Abstract
Benzene causes acute myeloid leukemia and probably other hematological malignancies. As benzene also causes hematotoxicity even in workers exposed to levels below the US permissible occupational exposure limit of 1 part per million, further assessment of the health risks associated with its exposure, particularly at low levels, is needed. Here, we describe the probable mechanism by which benzene induces leukemia involving the targeting of critical genes and pathways through the induction of genetic, chromosomal or epigenetic abnormalities and genomic instability, in a hematopoietic stem cell (HSC); stromal cell dysregulation; apoptosis of HSCs and stromal cells and altered proliferation and differentiation of HSCs. These effects modulated by benzene-induced oxidative stress, aryl hydrocarbon receptor dysregulation and reduced immunosurveillance, lead to the generation of leukemic stem cells and subsequent clonal evolution to leukemia. A mode of action (MOA) approach to the risk assessment of benzene was recently proposed. This approach is limited, however, by the challenges of defining a simple stochastic MOA of benzene-induced leukemogenesis and of identifying relevant and quantifiable parameters associated with potential key events. An alternative risk assessment approach is the application of toxicogenomics and systems biology in human populations, animals and in vitro models of the HSC stem cell niche, exposed to a range of levels of benzene. These approaches will inform our understanding of the mechanisms of benzene toxicity and identify additional biomarkers of exposure, early effect and susceptibility useful for risk assessment.
Introduction
Benzene, an aromatic hydrocarbon and a component of crude oil and gasoline, is produced at high levels and is widely used in the USA as an intermediate in the manufacture of plastics, resins, dyes, etc. (1). Occupational exposure to benzene occurs through solvent exposures in the chemical industry, in petroleum refineries, oil pipelines, on ships and tankers, auto repair shops and bus garages (1,2). In the USA, occupational exposure levels typically are below the Occupational Safety and Health Administration’s permissible exposure limit of 1 part per million (p.p.m.) (8 h time-weighted average) (3), but levels exceeding 100 p.p.m. have been reported in shoe factories in China (4,5). Environmental exposures among the general population are much lower than occupational exposures, ranging from <1 to 10 p.p.b., mainly arising from vehicle emissions and cigarette smoke (3). Smokers may be exposed to 10 times the level of non-smokers (6,7).
Benzene is associated with multiple adverse health effects. It was shown to induce hematotoxicity in humans over 100 years ago and recent studies have reported hematological effects at various benzene levels including those <1 p.p.m. in air (8,9), as shown in Table I. Benzene is a cause of acute myeloid leukemia (AML) and myelodysplastic syndrome and a probable cause of other hematological malignancies, such as non-Hodgkin lymphoma (39–41). In addition, epidemiological studies have also provided some evidence for an association with childhood leukemia (40,42). Although others have disputed a causal relationship for diseases other than AML (43–45), there is a need for further assessment of the health risks associated with benzene exposure particularly at low occupational and environmental levels.
Table I.
Summary of benzene studies applying OMICS approaches
| Systems biology | Study method | Study size | Benzene exposure (p.p.m.) | Major findings | Reference | |
| Exposed | Controls | |||||
