Abstract
Previous studies have shown that decorin expression is significantly reduced in colorectal cancer tissues and cancer cells, and genetic deletion of the decorin gene is sufficient to cause intestinal tumor formation in mice, resulting from a downregulation of p21, p27kip1 and E-cadherin and an upregulation of β-catenin signaling [Bi,X. et al. (2008) Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis, 29, 1435–1440]. However, the regulation of E-cadherin by decorin and its implication in cancer formation and metastasis is largely unknown. Using a decorin knockout mouse model (Dcn−/− mice) and manipulated expression of decorin in human colorectal cancer cells, we found that E-cadherin, a protein that regulates cell–cell adhesion, epithelial–mesenchymal transition and metastasis, was almost completely lost in Dcn−/− mouse intestine, and loss of decorin and E-cadherin accelerated colon cancer cell growth and invasion in Dcn−/− mice. However, increasing decorin expression in colorectal cancer cells attenuated cancer cell malignancy, including inhibition of cancer cell proliferation, promotion of apoptosis and importantly, attenuation of cancer cell migration. All these changes were linked to the regulation of E-cadherin by decorin. Moreover, overexpression of decorin upregulated E-cadherin through increasing of E-cadherin protein stability as E-cadherin messenger RNA and promoter activity were not affected. Co-immunoprecipitation assay showed a physical binding between decorin and E-cadherin proteins. Taken together, our results provide direct evidence that decorin-mediated inhibition of colorectal cancer growth and migration are through the interaction with and stabilization of E-cadherin.
Introduction
Decorin is a member of the small leucine-rich proteoglycan family that is primarily synthesized by fibroblasts and myofibroblasts and regulates collagen fibrillogenesis. It is also involved in a number of physiological and pathological processes including the control of osteogenic stem cells, muscular development, wound healing and cancer (1,2). Several studies have shown tumor suppressor function of decorin. Decorin expression levels are significantly reduced in human colorectal cancer tissues (3). Increasing decorin expression in colon, breast and squamous cancer cell lines suppresses their growth and progression. These effects are associated with the induction of p21 (4–6), interaction with transforming growth factor β (5,7,8) and epithelial growth factor receptor (EGFR) (9–11). Loss of decorin accelerates malignant lymphomas in p53 mutant mice, leading to early mortality (12). Low levels of decorin in invasive breast carcinomas are associated with larger tumor size, shortened time to progression and poor outcome (13). Similarly, low decorin levels are also correlated with accelerated lung cancer progression (14). In contrast, administration with human recombinant decorin or infection with a decorin-expressing adenovirus inhibited breast cancer cell growth and prevented pulmonary metastasis in nude mice through decorin’s long-term downregulation of the ErbB2 tyrosine kinase cascade (15,16).
Earlier study showed that the mice in a mixture background with a targeted disruption of the decorin gene do not develop spontaneous tumors (17). Our recent work showed decorin deficiency could cause spontaneous intestinal tumor formation in the mice that are in C57Bl/6 background (3). The mechanism of tumorigenesis involves downregulation of p21, p27 and E-cadherin and upregulation of the β-catenin signaling. Further study showed that decorin could interact with Met, which induces transient receptor activation, recruits E3 ubiquintin ligase c-Cbl and rapid intracellular degradation of Met and finally leads to a suppression of β-catenin, a downstream Met effector (18).
Numerous studies have demonstrated that loss of E-cadherin enhances cancer metastasis through modulation of epithelial–mesenchymal transition, and increase of E-cadherin expression could prevent tumor invasion and metastasis (19,20). However, how decorin affects E-cadherin and whether the effect of E-cadherin by decorin plays a role in tumor formation and metastasis are still unknown. Herein, we showed that decorin could stabilize E-cadherin protein and physically interacted with E-cadherin, leading to attenuation of colorectal cancer growth and migration in vitro and in decorin knockout mice.
Materials and methods
Mouse intestine collection and immunohistochemical staining
Mouse intestinal tissues from Dcn−/− and Dcn+/+ mice (C57Bl/6 background) were collected, fixed in 10% buffered formalin and embedded in paraffin, as reported by us (3). Four micrometer of sections were utilized for immunohistochemical staining using anti-E-cadherin antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The procedure was similar as reported (3). In brief, the sections were deparaffined, rehydrated, quenched with 3% H2O2 and blocked with 10% normal serum, then incubated with E-cadherin antibody overnight at 4°C, followed by incubation with secondary antibody and avidin–biotin complex (ABC kit, Vector Laboratory, Burlingame, CA). The staining was developed by using 3′, 5′-diaminobenzidine and finalized with lightly counter staining with hematoxylin. The stained slide image was captured by Aperio Image System (Vista, CA).
