetin-3-methyl ether suppressed ERK1 kinase activity. (B) Quercetin-3-methyl ether directly binds with ERK1 and ERK2. The ERK1- or ERK2-quercetin-3-methyl ether binding was confirmed by immunoblotting using an antibody against ERKs. Lane 1 (input control), ERK1 (left) or ERK2 (right) protein standard; lane 2 (control), Sepharose 4B was used to pull down ERK1 or ERK2 as described in Materials and methods and lane 3, ERK1 or ERK2 was pulled down using quercetin-3-methyl ether conjugated-Sepharose 4B beads as described in Materials and methods.
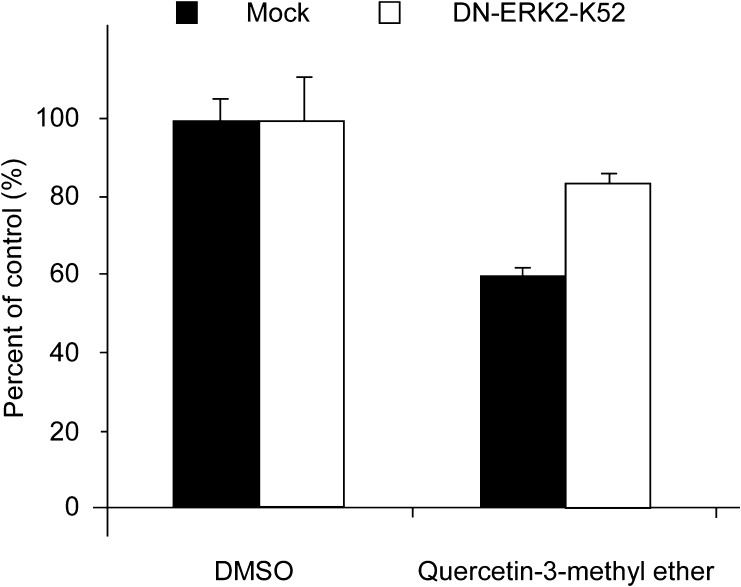
Quercetin-3-methyl ether inhibits cell proliferation by targeting ERKs
To confirm that ERKs are a molecular target for quercetin-3-methyl ether, we transiently transfected a plasmid expressing a dominant negative ERK2, pCEP4-DN-ERK2-K52, in JB6 P+ cells and compared the effect of quercetin-3-methyl ether in dominant negative-ERK2 and control cells. Results showed that quercetin-3-methyl ether was less effective in suppressing cell proliferation in dominant negative-ERK2 JB6 P+ cells (Figure 6). Results indicate that quercetin-3-methyl ether inhibited proliferation by targeting ERKs.
Fig. 6.
Quercetin-3-methyl ether inhibits cell proliferation by targeting ERKs. JB6 cells were transiently transfected with pCEP4-DN-ERK2-K52. Twenty-four hours after transfection, cells were treated with 10 μM quercetin-3-methyl ether or dimethyl sulfoxide vehicle and evaluated for cell growth at 48 h after drug treatment.
Discussion
The use of chemopreventive agents (especially naturally occurring plant products) to inhibit skin carcinogenesis, particularly at the promotion stage, is gaining attention. A variety of flavonoids have been reported to possess substantial skin photoprotective effects (2). Quercetin-3-methyl ether is one of those flavonoids and shares a similar structure with quercetin. Quercetin has been suggested as a potent skin cancer chemopreventive agent (22), whereas whether quercetin-3-methyl ether exhibits preventive effects in skin carcinogenesis is still unknown. In the present study, we show the chemopreventive effect of quercetin-3-methyl ether against UVB- or TPA-induced skin carcinogenesis and identified the molecular mechanism(s) and target(s). Cellular homeostasis, the equilibrium between cell proliferation and cell death, is controlled by cell cycle progression and apoptosis induction (23). Unchecked proliferative potential involving deregulation in cell cycle progression is generally described as a central process in the development of cancer (24). Suppression of cell proliferation is an important preventive approach (25). We examined the effects of quercetin-3-methyl ether on mouse epidermal JB6 P+ cell proliferation and cell cycle. The data showed that the inhibition of cell proliferation contributes to G2–M cell cycle accumulation induced by quercetin-3-methyl ether. Cell transformation arises from activation of oncoproteins and/or inactivation of tumor suppressor proteins. During cancer development, transformation occurs (26). Hence, inhibition of transformation is also a key preventive measure. Our result showed that quercetin-3-methyl ether (5 μM) significantly inhibited TPA-promoted cell transformation in JB6 P+ cells. Quercetin, which is widely distributed in nature, is a promising agent for cancer prevention. A recent study showed that quercetin exerted a strong anticarcinogenic effect in a 9,10-dimethyl-1,2-benzanthracene-initiated and TPA-promoted two-stage mouse skin cancer model (27). Here, we found that quercetin-3-methyl ether was more potent than quercetin in suppressing cell proliferation and transformation. This study suggested that inhibition of cell proliferation and transformation might be important mechanisms explaining the anticarcinogenic effects of quercetin-3-methyl ether.