| Phenomics | ||||||
| Hematotoxicity | CBC | 250 | 140 | <1, <10, >10 | Decrease in all blood cell counts | Lan et al. (8) |
| Colony | 24 | 29 | <10, >10 | Decrease in colony formation | Lan et al. (8) | |
| WBC, RBC | 657 | Daily exposure <5 to 34 | Decrease WBC, weak decrease RBC, no evidence of threshold | Ward et al. (10) | ||
| WBC | 437 | 150 | Mean 1.43 ± 1.93 | Reduced WBC | Zhang et al. (11) | |
| WBC and PB indices | 855 | 73 | <1, 1 to <10, >10 | Decrease in red cell indices >10 p.p.m. | Schnatter et al. (12) | |
| Decrease in neutrophils, MPV ∼8 p.p.m. | ||||||
| CBC | 130 | 51 | <5, >5 to 15, >15 to 30, >30 | Decrease WBC, RBC, neutrophils | Qu et al. (9) | |
| WBC | 701 | 1059 | <0.5, 0.5–1, >1 | No effect | Swaen et al. (13) | |
| WBC, RBC | 387 | 553 | ∼0.55 | No effect | Collins et al. (14) | |
| CBC | 1200 | 3227 | ∼0.6 (1977–1988) | No effect | Tsai et al. (15) | |
| ∼0.14 (1988–2002) | ||||||
| WBC, RBC, MPV | 153 | 50 | 0.01–23.9 | No effect | Pesatori et al. (16) | |
| Toxicogenomics | ||||||
| Transcriptomics | Affymetrix | 6 | 6 | ≥10 | 29 genes differentially expressed | Forrest et al. (17) |
| q-PCR | 13 | 15 | ≥10 | Validated 6 of 29 genes | ||
| Affymetrix & Illumina | 8 | 8 | ≥10 | Confirms Forrest findings | McHale et al. (18) | |
| Illumina | 83 | 42 | <1, <10, >10 | Immune and AML gene pathways identified at low and high exposures | McHale et al. (19) | |
| CSC-GE-80 | 7 | 7 | 2.9–60.3 | 40 genes altered in CBP, e.g. CYP43A and DNA-PKcs | Bi et al. 2010 (20) | |
| Proteomics | SELDI-TOF | 20 | 20 | 31.3 and 37.9 (mean of 2 groups of 10) | Identified two downregulated proteins as PF4 and CTAP-III | Vermeulen et al. (21) |
| 2-DE | 50 | 38 | t,t-MA (mg/g creatinine): exposed, 1.011 ± 0.249; Control, 0.026 ± 0.028 | Identified three upregulated proteins as TCR beta, FKBP51 and MMP13 | Joo et al. (22) | |
| Epigenomics | ||||||
| DNA methylation | Illumina (GoldenGate) | 6 | 4 | 8.9 ± 9.1 (mean ± SD) | Results preliminary (MSH3, RUNX3) | Zhang et al. (23) |
| Human TK6 cells | HQ (0, 10, 15, 20 μM) | Results preliminary (RUNX1, IL12) | ||||
| Pyrosequencing | 78 + 77 | 58 | ∼22 p.p.b. | Very small decreases in global methylation (LINE-1 and Alu) and in p15 and MAGE-1 | Bollati et al. (24) | |
| Immunochemistry | Human TK6 cells | HQ (0–20 μM) | Decrease in global methylation | Ji et al. (25) | ||
| miRNA | Agilent | 7 | 7 | <1 p.p.m. | Results preliminary (4 miRNAs) | Zhang et al. (23) |
| Genomics | ||||||
| PDA in yeast | ∼4600 homozygous deletion strains | HQ, CAT, BT | Oxidative stress response | North et al. (26) | ||
| RNAi | WRN | HQ | DNA repair HR pathway | Galvan et al. (27); Ren et al. (28) | ||
| Genotyping | ||||||
| Real-time PCR | 250 | 140 | 0, <1, <10, >10 | SNPs in MPO and NQO1 linked to WBC | Lan et al. (8) | |
| Illumina (GoldenGate) | 250 | 140 | 0, <1, <10, >10 | SNPs in DNA repair and genomic maintenance genes (BLM, TP53, RAD51, WDR79, WRN) linked to WBC | Lan et al. (29) | |
| Illumina (GoldenGate) | 250 | 140 | 0, <1, <10, >10 | SNPs in cytokine and cellular adhesion molecule genes (IL-1A, IL-4, IL-10, IL-12A, VCAM1) linked to WBC | Lan et al. 2005 (30) | |
| Illumina (GoldenGate) | 250 | 140 | 0, <1, <10, >10 | SNPs in DNA DSB repair pathway genes (WRN, BRCA2, TP53) linked to WBC | Shen et al. (31) | |
| Illumina (GoldenGate) | 250 | 140 | 0, <1, <10, >10 | SNPs in VEGF and ERCC3 linked to WBC | Hosgood et al. (32) | |
| PCR–RFLP | 39 CBP, 38 non-CBP | 37 | <12.4, 12.4–31, >31 | SNP in p21 associated with CBP | Sun et al. (33) | |
| PCR–RFLP | 152 CBP, 152 non-CBP | — | <12.4, 12.4–31, >31 | SNPs in oxidative stress-related DNA damage repair genes, hMTH1, hOGG1, associated with CBP | Wu et al. (34) | |