Cell lines
Human colon cancer cell line HCT116 was cultured in McCoy’s 5A Medium (Invitrogen, Carlsbad, CA). The murine colon adenocarcinoma cell line MC38 (derived from C57BL6/J mouse) (21) was cultured in Modified Eagle’s medium (Invitrogen). Human embryonic kidney HEK293 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen). All media were supplemented with 10% fetal bovine serum and antibiotics (10 000 U/ml penicillin, 10 μg/ml streptomycin). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Xenografts
Murine colon adenocarcinoma cells MC38 (2.5 × 106) were injected subcutaneously into the flank of the Dcn−/− or Dcn+/+ mice at age about 8 weeks (five mice per group). Both MC38 cell line and Dcn mice were in C57BL/6 background. The animals were maintained in a pathogen-free barrier facility at the University of Illinois at Chicago Biological Resources Laboratory and closely monitored by animal facility staff. Fourteen days after injection, the animals were killed and the xenografts were isolated, the weight (g) and volume (mm3) of the xenografts were determined. All procedures were conducted according to the Animal Care and Use guideline approved by the University of Illinois at Chicago Animal Care Committee.
Decorin overexpressing plasmid construction and transfection
A human decorin expression plasmid (pcDNA3-DCN) was generated using normal colon mucosa complementary DNA as template for polymerase chain reaction amplification and subcloning into the pcDNA3-HA vector (Clontech Laboratories, Mountain View, CA) using the following primers: forward 5′-CCGCTCGAGATGAA GGCCACTATCATCCTC-3′ and reverse 5′-GAAGATCTTTACTTATAGTT TCCGAGTT-3′. The polymerase chain reaction product was digested with XhoI and Bgl II and the digested products were ligated into the vector. Transient transfection of HCT116 cells with pcDNA3-DCN or pcDNA3-HA vector control was performed via Lipofectamine 2000 (Invitrogen). Cells were subject to different assays, 24–72 h after transfection.
Small interfering RNA and transfection
A small interfering RNA (siRNA) targeting human decorin (si-DCN) and a scramble control siRNA were purchased from the Integrated DNA Technologies (Skokie, IL). The siRNAs were transfected into the HEK293 cells using Lipofectamine 2000 (Invitrogen). The cells were collected after 48 h transfection and were subjected for immunoblotting to determine the changes of E-cadherin.
Immunoblotting
For immunoblotting, cells were collected 72 h after transfection. Cells were lysed using 1X RIPA buffer (Upstate Biotechnology, Lake Placid, NY) containing a protease inhibitor cocktail (Sigma, St Louis, MO). After cell lysis, 30 μg of protein was loaded on a 10% sodium dodecyl sulfate gel followed by transfer to polyvinylidene difluoride membrane. Antibodies against decorin (a gift from Dr Fisher, NIH), E-cadherin, p21, β-catenin (Santa Cruz) and β-actin (Tianjin Sungene Biotech, Tianjin, China) were used. Secondary antibody was purchased from Santa Cruz Biotechnology. The detected signals of immunoblotting were visualized by an enhanced chemiluminescence reaction system (ECL Plus, Amersham, Piscataway, NJ), as recommended by the manufacturer.
Proliferation, apoptosis and migration analysis
Cell proliferation was analyzed by MTS assay according to the manufacture’s protocol (Promega, Madison, WI). To detect apoptosis, cells treated with recombinant decorin core protein (Sigma) or phosphate-buffered saline, respectively, were harvested and fixed with 70% ethanol followed by propidium iodide buffer staining. Cells were then counted by flow cytometry (FACScan, BD Biosciences, San Jose, CA). Cell migration was detected using a transwell plate (Corning, Lowell, MA) followed by 4′,6-diamidino-2-phenylindole staining after 48 h of transient transfection with pcDNA3-DCN or pcDNA3-HA vector, respectively.
Wound healing assay
HCT116 cells transiently transfected with pcDNA3-DCN or pcDNA3-HA vector were seeded in a 100 mm Petri dish. A wound was made by scratching on the Petri dish bottom, followed by another 24 h growth. The distance of the wound was measured under microscope.
