Abstract
Naturally occurring allyl isothiocyanate (AITC) was recently shown to be selectively delivered to bladder cancer tissue via urinary excretion and to inhibit bladder cancer growth and muscle invasion in an animal model. AITC is excreted in urine mainly as N-acetyl-S-(N-allylthiocarbamoyl)cysteine, more commonly known as the N-acetylcysteine conjugate (NAC-AITC). We show here that treatment of human bladder cancer UM-UC-3 cells or rat bladder cancer AY-27 cells with NAC-AITC at 15 μM results in significant inhibition of cell growth and proliferation, together with cell cycle arrest and apoptosis. We also show that NAC-AITC administered orally at 10 μmol/kg body wt inhibits cancer growth by 40% and muscle invasion by 49% in an orthotopic rat bladder cancer model. Furthermore, the anticancer activity of NAC-AITC is associated with the modulation of several important molecular targets, including downregulation of both α-tubulin and β-tubulin, activation of caspase-3 and downregulation of vascular endothelial growth factor. These results are similar to those shown previously for AITC and are consistent with the understanding that NAC-AITC is a carrier of AITC. Furthermore, comparison of the pharmacokinetic and physical properties of NAC-AITC with those of AITC suggests that NAC-AITC is superior to AITC for potential use for prevention and therapy of bladder cancer.
Introduction
Allyl isothiocyanate (AITC) occurs in many common cruciferous vegetables and is particularly abundant in mustard, horseradish and wasabi (1,2). We have recently shown that both AITC and AITC-rich mustard seed powder inhibited the survival and proliferation of bladder cancer cells in vitro and inhibited cancer growth and muscle invasion in an orthotopic bladder cancer model in vivo (3,4). Both AITC and the AITC-rich mustard seed powder arrested cells in the G2/M phase and activated apoptosis in bladder cancer cells and cancer tissues (3,4). Further study showed that AITC promoted ubiquitination and degradation of both α-tubulin and β-tubulin and arrested bladder cancer cells in mitosis, which in turn activated mitochondria-mediated apoptosis via Bcl-2 phosphorylation (5). We also found that AITC was significantly less toxic to normal human bladder epithelial cells than to human bladder carcinoma cells and was selectively delivered to bladder cancer tissues through urinary excretion (3). These findings show that AITC is a highly promising agent for bladder cancer prevention and therapy. AITC is primarily metabolized through the mercapturic acid pathway in vivo. Initial conjugation of the –N=C=S group with the cysteine thiol of glutathione gives rise to the corresponding conjugate, which is further metabolized successively to the cysteinylglycine conjugate and the cysteine conjugate, ultimately yielding N-acetyl-S-(N-allylthiocarbamoyl)cysteine, commonly known as the N-acetylcysteine conjugate (NAC-AITC; see Figure 1A for its chemical structure), which is excreted in the urine (6). In rats dosed orally with AITC or [14C]AITC, 60–65% of the 14C in the urine was in the form of NAC-AITC (7–9). NAC-AITC apparently is also the major urinary metabolite in humans, as 42–54% of an oral AITC dose was recovered in the urine as the conjugate within 10–12 h after dosing (10,11). These results suggest that NAC-AITC excreted in urine may be ultimately responsible for the in vivo anticancer activity of AITC in the bladder.
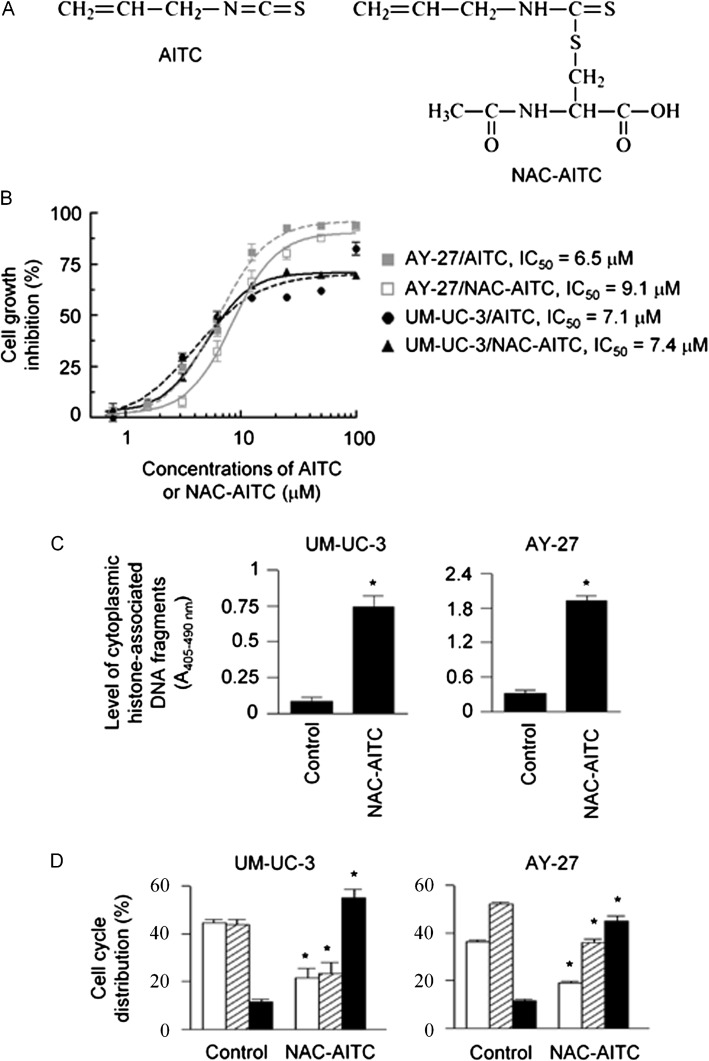
Fig. 1.