| Real-time PCR | 268 CBP, 268 non-CBP | <12.4, 12.4–31, >31 | SNP in EPHX1 associated with CBP | Sun et al. (35) | ||
| PCR–RFLP | 152 CBP, 152 non-CBP | <12.4, 12.4–31, >31 | SNP in XRCC1 associated with CBP | Zhang et al. (36) | ||
| Chromosomics | ||||||
| OctoChrome FISH | 6 | 5 | >5 p.p.m. | Monosomy 5,6,7,10 and trisomy 8,9,17,21,22 significantly increased | Zhang et al. (37) | |
| CWAS | 47 | 27 | 0, <10, ≥10 p.p.m. | Monosomy 5, 6, 7, 10, 16 and 19 and trisomy 5, 6, 7, 8, 10, 14, 16, 21 and 22 | Zhang et al. (38) | |
BT, 1,2,4-benzenetriol; CAT, catechol; CBC, complete blood count; CBP, chronic benzene poisoning; CWAS, chromosome-wide anueploidy study; EPHX1, epoxide hydrolase 1, microsomal; ERCC3, excision repair cross-complementing rodent repair deficiency, complementation group 3; miRNA, microRNA; MPV, mean platelet volume; PB, peripheral blood; PCR–RFLP, restriction length fragment polymorphism; PDA, parallel deletion assay; RBC, red blood cell; RNAi, RNA interference; SELDI-TOF, surface-enhanced laser desorption/ionization time-of-flight; SNP, single-nucleotide polymorphism; VEGF, vascular endothelial growth factor; WBC, white blood cell; XRCC1, x-ray repair complementing defective repair in Chinese hamster cells 1; 2-DE, 2-dimensional gel electrophoresis.
Leukemias arising de novo and secondary to known leukemogen exposures are similar
Among the few known inducers of AML, other than benzene, are a group of agents used in the treatment of cancer and non-cancer disorders: alkylating agents, topoisomerase II (topo II) inhibitors and ionizing radiation. A small percentage of AML and myelodysplastic syndrome cases are a consequence of such therapies, as reviewed (46). Depending on the specific therapeutic regimen, these diseases, termed therapy-related acute myeloid leukemia (t-AML) and therapy-related myelodysplastic syndrome (t-MDS), comprise distinct cytogenetic subtypes, exhibit different latencies and may arise in cells at different stages of hematopoiesis (47–50). For example, t-MDS/t-AML following alkylating agent therapy is characterized by abnormalities of chromosomes 5 and/or 7 (47–49) and is thought to arise in a multipotent hematopoietic stem cell (HSC) or progenitor cell, whereas t-AML following treatment with topo II inhibitors is characterized by balanced translocations involving MLL at 11q23, RUNX1 at 21q22, CBFB at 16q22 or PML (15q22) and RARA (17q12) which probably arise in a lineage committed progenitor cell. These translocations, which result in the formation of chimeric oncoproteins, are thought to be generated by repair of chromosomal breakpoints by the non-homologous end joining (NHEJ) DNA repair pathway following topo II-mediated cleavage (50). The characteristic clonal chromosomal abnormalities, in cooperation with genetic and epigenetic mutations, are thought to deregulate a limited number of critical pathways such as cytokine signaling and proliferation, survival and differentiation of hematopoietic cells, leading to leukemogenesis (51). De novo and t-MDS and t-AML are considered similar diseases as they exhibit similar patterns of cytogenetic abnormalities and gene mutations, albeit with different frequencies among subtypes (51). Eight different genetic pathways to t-MDS and t-AML, defined by the specific abnormalities present in each, were proposed (52). There is evidence that benzene induces many of the specific abnormalities associated with these pathways (40), but the multiple mechanisms underlying benzene-induced leukemia have not been fully characterized.