Messenger RNA levels analysis
Total RNA was extracted from the HCT116 cells and messenger RNA (mRNA) levels of E-cadherin and decorin were analyzed using real-time quantitative reverse transcription–polymerase chain reaction as described recently (22). The primers for E-cadherin were: forward 5′-tgcccagaaaatgaaaaagg-3′ and reverse 5′-gtgtatgtggcaatgcgttc-3′. The primers for decorin were: forward 5′-cgagtggtccagtgttctga-3′ and reverse 5′-aaagccccattttcaattcc-3′. Actin was used as internal control (3).
E-cadherin promoter activity analysis
HCT116 cells were co-transfected with a pGL4 empty vector or pGL4-E-cadherin-Luc plasmid, which contains the full-length E-cadherin promoter (a gift from Dr M.Saitoh) (23), with pcDNA3-DCN or pcDNA3-HA vector. Renilla was co-transfected as an internal control. Forty-eight hours after transfection, dual luciferase activity kit (Promega, Madison, WI) was used in combination with a luminometer to measure luciferase activity of the cells according to the manufacturers’ protocol.
E-cadherin protein stability analysis
HCT116 cells were transiently transfected with pcDNA3-DCN or pcDNA3-HA vector, followed by exposure to protein synthesis inhibitor cycloheximide (50 μg/ml; Sigma). The cells were harvested after 4, 6 and 8 h. E-cadherin protein levels were analyzed by immunoblotting.
Co-immunoprecipitation analysis
Similar as described recently (24), HCT116 cells with transfection of decorin expression plasmid pcDNA3-DCN or empty vector pcDNA3-HA were harvested after 24 h transfection, the cells were lysed in RIPA lysis buffer (20 mM Tris–HCL, pH 8.0, 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail), and then the cell lysates were immunoprecipitated with anti-E-cadherin antibody, and the immunocomplex was then probed with anti-HA antibody (for decorin). Vice versa, the cell lysates were immunoprecipitated with anti-HA antibody (recognition of decorin), the immunocomplex was then probed with anti-E-cadherin antibody. The immunocomplex was visualized using enhanced chemiluminescence reagent (Amersham Biosciences, Piscataway, NJ). The signal was quantified using Quantity One software (Bio-Rad, Hercules, CA)
Results
E-Cadherin was dramatically reduced in Dcn−/− mouse intestine
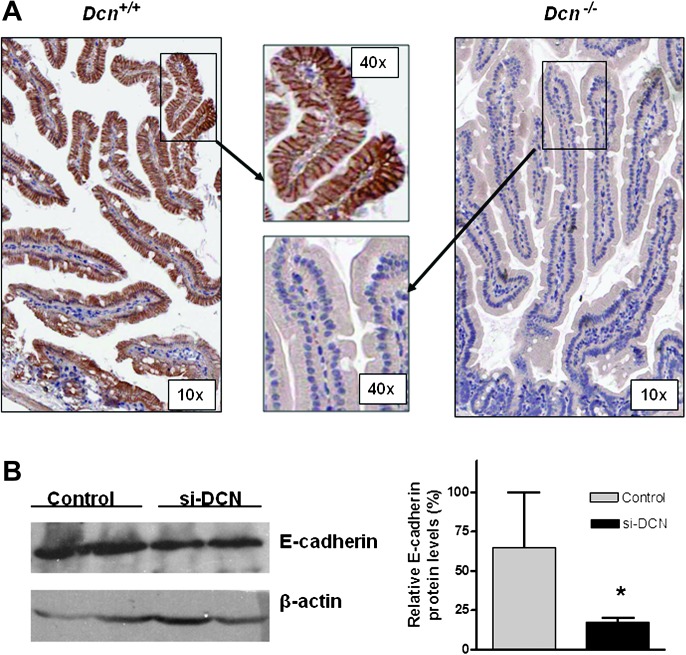
Early characterization of Dcn−/− mice has shown intestinal tumor formation and potential changes at molecular levels (3), in which cyclin-dependent inhibitors p21 and p27 were decreased, and Wnt/β-catenin signaling was increased, and in contrast, E-cadherin, a critical partner of β-catenin, was decreased. To determine the effect of E-cadherin by decorin in situ, we performed immunohistochemical staining. As shown in Figure 1A, E-cadherin was dramatically reduced or almost absent in the small intestine of the Dcn−/− mice compared with that in the Dcn+/+ mice.
Fig. 1.