The effect of NAC-AITC on cell survival and proliferation. NAC-AITC was evaluated in both UM-UC-3 cells and AY-27 cells. (A) Chemical structures of AITC and NAC-AITC. (B) Cell growth and proliferation, measured by 3-(4,6-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, 72 h treatment with AITC or NAC-AITC at indicated concentrations. IC50 was calculated from the non-linear regression curve fit. (C) Apoptosis, measured by an enzyme-linked immunosorbent assay, 24 h NAC-AITC treatment at 15 μM. (D) Cell cycle (open bars, G1; shaded bars, S; closed bars, G2/M), measured by flow cytometry, 24 h NAC-AITC treatment at 15 μM. *P < 0.005, compared with control.
In the present study, we have examined the anticancer activity of NAC-AITC against bladder cancer cells growing in vitro and in a syngeneic orthotopic rat bladder cancer model in vivo. We have also compared the pharmacokinetic profiles of AITC and NAC-AITC. Our study demonstrates the potent anticancer activity of NAC-AITC and suggests that NAC-AITC is a promising and perhaps better agent than AITC itself for bladder cancer prevention and therapy.
Materials and methods
Materials
AITC was purchased from Sigma–Aldrich (St Louis, MO). NAC-AITC was synthesized and purified by the method of Vermeulen et al. (12), using redistilled AITC and N-acetyl cysteine (Sigma–Aldrich), and verified by mass spectrometry. The syntheses of AITC metabolites, including the glutathione conjugate (GS-AITC), the cysteinylglycine conjugate (Gly-Cys-AITC) and the cysteine conjugate (Cys-AITC), followed a similar strategy. Antibodies specific for cleaved caspase-3, cleaved caspase-9 and β-tubulin were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for α-tubulin, vascular endothelial growth factor (VEGF) and GAPDH were purchased from EMD/Calbiochem (Gibbstown, NJ), Santa Cruz Biotechnology (Santa Cruz, CA) and Millipore (Bellerica, MA), respectively.
Cells and animals
Human bladder cancer UM-UC-3 cell line and rat bladder cancer AY-27 cell line were used in the study; their origin and culture conditions have been reported previously (3). Female F344 rats were purchased from Harlan Laboratories (Indianapolis, IN) and were acclimatized for ∼1 week before experiments. The animals were maintained at 21–23°C and a 12 h light/dark cycle with free access to food (Harlan Teklad LM-485 mouse/rat sterilizable diet) and water. All animal protocols and procedures were approved by the Roswell Park Cancer Institute Animal Care and Use committee.
Assays for cell proliferation, cell cycle arrest and apoptosis
To determine the antiproliferative activity of NAC-AITC, AY-27 cells were grown in 96-well microtiter plates (5 × 106 cells with 0.15 ml medium per well) for 24 h and then grown for 72 h in fresh medium (0.2 ml per well) containing a series of concentrations of NAC-AITC or solvent. Cell growth was measured at the end of treatment using the 3-(4,6-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (13), from which the half maximal inhibitory concentration (IC50) of NAC-AITC was calculated. AITC was also evaluated for comparison.
The effect of NAC-AITC on cell cycle progression was measured by flow cytometry as described previously (14). Briefly, 1.5 × 106 AY-27 cells were grown in each 10 cm plate with 10 ml medium for 24 h and then treated with NAC-AITC or solvent for 24 h before analysis.
Induction of apoptosis by NAC-AITC was measured using the Cell Death Detection ELISAplus kit (Roche Diagnostics, Indianapolis, IN), following the manufacturer’s instruction. Briefly, cells were cultured in 96-well plates as described above and treated with NAC-AITC or solvent for 24 h. After this time, the cells were treated with lysis buffer, and after a low-speed centrifugation, a portion of the supernatant fraction was used for spectroscopic measurement (expressed as A405–490 nm) of cytoplasmic levels of histone-associated mononucleosomes or oligonucleosomes by an enzyme-linked immunosorbent assay.