Benzene causes hematotoxicity and leukemia through multiple toxic metabolites
Although a recent study has shown that adding very high concentrations of benzene alone to HL60 cells can increase reactive oxygen species (ROS) levels and cause cytotoxicity (53), metabolism of benzene to its toxic metabolites is generally thought to be a critical factor in benzene toxicity (54). This is supported by several key studies. Benzene metabolism and toxicity were shown to be reduced in rats following partial hepatectomy (55) and in transgenic cytochrome-P450 2E1 (CYP2E1) knockout mice (56). Benzene metabolism and toxicity were reduced in mice following co-administration of high concentrations of toluene, a competitive inhibitor of benzene metabolism (57), although a recent study suggested that toluene enhances the genotoxic effects of benzene in mice at lower doses (58,59).
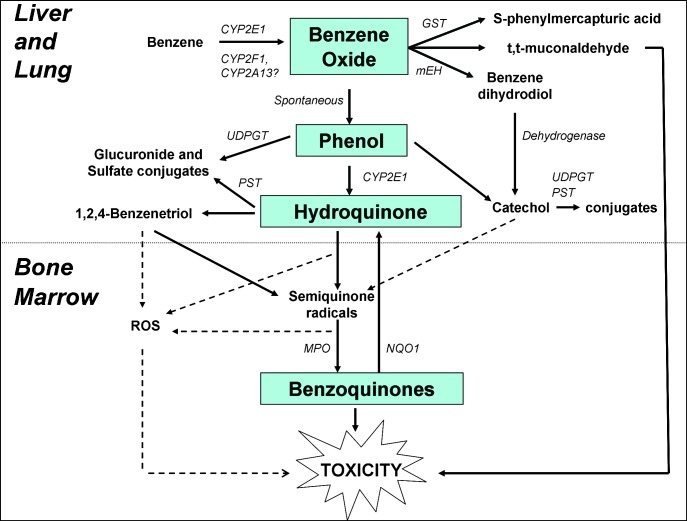
Benzene metabolism is inherently complex (1,60) and occurs principally in the liver (55) and also in the lung (61,62), with secondary metabolism occurring in the bone marrow (63–66). A simplified schematic is illustrated in Figure 1. The initial step involving cytochrome-P450 (CYP)-dependent oxidation of benzene to benzene oxide is primarily catalyzed at high levels of exposure by liver CYP2E1 (56,67). However, CYP2F1 and CYP2A13 are both highly active in the human lung (68–70) and are candidates for the high-affinity low capacity metabolic enzymes recently proposed to be active at benzene levels <1 p.p.m. (5,71), suggesting that the lung may be the primary site for low-dose metabolism of benzene. The majority of benzene oxide, which exists in equilibrium with its tautomer, oxepin, spontaneously rearranges to phenol (PH), and the remainder is either hydrolyzed to produce catechol and 1,2-benzoquinone, via benzene dihydrodiol, or reacts with glutathione to eventually produce S-phenylmercapturic acid. PH is excreted or further metabolized to hydroquinone (HQ) and 1,4-benzoquinone (BQ). HQ is converted to the reactive metabolite 1,2,4-benzenetriol via CYP2E1 catalysis (72). Metabolism of oxepin is thought to open the aromatic ring, yielding the reactive muconaldehydes, which cause hematotoxicity in exposed mice (73) and E,E-muconic acid. Benzene oxide, the benzoquinones and muconaldehydes are electrophiles that readily react with peptides and proteins and can thereby interfere with cellular function (74). Semiquinone radicals and quinones produced from phenolic metabolites by peroxidases in the bone marrow are highly toxic by directly binding to cellular macromolecules and generating oxygen radicals through redox cycling (74,75). In particular, BQ formation from HQ in the bone marrow via myeloperoxidase may be a key in benzene carcinogenicity, as shown in Figure 1 (74). In support of this is the evidence showing that the BQ-detoxifying enzyme NAD(P)H:quinone oxidoreductase 1 (NQO1) protects mice against benzene-induced myelodysplasia (76,77) and protects humans against benzene hematotoxicity (78). Glutathione conjugates of HQ also redox cycle and may be involved in benzene toxicity (79–81).
Fig. 1.
Simplified metabolic scheme for benzene showing site-specific metabolism from liver to bone marrow including major pathways and metabolizing enzymes leading to toxicity. GST, glutathione-S-transferase; mEH, microsomal epoxide hydrolase; MPO, myeloperoxidase; PST, phenol sulfotransferase; UDPGT, uridine diphosphate glucuronosyltransferase.