Loss of decorin reduced E-cadherin expression in mouse and in vitro. (A) E-cadherin was dramatically reduced in intestinal epithelial cells in Dcn−/− mice by immnunohistochemical staining (anti-E-cadherin antibody dilution 1:100) (magnification ×10 and ×40, respectively). (B) Knockdown decorin expression via decorin siRNA (si-DCN) led to reduced expression of E-cadherin in HEK293 cells compared with the control. The first two lanes stood for that the cell lysated was obtained from duplicated experiments. So did the last two lanes of control. The average levels of E-cadherin were quantified and showed in the right panel. *P < 0.05 compared with the control group.
Knockdown decorin expression led to reduced expression of E-cadherin in vitro
To validate decorin-caused reduction of E-cadherin in vitro, we transfected the HEK293 cells with a siRNA targeting decorin (si-DCN) to knockdown decorin expression and found that E-cadherin was also reduced compared with the control (Figure 1B).
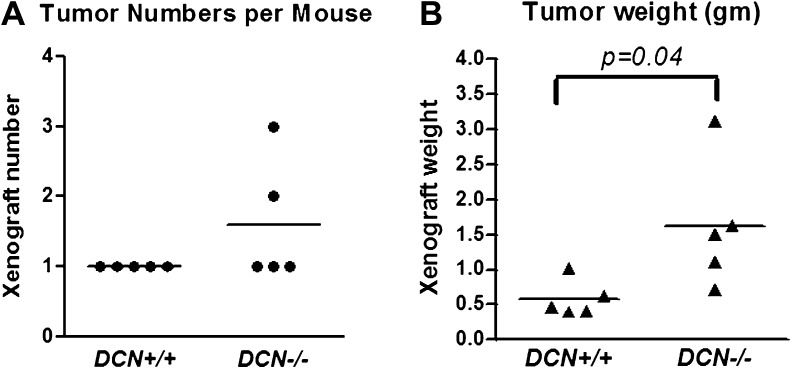
Loss of decorin accelerated cancer cell growth and invasion in mice
To determine whether decorin-mediated loss of E-cadherin influences tumor growth and invasion in mice, we inoculated murine colon carcinoma MC38 cells (C57BL/6 background) (2.5 × 106 cells per mice) subcutaneously in Dcn−/− and +/+ mice (C57BL/6 background) for 14 days. Compared with the wild-type mice that had single and smaller tumor as usually seen in nude mice, one Dcn−/− mouse had two tumors (xenografts) and another one had three tumors (Figure 2A, P > 0.05). In addition, the sizes (weight in grams) of the tumors in the decorin knockout mice were larger than those in decorin wild-type mice (1.62 ± 0.41 g per mouse in Dcn−/− mice versus 0.58 ± 0.12 g per mouse in Dcn+/+ mice) (Figure 2B, P = 0.04). Impressively, tumor invasions to the muscle and abdominal were also observed in Dcn−/− mice (image not shown).
Fig. 2.
Loss of decorin led to enhancement of colon cancer cell growth in mouse xenografts. Murine colon cancer cells MC38 (2.5 ×106) (250 μl) were subcutaneously injected into the flank of Dcn+/+ or −/− mice (five mice per group). Fourteen days post inoculation, the xenografts were excised and weighed. Each spot represents one mouse.
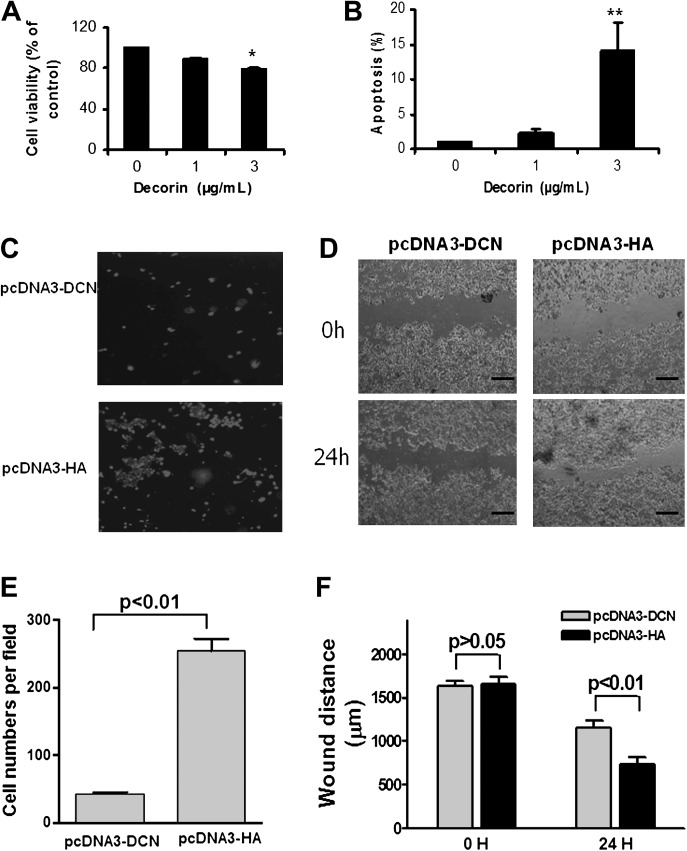
Overexpression of decorin inhibited cancer cell growth and migration and slowered wound healing in vitro
To elucidate whether restore decorin expression attenuates cancer cell malignancy, we treated human colon cancer cells HCT116 with 1 μg/ml or 3 μg/ml of recombinant decorin protein (Sigma) for 24 h. We found that decorin protein indeed inhibited cell proliferation by 22% (Figure 3A, P < 0.05) and promoted apoptosis by 14-fold (Figure 3B, P < 0.01). Decorin overexpression plasmid pcDNA3-DCN was then transfected into the HCT116 cell. We found that increasing expression was able to inhibit cancer cell migration using transwell (Figure 3C and E) and wound healing approaches (Figure 3D and F).