In all experiments, NAC-AITC and AITC were dissolved in dimethyl sulfoxide (DMSO) and then diluted in water. The DMSO concentration in culture medium was ≤0.1%.
A syngeneic orthotopic rat bladder cancer model
The anticancer activity of NAC-AITC was evaluated in a syngeneic orthotopic rat bladder cancer model as described previously (3), with a minor modification. Briefly, after priming the bladder mucosa with 0.3 ml of 0.1 N HCl for 15 s, followed first by treatment with 0.3 ml of 0.1 N KOH for 15 s to neutralize the acid and then phosphate-buffered saline wash, female F344 rats (8–10 weeks of age) were inoculated orthotopically via a catheter (BD Insyte™ Autoguard™ shielded IV catheter, 18 G × 48 mm) through the urethra with AY-27 cells (1 × 106 cells in 0.5 ml serum-free medium per rat). Female rats were used in the experiment because urethral catheterization in male rats is difficult. One day after the inoculation, the rats were randomly assigned to receive by gavage either vehicle (1.33 ml water containing 5% DMSO/kg body wt) or NAC-AITC in an equal volume of water/DMSO once daily for 3 weeks. NAC-AITC was freshly dissolved in DMSO and diluted in water. The animals were monitored daily and were euthanized 24 h after the last dose of NAC-AITC or vehicle; the bladders were quickly removed, and after opening, were examined for macroscopic lesions, weighed and photographed with a digital camera. Tumor was present in all the bladders; tumor weight was calculated by subtracting the average normal bladder weight (from untreated rats at the same age) from the tumor-bearing bladder weight. Some bladders that showed significant edema/inflammation were excluded to ensure accurate measurement of tumor weight. Approximately, half of each bladder was fixed in formalin for histological analysis and the other half was frozen in liquid nitrogen for western blot analysis.
Measurement of pharmacokinetic profiles of AITC and NAC-AITC
The experiment was carried out in female F344 rats since these animals were used in the rat bladder cancer model described above. Groups of three to five rats (8–9 weeks of age) were given a single oral dose of AITC, NAC-AITC or vehicles. AITC was administered in 0.5 ml of soy oil per rat, whereas NAC-AITC was dissolved in DMSO and diluted with water and administered in 0.2 ml volume (5% DMSO) per rat. The rats were immediately transferred to metabolism cages (one rat per cage), with free access to food and water, for urine collection over four consecutive periods of 0–1.5, 1.5–3, 3–6 and 6–24 h. Additional groups of female F344 rats that were treated with the same doses of AITC, NAC-AITC or the vehicles were used for blood drawing, to avoid potential impact of blood loss on the pharmacokinetics of the compounds. One group of animals was killed at 1.5, 3, 6 and 24 h after dosing for blood collection and plasma preparation.
AITC and NAC-AITC contents in the plasma and urine were determined using the high-performance liquid chromatography (HPLC)-based cyclocondensation assay, as described previously (15), and are expressed as AITC equivalent or NAC-AITC equivalent. The cyclocondensation assay detects AITC, NAC-AITC and other metabolites of AITC formed in the mercapturic acid pathway (16).
To specifically measure urinary levels of NAC-AITC, groups of five rats were dosed orally with either AITC or NAC-AITC at 300 μmol/kg and immediately moved to metabolism cages (one rat per cage) for 24 h urine collection. AITC and NAC-AITC were freshly prepared in soy oil or 5% aqueous DMSO, as described above. Urine samples were fractionated by HPLC, using an Agilent system with a diode-array detector, to separate NAC-AITC from AITC and other metabolites including GS-AITC, Gly-Cys-AITC and Cys-AITC before analysis of the fractions by the cyclocondensation assay. The mobile phase consisted of acetonitrile and 20 mM aqueous potassium phosphate (pH 3). The system was operated at a flow rate of 1.75 ml/min, using a Partisil 10 ODS-2 reverse-phase column (4.6 × 250 mm; Whatman), beginning with an isocratic phase of 15% acetonitrile for 15 min, followed by 100% acetonitrile for 5 min. The compounds were monitored at both 230 nm (AITC) and 254 nm (its metabolites). Pure synthetic standards were used to set up the HPLC conditions and to establish retention times for each compound; GS-AITC, Gly-Cys-AITC, Cys-AITC, NAC-AITC and AITC eluted at 3.3, 4.4, 6.5, 8.9 and 19.5 min, respectively. The fraction corresponding to NAC-AITC was collected and quantified by the cyclocondensation assay; its identity was confirmed by infusion electrospray ionization mass spectrometry.