AP-1 is extremely important in the processes of cell proliferation, differentiation, inflammation, survival and transformation (6–9,28). Therefore, AP-1 plays an important role in tumorigenesis and MAPKs play a critical role in the transcriptional activity of AP-1. AP-1 and MAPKs are key molecules activated after UVB or TPA exposure. Therefore, we performed a luciferase assay to detect AP-1 transcription activity and western blotting to assess MAPKs phosphorylation. Our results showed that quercetin-3-methyl ether inhibits AP-1 transcription activation through the suppression of ERKs phosphorylation. Quercetin-3-methyl ether inhibited UVB- or TPA-induced phosphorylation of ERKs but not JNKs or p38. Based on virtual screening results and because quercetin-3-methyl ether strongly suppressed the activation of ERKs signaling, we hypothesized that the molecular target of quercetin-3-methyl ether might be ERKs. Our results clearly showed that quercetin-3-methyl ether strongly inhibited ERKs kinase activity and that the inhibition resulted from the binding of quercetin-3-methyl ether with ERKs. ERKs phosphorylation plays an important role in cell proliferation. Loss-of-function ERK2 mutation inhibited the effectiveness of the quercetin-3-methyl ether. These results suggest that quercetin-3-methyl ether inhibits UVB- or TPA-induced skin cell carcinogenesis through the inhibition of the AP-1 transcription factor and MAPKs by targeting ERKs.
Besides skin cancer, mitogen-activated protein kinase kinase-ERK signaling pathway is upregulated in a broad spectrum of human tumor cells such as breast, colon, pancreatic, ovarian, prostate, non-small cell lung and glioma (5). The activation of ERK signaling pathway is important for AP-1 activity (29). ERK signaling and AP-1 seem to be important targets for chemoprevention in other cancers. Based on our study, quercetin-3-methyl ether may be also a good chemopreventive agent in other cancers besides skin cancer.
In summary, quercetin-3-methyl ether inhibits skin cell carcinogenesis. This inhibition occurs mainly through the suppression of proliferation by inducing G2–M accumulation, the inhibition of transformation and the regulation of AP-1 by blocking MAPKs signaling. Quercetin-3-methyl ether strongly inhibits ERKs activity by directly binding with ERK1 and ERK2 making it an ideal chemopreventive agent for skin cancer.
Funding
The Hormel Foundation and National Institutes of Health (CA0120388, CA027502, R37CA081064 and ES016548).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AP-1
activator protein-1
- DTT
dithiothreitol
- EMEM
Eagle's minimum essential medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-buffered saline
- TPA
12-O-tetradecanoylphorbol-13-acetate
- UV
ultraviolet
References
- 1.Jung SK, et al. Myricetin suppresses UVB-induced skin cancer by targeting Fyn. Cancer Res. 2008;68:6021–6029. doi: 10.1158/0008-5472.CAN-08-0899. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JA, et al. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assefa Z, et al. Differential stimulation of ERK and JNK activities by ultraviolet B irradiation and epidermal growth factor in human keratinocytes. J. Invest. Dermatol. 1997;108:886–891. doi: 10.1111/1523-1747.ep12292595. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, et al. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 5.Sebolt-Leopold JS, et al. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 6.Dong Z, et al. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc. Natl Acad. Sci. USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, et al. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc. Natl Acad. Sci. USA. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu G, et al. Two novel glycosides from the fruits of Morinda citrifolia (noni) inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001;61:5749–5756. [PubMed] [Google Scholar]
- 9.Young MR, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc. Natl Acad. Sci. USA. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EK, et al. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Suzukawa K, et al. AP-1, NF-kappa-B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ. Health Perspect. 2002;110:865–870. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gioeli D, et al. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 13.Oka H, et al. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995;55:4182–4187. [PubMed] [Google Scholar]
- 14.Rubio S, et al. Acetyl derivative of quercetin 3-methyl ether-induced cell death in human leukemia cells is amplified by the inhibition of ERK. Carcinogenesis. 2007;28:2105–2113. doi: 10.1093/carcin/bgm131. [DOI] [PubMed] [Google Scholar]
- 15.Lee EH, et al. Constituents of the stems and fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. 2003;26:1018–1023. doi: 10.1007/BF02994752. [DOI] [PubMed] [Google Scholar]
- 16.Takeara R, et al. Trypanocidal activity of Lychnophora staavioides Mart. (Vernonieae, Asteraceae) Phytomedicine. 2003;10:490–493. doi: 10.1078/094471103322331430. [DOI] [PubMed] [Google Scholar]
- 17.Wei BL, et al. In vitro anti-inflammatory effects of quercetin 3-O-methyl ether and other constituents from Rhamnus species. Planta Med. 2001;67:745–747. doi: 10.1055/s-2001-18339. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, et al. Glucose-containing flavones—their synthesis and antioxidant and neuroprotective activities. Bioorg. Med. Chem. Lett. 2009;19:6009–6013. doi: 10.1016/j.bmcl.2009.09.062. [DOI] [PubMed] [Google Scholar]
- 19.Bode AM, et al. Signal transduction pathways in cancer development and as targets for cancer prevention. Prog. Nucleic Acid Res. Mol. Biol. 2005;79:237–297. doi: 10.1016/S0079-6603(04)79005-4. [DOI] [PubMed] [Google Scholar]
- 20.Colburn NH, et al. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc. Natl Acad. Sci. USA. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plesca D, et al. E2F4 function in G2: maintaining G2-arrest to prevent mitotic entry with damaged DNA. Cell Cycle. 2007;6:1147–1152. doi: 10.4161/cc.6.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KW, et al. Raf and MEK protein kinases are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–955. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RP, et al. Natural flavonoids targeting deregulated cell cycle progression in cancer cells. Curr. Drug Targets. 2006;7:345–354. doi: 10.2174/138945006776055004. [DOI] [PubMed] [Google Scholar]
- 24.Deep G, et al. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 25.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Kang NJ, et al. Cocoa procyanidins suppress transformation by inhibiting mitogen-activated protein kinase kinase. J. Biol. Chem. 2008;283:20664–20673. doi: 10.1074/jbc.M800263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soleas GJ, et al. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin. Biochem. 2002;35:119–124. doi: 10.1016/s0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 28.Cooper SJ, et al. Ultraviolet B regulation of transcription factor families: roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets. 2007;7:325–334. doi: 10.2174/156800907780809714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]