Fig. 3.
Decorin core protein treatment suppressed cell proliferation (A) promoted apoptosis (B) in HCT116 cells. *P < 0.05 and **P < 0.01 compared with the control group. Increasing decorin expression in colon cancer cells HCT116 inhibited cell migration (C) and wound healing (D). The migrated cell numbers (mean ± SD) were quantified in (E), and the wound distances (mean ± SD) were measured and presented in (F) (‘—’ equals to 1000 μm). All experiments were triplicated independently. pcDNA3-DCN indicated the cells with transfection of decorin overexpression plasmid, pcDNA3-HA indicated the cells with transfection of pcDNA3-HA vector control.
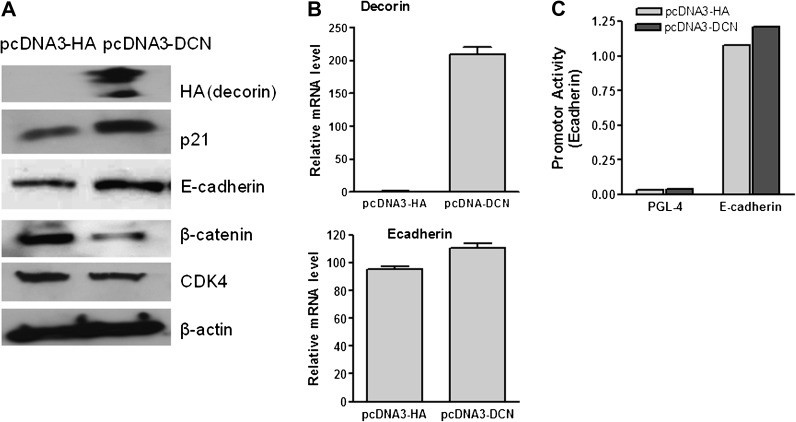
Increasing decorin expression upregulated E-cadherin protein level and did not affect E-cadherin mRNA and promoter activity
To determine whether decorin involved inhibition of malignancy was associated with E-cadherin, we first determined the changes of E-cadherin at protein level and found that overexpression of decorin upregulated the expression of E-cadherin and p21WAF1 in HCT116 cells. However, overexpression of decorin suppressed β-catenin and CDK4 proteins (Figure 4A).
Fig. 4.
Increasing expression of decorin in HCT116 cells upregulated the protein levels of p21 and E-cadherin and downregulated β-catenin and cdk4 (A) but only slightly affected E-cadherin mRNA levels (B) and E-cadherin promoter activity (C). pcDNA3-DCN indicated the cells with transfection of decorin overexpression plasmid, pcDNA3-HA indicated the cells with transfection of pcDNA3-HA vector control. β-actin was used as internal loading control.
We then determined whether decorin affected E-cadherin at transcriptional levels by analyzing E-cadherin mRNA and promoter activity. Unexpected, E-cadherin mRNA level, unlike E-cadherin protein level, was only slightly increased by increasing overexpression of decorin (Figure 4B, P > 0.05), and E-cadherin promoter activity was not significantly increased either (Figure 4C, P > 0.05), assayed by quantitative reverse transcription–polymerase chain reaction and measuring promoter luciferase activities, respectively.