Measurement of dissociation of NAC-AITC
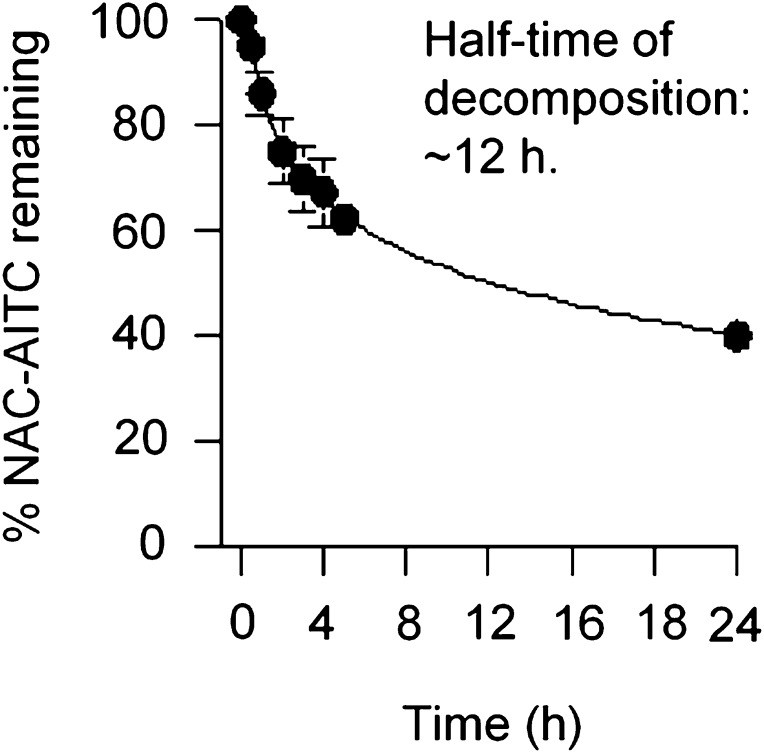
The assay was based on a protocol by Conaway et al. (17). Briefly, a solution of NAC-AITC was prepared as rapidly as possible by dilution of 0.2 ml of 5 mM NAC-AITC freshly prepared in 20 mM sodium phosphate buffer (pH 7.4): DMSO (1:1) with 20 mM sodium phosphate buffer (pH 7.4) to 10 ml, resulting in a 0.1 mM NAC-AITC solution in 1% DMSO. An aliquot (0.05 ml) was immediately analyzed by HPLC; the remainder of the solution was incubated at 37°C on a shaker, and an aliquot (0.05 ml) was analyzed by HPLC at different time points up to 24 h. The HPLC condition was the same as used in the urine analysis described above. A plot of the conjugate peak area remaining versus time was generated to determine the half-time of decomposition.
Western blot analysis
Cells after harvest were washed with ice-cold phosphate-buffered saline, suspended in radioimmunoprecipitation assay lysis buffer supplemented with a protease inhibitor cocktail (Sigma–Aldrich) and further lysed by sonication. Tissue specimens were washed with ice-cold phosphate-buffered saline and homogenized in radioimmunoprecipitation assay lysis buffer supplemented with the protease inhibitor cocktail in glass homogenizers. After removal of the debris from both cell lysates and tissue homogenates by centrifugation and measurement of protein contents by a bicinchoninic acid protein assay kit (Pierce, Rockford, IL), the samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (8–12%), followed by transfer to polyvinylidene difluoride membranes. The membranes were then probed by a specific antibody and stained using SuperSignal West Pico Chemiluminescence detection system (Thermo Scientific, Rockford, IL).
Histological analysis
Rat bladder specimens fixed in formalin were paraffin embedded, cut at ∼4 μm and stained with hematoxylin and eosin. The slides were examined for bladder and tumor histology using a Nikon 50i light microscope.
Statistical analysis
All numerical values are presented as mean ± SE. The difference between the means of two groups was analyzed for statistical significance using unpaired two-tailed Student t-test. One-way analysis of variance was used for multigroup comparison, followed by Dunnett’s multiple comparison test. P < 0.05 was considered significant.