More work is needed to elucidate the different roles of the various metabolites in benzene toxicity and the pathways that lead to their formation. In human populations, the proportions of metabolites are likely to differ at the individual level according to genetic variation in metabolic factors, such as CYP2E1 (56), epoxide hydrolase, glutathione-S-transferases, myeloperoxidase and NQO1, which influence susceptibility to the toxic effects of benzene (82). Lifestyle factors, such as diet and smoking, can also modulate metabolite proportions (83).
Benzene induces leukemia-related cytogenetic changes
It is well established that benzene and/or its metabolites cause chromosomal aberrations in the peripheral blood lymphocytes of chronically exposed humans (84–98). Benzene exposure has been associated with higher levels of chromosomal changes commonly observed in AML, including 5q−/−5 or 7q−/−7, +8 and t(8;21) in the peripheral blood lymphocytes of highly exposed workers (98–100). AML-related chromosomal changes are also produced by benzene metabolites in human cell cultures, including cultures of CD34+ progenitor cells (101,102). Together, these data provide strong evidence for the induction of AML by benzene through previously proposed genetic pathways (52).
Selective chromosomal aneuploidy was found to be induced by benzene using classical cytogenetic methods (89,103,104) and, more recently, by OctoChrome fluorescent in situ hybridization, a novel chromosome-wide aneuploidy study approach which analyses both numerical and structural changes in all 24 human chromosomes simultaneously (37,38), Table I. Applying the latter approach in a pilot study, monosomy of chromosomes 5, 6, 7 and 10 and trisomy of chromosomes 8, 9, 17, 21 and 22 was shown to be selectively induced in the peripheral blood lymphocytes of benzene-exposed subjects (37). In a larger study of 47 workers and 27 unexposed controls, statistically significant increases in the rates of monosomy [5, 6, 7, 10, 16 and 19] and trisomy [5, 6, 7, 8, 10, 14, 16, 21 and 22] were found to be dose dependently associated with benzene exposure (38). Increased aneuploidy of chromosomes 7 and 9 was detected recently in workers occupationally exposed to low-dose (0.5 p.p.m.) benzene, using a micronucleus-centromere assay in conjunction with fluorescent in situ hybridization and chromosome-specific centromeric probes (105). In addition, using a pan-centromeric probe and scoring centromere-positive and -negative micronuclei, the study showed that benzene-induced micronuclei originated from chromosome breaks rather than chromosome non-disjunction. Inhibition of microtubule assembly was proposed as a mechanism of benzene-associated aneuploidy (74), as this is a known effect of HQ and 1,2,4-benzenetriol (106,107). However, selective chromosome loss would probably not be caused by this process as effects on the mitotic spindle should be non-selective.
Topo II inhibition is the likely mechanism through which benzene induces leukemia- and lymphoma-related chromosomal translocations, such as t(8;21) and t(14;18) (97,99), and possibly inversions, in exposed workers. Inhibition of the functionality of topo II and enhanced DNA cleavage was associated with exposure to benzene in vivo in mice (108) and exposure to HQ and BQ in vitro (109,110–115). Bioactivation of HQ to BQ by peroxidase was found to enhance topo II inhibition (116) and BQ was more potent than HQ in a cell-free assay system (112,117). A quantitative structure activity relationship model of the interaction of benzene metabolites with human topo II alpha further supports the inhibition of topo II as a mechanism of benzene-induced genotoxicity (118). Recently, both HQ and the well-known topo II poison, etoposide, were found to significantly induce endoreduplication, a phenomenon that involves DNA duplication without corresponding cell division, in a dose-dependent manner in the lymphoblastoid TK6 cell line (119). Since endoreduplication may underlie the acquisition of high chromosome numbers by tumor cells, it may play a role in HQ-induced genomic instability and subsequent carcinogenesis. Benzene exposure was found to induce gene-duplicating mutations at the glycophorin A locus in the peripheral red blood cells of exposed humans (120,121), causing a phenotype associated with topo II inhibitors (122) that could arise from mitotic recombination. These findings reflect a mutational effect on precursor erythroid cells or stem cells in the bone marrow since mature red blood cell lack a nucleus. Recently, topo II alpha activity, protein and messenger RNA (mRNA) expression were shown to be reduced in patients with chronic benzene exposure, accompanied by alterations in histone acetylation and methylation of regulatory regions of the topo II alpha promoter (123). Together, these findings suggest that benzene could cause leukemias with chromosome translocations and inversions known to be induced by topo II inhibitors, including AMLs harboring t(21q22), t(15;17), inv(16) and t(16;16) in a manner consistent with previously proposed genetic pathways (51,124–127).