Decorin stabilized E-cadherin protein
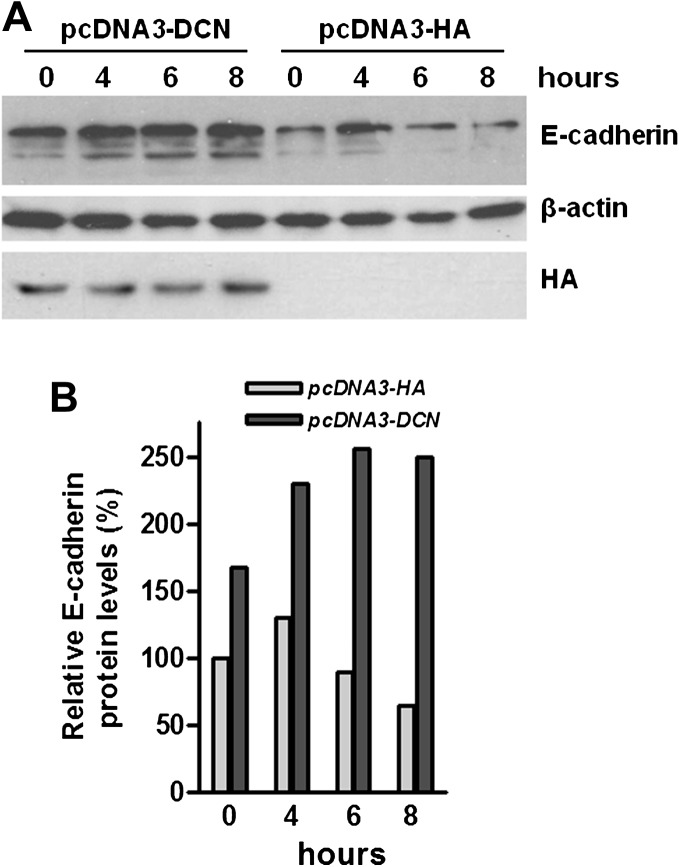
To elucidate the underlying mechanism of that decorin upregulated E-cadherin at protein level but did not alter E-cadherin at transcriptional levels, we conducted protein stability experiments. We transfected the HCT116 cells with decorin-expressing plasmid pcDNA3-DCN or empty vector pcDNA3-HA and treated the cells with protein synthesis inhibitor cycloheximide (50 μg/ml). As shown in Figure 5, increasing decorin expression indeed prolonged the half life of E-cadherin protein, indicating that decorin could stabilize E-cadherin protein.
Fig. 5.
Increasing decorin expression enhanced endogenous E-cadherin protein stability in HCT116 cells. HCT116 cells were transfected with decorin expression plasmid (pcDNA3-DCN) or corresponding empty vector (pcDNA-HA), followed by exposure to the protein synthesis inhibitor cycloheximide (50 μg/ml) for different times. Protein levels were analyzed by immunoblotting (A) and the changes of proteins were quantified (B). β-actin was used as internal loading control.
Decorin physically bound to E-cadherin
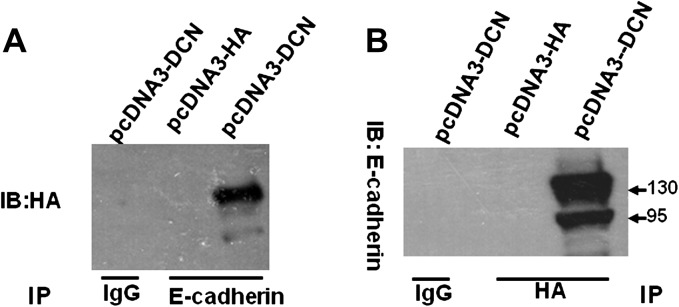
To determine whether there is an interaction between decorin and E-cadherin proteins, we performed co-immunoprecipitation assay. Since decorin is almost undetectable in the HCT116 cells, we transfected the HCT116 cells with decorin-expressing plasmid, precipitated the cell lysates with anti-E-cadherin and probed with anti-HA antibody to recognize decorin. We found an immunocomplex of E-cadherin and decorin (Figure 6A). Vice versa, an immunocomplex was also seen using anti-HA antibody to pull down and anti-E-cadherin antibody to probe. Both approaches demonstrated the physical binding between decorin and E-cadherin proteins.
Fig. 6.
There was a physical binding between decorin and E-cadherin proteins. The HCT116 cells were transfected with decorin-expressing plasmid (pcDAN3-DCN) or vector (pcDNA3-HA) for 48 h and the cells were harvested for lysate. The cell lysates were precipitated by anti-E-cadherin antibody and probed with anti-HA antibody to recognize decorin (A). Vice versa, the cell lysates were precipitated by anti-HA antibody and probed with anti-E-cadherin antibody to recognize E-cadherin (B). IgG was used as negative control.