Results and discussion
NAC-AITC inhibits the survival and proliferation of bladder cancer cells
Treatment of UM-UC-3 and AY-27 cells with NAC-AITC led to dose-dependent inhibition of cell proliferation, with an IC50 value of 7.4 μM in UM-UC-3 cells and 9.1 μM in AY-27 cells; AITC itself was of similar efficacy (Figure 1B). Inhibition of cell proliferation by NAC-AITC was associated with significant cell cycle arrest and induction of apoptosis. Treatment of UM-UC-3 and AY-27 cells with NAC-AITC at 15 μM for 24 h resulted in 8.8- and 10.6-fold increase in apoptotic activity, respectively (Figure 1C). Cells were arrested by NAC-AITC in the G2/M phase; 55% UM-UC-3 cells and 45% AY-27 cells were detected in G2/M phase after treatment with NAC-AITC at 15 μM for 24 h compared with only 11–12% of control cells present in this phase (Figure 1D). The effects of NAC-AITC on cell cycle and apoptosis were similar to those recorded with AITC (3). Previous studies have suggested that N-acetylcysteine conjugates of isothiocyanates, including NAC-AITC, are not biologically active themselves and cannot be taken up by cells, but serve as the carriers of their parent compounds (17–19). Indeed, the half-time of dissociation of NAC-AITC in phosphate buffer (pH 7.4, 37°C) was ∼12 h (Figure 2). However, we found that NAC-AITC when kept dry was completely stable for at least 16 months at room temperature (data not shown).
Fig. 2.
Dissociation of NAC-AITC. NAC-AITC at 100 μM was freshly prepared in 20 mM sodium phosphate buffer (pH 7.4) containing 1% DMSO and incubated at 37°C for 0, 0.5, 1, 2, 3, 4, 5 and 24 h. At each time point, an aliquot of the solution was analyzed for remaining NAC-AITC by HPLC. Each value is a mean ± SD (n = 3).
NAC-AITC inhibits bladder cancer growth in vivo
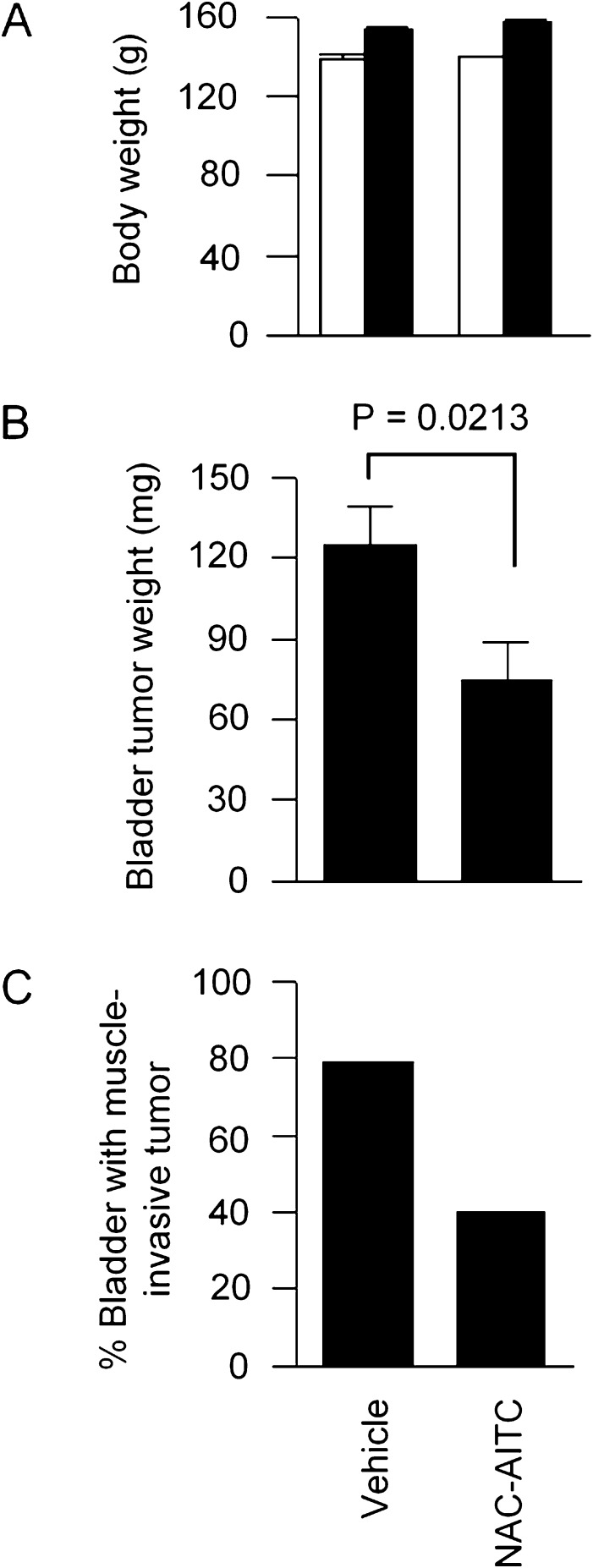
NAC-AITC was next evaluated in an orthotopic rat bladder cancer model. AITC was shown previously to significantly inhibit bladder cancer growth and muscle invasion in this model (3). Bladder cancer AY-27 cells were inoculated intravesically via a urethral catheter. Daily oral administration of NAC-AITC at 10 μmol/kg body wt was initiated 1 day after AY-27 cell inoculation and continued for 3 weeks. The same dose and treatment time were previously used when AITC was evaluated in the same animal model. All rats behaved normally during NAC-AITC treatment, and no significant effect on body weight was detected (Figure 3A). All rats developed bladder tumors, but treatment with NAC-AITC inhibited tumor growth by 40% (Figure 3B). Moreover, although 79% of the bladders in the control group showed tumor invasion into the musculature, muscle invasion occurred in only 30% of the bladders in the NAC-AITC group (Figure 3B). In comparison, AITC, which was previously evaluated in the same animal model at the same dose level and treatment duration, inhibited tumor growth and muscle invasion by 30 and 73%, respectively (3). Thus, the anticancer efficacy of NAC-AITC is similar to that of AITC.