Discussion
Colorectal cancer is the third most common cancer and is the second leading cause of cancer-related deaths in the USA (25), mainly due to invasion and metastasis. However, the underlying mechanisms of metastasis are largely unknown. Herein, we report that loss of decorin accelerated colorectal cancer cell growth and invasion in mice and increase of decorin inhibited cancer cell proliferation and migration, and promoted apoptosis, which was directly linked to E-cadherin—stabilization by decorin and physical binding with decorin.
E-cadherin is a single-span transmembrane glycoprotein that mediates cell–cell adhesion through calcium-dependent homophilic interactions of extracellular domains (26). Perturbation of E-cadherin-mediated cell adhesion is involved in tumor progression, metastasis and poor prognosis (27–32), and loss of E-cadherin expression or function in vitro has been associated with decreased differentiation and increased invasive capacity of cancer cell lines (26). Growing evidence suggests the importance of E-cadherin in carcinogenesis and cancer metastasis and the potential regulation of E-cadherin by GSK3β, snail/slug and Akt/PKB (33–35). E-cadherin loss promotes metastasis by disaggregating cancer cells from another, activates specific downstream signal transduction pathways and causes epithelial–mesenchymal transition, which facilitates metastasis. However, maintenance of E-cadherin expression could restore epithelial morphology and prevent tumor invasion and metastasis (19,20).
In colorectal cancer, both Apc mutations and E-cadherin downregulation are linked to increased β-catenin expression (36). The APC gene is mutated in up to 80% of sporadic human colon cancers and loss of APC has been shown to induce irregular stabilization and accumulation of β-catenin because of a decrease in the binding among the APC/GSK3β/Axin complex, the complex in the cytoplasm that controls β-catenin stability via phosphorylation and proteasomal degradation (36). β-catenin is a key mediator in the Wnt signaling pathway and plays an important role in intestinal cancer development (37). β-catenin level and localization throughout the cell are controlled by E-cadherin.
Our characterization on the decorin knockout mice has demonstrated the critical role of decorin in regulating of intestinal cell homeostasis and tumor suppressor function, also demonstrated that β-catenin signaling is activated through a non-canonic Wnt/β-catenin pathway (3). Present study again revealed that dramatic loss of E-cadherin by loss of decorin in vivo resulted in the acceleration of tumor growth and invasion in Dcn−/− mice, but upregulation of E-cadherin by increase of decorin expression in vitro led to the attenuation of colorectal cancer cell malignancy. Both in vivo and in vitro results strongly suggested the tumor suppression and anti-metastasis of decorin in stromal and epithelial cells, respectively, which was through regulatory interaction and stabilization of E-cadherin.
Although upregulation of E-cadherin protein by decorin was seen, alterations of E-cadherin at transcriptional levels by decorin were not significant. Besides protein stabilization, whether there are additional mechanisms involved in the regulation of E-cadherin by decorin and how these two proteins interacted are not completely clear. Since HCT116 cell line is one of the most difficulty cell lines for transfection, the transfection efficiency might affect the results, and thus, should take consideration. An improvement of transfection efficiency is needed for further investigation. Earlier studies found E-cadherin could be ubiquitinated by a c-Cbl-like E3 ubiquitin ligase Hakai and then is induced to the internalization of E-cadherin (38,39). The latest study showed proteasome-dependent events are likely to be involved in stabilization of cell surface E-cadherin (40). As mentioned above, decorin in tumor suppression is also associated with EGFR and cell cycle inhibitors p21 and p27. Importantly, the regulation between E-cadherin and EGFR, p21 and p27, also has been reported (41–44). Our data showed a direct interaction between decorin and E-cadherin, but whether the proteasome pathway is also involved in the direct regulation of those two proteins or whether the regulation is mediated by other factors like EGFR, p21 or p27 are unknown and need further investigation.
In summary, this study identified additional antitumor and anti-metastasis functions of decorin, which was linked to the E-cadherin stabilization and regulatory interaction. This study also demonstrated translational potential of decorin as a therapeutic and preventive agent for malignant diseases.
Funding
National Cancer Institute, National Institutes of Health, USA (CA112081); Faculty Startup Fund from the University of Illinois at Chicago (to W.Y.) and China National Natural Science Foundation (81001003 to X.B.).