Fig. 3.
Inhibition of bladder cancer development by NAC-AITC. Bladder cancer was initiated in female F344 rats by orthotopic inoculation of bladder cancer AY-27 cells. Oral administration of NAC-AITC at 10 μmol/kg body wt once daily was started 1 day after cancer cell inoculation and ended 3 weeks later. There were 23 rats in the control group and 15 rats in the treatment group. (A) The initial (open bars) and final (closed bars) body weight. (B) Bladder tumor weight. Each value is a mean ± SE. (C) Percentage of bladders where the tumor invaded the muscle tissue.
Molecular targets of NAC-AITC
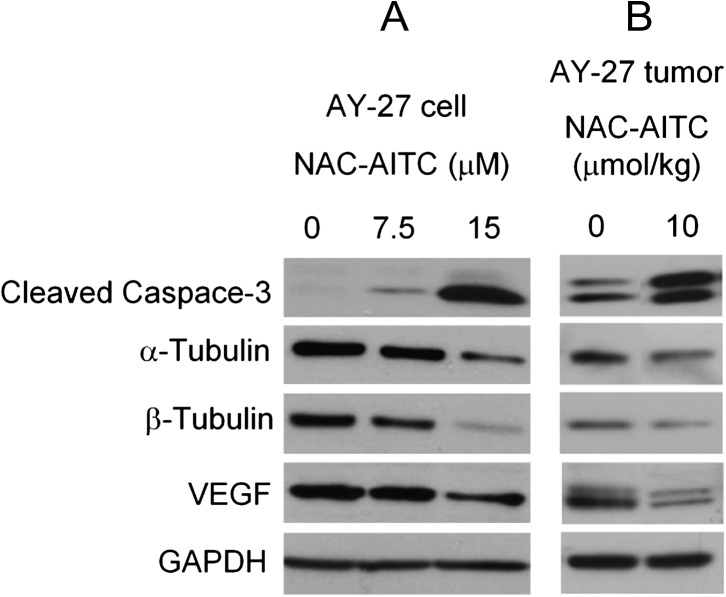
We next examined the effect of NAC-AITC on several proteins, which are known to play important roles in cancer cell survival, proliferation and invasion, including caspase-3, α-tubulin, β-tubulin and VEGF. These proteins were recently shown to be modulated by AITC or an AITC-containing mustard seed powder in bladder cancer cells (4,5). Caspase-3 and VEGF were also modulated by either AITC or the mustard seed powder in AY-27 cells and bladder tumors derived from AY-27 cells in vivo (3,4). Treatment of AY-27 cells with NAC-AITC at 7.5 and 15 μM caused dose-dependent cleavage/activation of caspase-3 and downregulation of α-tubulin, β-tubulin and VEGF (Figure 4A). Moreover, similar changes in these proteins were also detected in bladder tumors removed from rats treated with NAC-AITC at 10 μmol/kg (Figure 4B). Although it remains to be determined to what extent modulation of these proteins by NAC-AITC may account for inhibition of tumor growth and muscle invasion, the results described above make it clear that NAC-AITC replicates the anticancer mechanism of AITC.
Fig. 4.
Molecular targets of NAC-AITC in bladder cancer. AY-27 cells in culture were treated with NAC-AITC for 24 h. The results are representative of at least two experiments. The bladder tumors were removed from rats, which were treated with NAC-AITC orally at 10 μmol/kg once daily for 3 weeks, starting 1 day after intravesicular inoculation of AY-27 cells. The results are representative of three to five tumors assayed in each group. Cell lysates and tumor tissue homogenates were analyzed by western blot analysis, using GAPDH as a loading control.