Acknowledgments
We would like to thank Dr M.Saitoh (University of Tokyo, Japan) for providing E-cadherin promoter reporter plasmid, Dr L.Fisher (NIH, Bethesda, MD) for providing the anti-decorin antibody, Drs D.Hu, W.Fang and Ms B.Mihelich (University of Illinois at Chicago, IL) for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- EGFR
epithelial growth factor receptor
- mRNA
messenger RNA
- siRNA
small interfering RNA
References
- 1.Iozzo RV, et al. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldoni S, et al. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int. J. Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 3.Bi X, et al. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santra M, et al. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci U S A. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stander M, et al. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221–227. doi: 10.1007/s004410051283. [DOI] [PubMed] [Google Scholar]
- 6.De Luca A, et al. Decorin-induced growth suppression is associated with up-regulation of p21, an inhibitor of cyclin-dependent kinases. J. Biol. Chem. 1996;271:18961–18965. doi: 10.1074/jbc.271.31.18961. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee AG, et al. Aberrant expression and localization of decorin in human oral dysplasia and squamous cell carcinoma. Cancer Res. 2003;63:7769–7776. [PubMed] [Google Scholar]
- 8.Asakura S, et al. Role of transforming growth factor-beta1 and decorin in development of central fibrosis in pulmonary adenocarcinoma. Hum. Pathol. 1999;30:195–198. doi: 10.1016/s0046-8177(99)90275-7. [DOI] [PubMed] [Google Scholar]
- 9.Iozzo RV, et al. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 10.Csordas G, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 11.Reed CC, et al. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 12.Iozzo RV, et al. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc. Natl Acad. Sci. U S A. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troup S, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin. Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 14.Campioni M, et al. Identification of genes down-regulated during lung cancer progression: a cDNA array study. J. Exp. Clin. Cancer Res. 2008;27:38. doi: 10.1186/1756-9966-27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldoni S, et al. An antimetastatic role for decorin in breast cancer. Am. J. Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed CC, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 17.Danielson KG, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldoni S, et al. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmalhofer O, et al. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev., 28,151--166. 2009 doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 20.Nelson WJ, et al. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SA, et al. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 22.Bi X, et al. Black Raspberries inhibit intestinal tumorigenesis in Apc1638+/− and Muc2−/− mouse models of colorectal cancer. Cancer Prev. Res. (Phila) 2010;3:1443–1450. doi: 10.1158/1940-6207.CAPR-10-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiguchi K, et al. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J. Biol. Chem. 2009;284:245–253. doi: 10.1074/jbc.M804777200. [DOI] [PubMed] [Google Scholar]
- 24.Fang W, et al. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis. 2010;31:1360–1366. doi: 10.1093/carcin/bgq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelloff GJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—a plan to move forward. Clin. Cancer Res. 2006;12:3661–3697. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 26.Wheelock MJ, et al. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 27.Doki Y, et al. Correlation between E-cadherin expression and invasiveness in vitro in a human esophageal cancer cell line. Cancer Res. 1993;53:3421–3426. [PubMed] [Google Scholar]
- 28.Oka H, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- 29.Umbas R, et al. Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res. 1994;54:3929–3933. [PubMed] [Google Scholar]
- 30.Derksen PW, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Frixen UH, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onder TT, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 33.Baranwal S, et al. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem. Biophys. Res. Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CC, et al. Inflamed snail speeds metastasis. Cancer Cell. 2009;15:355–357. doi: 10.1016/j.ccr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, et al. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Logan CY, et al. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 37.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 38.Fujita Y, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 39.Pece S, et al. E-cadherin and Hakai: signalling, remodeling or destruction? Nat. Cell Biol. 2002;4:E72–E74. doi: 10.1038/ncb0402-e72. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh M, et al. Regulation of the stability of cell surface E-cadherin by the proteasome. Biochem. Biophys. Res. Commun. 2009;381:560–565. doi: 10.1016/j.bbrc.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 41.Theard D, et al. Formation of E-cadherin/beta-catenin-based adherens junctions in hepatocytes requires serine-10 in p27(Kip1) Mol. Biol. Cell. 2008;19:1605–1613. doi: 10.1091/mbc.E07-07-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller S, et al. p21WAF1 regulates anchorage-independent growth of HCT116 colon carcinoma cells via E-cadherin expression. Cancer Res. 2000;60:156–163. [PubMed] [Google Scholar]
- 43.Fedor-Chaiken M, et al. E-cadherin binding modulates EGF receptor activation. Cell Commun. Adhes. 2003;10:105–118. [PubMed] [Google Scholar]
- 44.Lu Z, et al. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4:499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]