NAC-AITC is selectively delivered to bladder through urinary excretion
We have also compared the pharmacokinetic profiles of NAC-AITC and AITC as shown in Table I. Total levels of AITC, NAC-AITC and other potential AITC metabolites formed in the mercapturic acid pathway were measured in blood and urine by the cyclocondensation assay and were expressed as AITC equivalent or NAC-AITC equivalent. In rats that were given a single oral dose of NAC-AITC (10 μmol/kg), the plasma concentration of NAC-AITC equivalent was 1.2 and 0.7 μM at 1.5 and 3 h post-dosing, respectively, and was undetectable by 6 h. In contrast, the average urinary concentrations of NAC-AITC equivalent were 398 and 1510 μM during the first 1.5 h and the 1.5–3 h interval after NAC-AITC dosing, respectively, which are 332- and 2157-fold higher than the plasma concentrations measured at 1.5 and 3 h, as mentioned above. The average urinary concentration of NAC-AITC equivalent remained high at 337 μM, 3–6 h after the dosing, although it decreased >20-fold to 16.6 μM in the urine collected at 6–24 h after dosing.
Table I.
Pharmacokinetic profiles of NAC-AITC and AITC
| Rat treatment | Plasma |
Urine |
||
| Time (h) | AITC or NAC-AITC equivalent (μM) | Time (h) | AITC or NAC-AITC equivalent (μM) | |
| Controla | <0.5 | <0.2 | ||
| NAC-AITC (10 μmol/kg) | 1.5 | 1.2 ± 0.3 | 0–1.5 | 398 ± 118 |
| 3 | 0.7 ± 0.2 | 1.5–3 | 1510 ± 101b | |
| 6 | <0.5 | 3–6 | 337 ± 99.3c | |
| 24 | <0.5 | 6–24 | 16.6 ± 1.1 | |
| AITC (10 μmol/kg) | 1.5 | 1.5 ± 0.2 | 0–1.5 | 115 ± 44.3 |
| 3 | <0.5 | 1.5–3 | 415 ± 86.7b | |
| 6 | <0.5 | 3–6 | 744 ± 109c | |
| 24 | <0.5 | 6–24 | 34.7 ± 6.8 | |
The two values marked with the same alphabets (b,c) are statistically significant.
Control animals were given vehicle from which blood and urine were collected at corresponding times. Each value is mean ± SE (n = 5).
The profound difference in concentrations of NAC-AITC equivalent between the plasma and urine in rats dosed with NAC-AITC, as described above, resembles that of AITC. In rats given a single oral dose of AITC (10 μmol/kg), the plasma concentration of AITC equivalent was 1.5 μM at 1.5 h post-dosing and was undetectable thereafter. In contrast, urinary concentrations of AITC equivalent ranged from 115–744 μM within 6 h of AITC dosing and decreased to 30 μM in the urine collected at 6–24 h after AITC dosing. Moreover, following treatment with NAC-AITC or AITC (a single oral dose at 10 μmol/kg), the 24 h cumulative urinary recovery for NAC-AITC and AITC in rats, as detected by the cyclocondensation assay, was 62.5 ± 6.5% and 54.1 ± 3.7% (mean ± SE), respectively, and the difference is not statistically significant. Further analysis of urine samples by HPLC and mass spectrometry showed that NAC-AITC was the principal excretion product whether rats were given AITC or NAC-AITC. Hence, in the 24 h urine collected from rats given a single oral dose of AITC or NAC-AITC at 300 μmol/kg (a relatively high dose was used to facilitate detection of NAC-AITC), 85.8% of the urinary AITC equivalent and 83.3% of the urinary NAC-AITC equivalent were NAC-AITC, respectively (Table II). It is not known at the present time if orally dosed NAC-AITC is excreted in urine intact or first dissociates to AITC, which is then metabolized via the mercapturic acid pathway to reform NAC-AITC. However, orally dosed NAC-AITC was excreted in urine at a significantly faster rate than AITC, based on the comparison of their urinary concentrations at different time points, as shown in Table I. Furthermore, the peak urinary concentration of AITC or NAC-AITC equivalent was reached at between 1.5 and 3 h following NAC-AITC dosing, but reached until 3 to 6 h after AITC administration.
Table II.
Urinary recovery in rats given AITC and NAC-AITC
| Rat treatment | 24 h urinary recovery |
||
| Total recoverya % of administered dose | Recovered as NAC-AITC |
||
| % Of adminstered dose | % Of total recovery | ||
| AITC | 54.8 ± 5.0 | 47.0 ± 9.3 | 85.8 ± 3.8 |
| NAC-AITC | 56.8 ± 3.1 | 47.3 ± 1.8 | 83.3 ± 4.5 |
As AITC equivalent or NAC-AITC equivalent measured by the cyclocondensation assay. Rats were given a single oral dose of AITC or NAC-AITC at 300 μmol/kg, followed by 24 h urine collection. Each value is a mean ± SE (three to five rats per group). To measure NAC-AITC, urine samples were fractionated by HPLC, and the NAC-AITC fraction was then subjected to the cyclocondensation assay.
In summary, both AITC and NAC-AITC after oral administration are rapidly excreted and concentrated in urine as NAC-AITC; urinary NAC-AITC serves as a carrier of AITC, which is ultimately responsible for bladder cancer inhibition in rats treated with AITC or NAC-AITC. NAC-AITC may be a more suitable agent than AITC for potential bladder cancer prevention and therapy, in view of its pharmacokinetic profile and favorable physical properties—a stable gum with little odor, compared with AITC which is an unstable, pungent and volatile liquid. In fact, we subsequently found that NAC-AITC could be readily formed into a tablet by conventional tableting technology. NAC-AITC is particularly attractive for potential use against recurrence of superficial bladder cancer, as it can be administered orally and delivers AITC to the bladder intravesicularly via urinary excretion. The majority of bladder cancers (∼80%) are initially diagnosed without muscle invasion (superficial bladder cancer), which are typically treated with transurethral resection, but most patients experience recurrence and disease progression. Intravesical therapies including Bacillus Calmette–Guerin and certain cancer chemotherapeutic agents are currently used to prevent recurrence and progression of superficial bladder cancer in high-risk patients. Although these therapies have shown significant clinical efficacy, they are not always effective and mandate urethral catheterization for delivery of the agents, and Bacillus Calmette–Guerin use is often associated with many side effects (20–22). Moreover, for patients deemed to be at relatively low risk of cancer recurrence, no post-transurethral resection therapy is currently available, and for these individuals, NAC-AITC could prove a particularly valuable therapeutic.
Funding
National Cancer Institute (R01CA124627 and P30CA016056).
Acknowledgments
We would like to thank Ma Khin Marlar and A.Latif Kazim for technical support.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AITC
allyl isothiocyanate
- DMSO
dimethyl sulfoxide
- HPLC
high-performance liquid chromatography
- NAC-AITC
N-acetyl-S-(N-allylthiocarbamoyl)cysteine or N-acetylcysteine conjugate of allyl isothiocyanate
- VEGF
vascular endothelial growth factor
References
- 1.Uematsu Y, et al. Determination of isothiocyanates and related compounds in mustard extract and horseradish extract used as natural food additives. Shokuhin Eiseigaku Zasshi. 2002;43:10–17. doi: 10.3358/shokueishi.43.10. [DOI] [PubMed] [Google Scholar]
- 2.Sultana T, et al. Effects of fertilisation on the allyl isothiocyanate profile of above-ground tissue of New Zealand-grown wasabi. J. Sci. Food Agric. 2002;82:1477–1482. [Google Scholar]
- 3.Bhattacharya A, et al. Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis. 2010;31:281–286. doi: 10.1093/carcin/bgp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya A, et al. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis. 2010;31:2105–2110. doi: 10.1093/carcin/bgq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng F, et al. Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via BCL-2 phosphorylation. J. Biol. Chem. 2011;286:32259–32267. doi: 10.1074/jbc.M111.278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannou YM, et al. Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol. Appl. Pharmacol. 1984;75:173–181. doi: 10.1016/0041-008x(84)90199-6. [DOI] [PubMed] [Google Scholar]
- 8.Bollard M, et al. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem. Toxicol. 1997;35:933–943. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 9.Munday R, et al. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr. Cancer. 2006;54:223–231. doi: 10.1207/s15327914nc5402_9. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro TA, et al. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomarkers Prev. 1998;7:1091–2100. [PubMed] [Google Scholar]
- 11.Jiao D, et al. Identification and quantification of the N-acetylcysteine conjugate of allyl isothiocyanate in human urine after ingestion of mustard. Cancer Epidemiol. Biomarkers Prev. 1994;3:487–492. [PubMed] [Google Scholar]
- 12.Vermeulen M, et al. Synthesis of isothiocyanate-derived mercapturic acids. Eur. J. Med. Chem. 2003;38:729–737. doi: 10.1016/s0223-5234(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 13.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, et al. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 15.Munday R, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, et al. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal. Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 17.Conaway CC, et al. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem. Res. Toxicol. 2001;14:1170–1176. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 18.Bruggeman IM, et al. Glutathione- and cysteine-mediated cytotoxicity of allyl and benzyl isothiocyanate. Toxicol. Appl. Pharmacol. 1986;83:349–359. doi: 10.1016/0041-008x(86)90312-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 20.Jacobs BL, et al. Bladder cancer in 2010: how far have we come? CA Cancer J. Clin. 2010;60:244–272. doi: 10.3322/caac.20077. [DOI] [PubMed] [Google Scholar]
- 21.Amling CL. Diagnosis and management of superficial bladder cancer. Curr. Probl. Cancer. 2001;25:219–278. doi: 10.1067/mcn.2001.117539. [DOI] [PubMed] [Google Scholar]
- 22.Pow-Sang JM, et al. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7:335–339. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]