Critical genes are targeted through multiple processes in HSCs
Benzene-induced leukemia is thought to be initiated when benzene metabolites target genes or pathways that are critical to hematopoiesis in an HSC. As discussed above, benzene induces cytogenetic alterations such as aneuploidy, which can lead to altered gene expression (128) and DNA methylation (129) and translocations, which produce chimeric oncoproteins. Critical genes may also be targeted through gene mutation and/or epigenetic alteration. These effects, or the mechanisms which cause them, have been assessed in accessible surrogate cells such as peripheral blood lymphocytes.
Gene mutation
Gene mutations commonly associated with AML such as NPM1, AML1, FLT3, RAS and C/EBPα (130,131) have not specifically been reported in association with benzene or its metabolites. Gene-duplicating mutation but not gene-inactivating mutation, such as point mutations, was found at the glycophorin A locus in peripheral erythrocytes of humans exposed to high benzene levels (120). Wild-type and p53 heterozygous mice chronically exposed to high levels of benzene by inhalation, exhibited similar levels of mutations in the hypoxanthine-guanine phosphoribosyl transferase gene in T-lymphocytes (132). Mullin et al. (133) found a statistically significant increase in the mutant frequencies of the bacteriophage lambda lacI transgene in lung and spleen of male C57BL/6 mice chronically exposed to high levels of benzene by inhalation. Based on the lacI mutant frequency and fraction of unique mutations, lung tissues of benzene-exposed mice were estimated to have a 1.8-fold increase in lacI mutation frequency compared with lung tissues of unexposed control mice (134). The spectrum of p53 mutations induced by benzene exposure in human lung cells was recently examined and showed that A > G transition could be a potential fingerprint of benzene exposure in tumors (135), in particular AML (136). Despite the limited direct evidence of benzene-induced mutation, particularly in humans, two mechanisms known to cause DNA mutations, oxidative stress and error-prone DNA repair, are associated with benzene exposure.
Oxidative stress.
Oxygen radicals are produced during benzene metabolism and can induce direct toxic effects. DNA strand breaks and point mutations induced by benzene-initiated toxicity have been linked to the production of ROS in several studies in vitro (137–139). A potential role for oxidative stress in the induction of homologous recombination initiated by benzene metabolites has also been demonstrated in vitro (140). Oxidative DNA damage, measured as 8-hydroxy-2′-deoxyguanosine, was shown to be induced by PH, HQ and 1,2,4-benzenetriol in HL60 cells in vitro and by benzene in the bone marrow of mice in vivo (138). Benzene exposure was associated with increased mitochondria DNA copy number in exposed workers (141), possibly in response to oxidative stress caused by benzene. NQO1 has been implicated in influencing oxygen radical levels in cell lines in vitro (142). In a recent study analyzing the peripheral blood transcriptomes of 83 individuals occupationally exposed to a range of benzene levels and 42 unexposed matched controls, we reported that the oxidative phosphorylation pathway was significantly dysregulated by benzene across several exposure categories including <1 p.p.m. (19). Impaired oxidative phosphorylation can result from depolarization of inner mitochondrial membrane potential induced by oxidative stress and damaged mitochondria in turn produce more ROS. In the same study, we found that the expression of superoxide dismutase was upregulated between 50 and 100% at all levels of exposure examined.
Error-prone DNA repair.
The removal of benzene-induced DNA lesions such as oxidative DNA lesions, DNA adducts and apurinic sites has been shown to involve the major DNA repair pathways such as base excision repair, nucleotide excision repair and double-strand break (DSB) repair, as recently reviewed (143). DSB repair is error prone and may contribute to benzene-induced genomic instability. A recent microarray study in benzene-poisoning patients found consistently increased expression of DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which regulates NHEJ in DNA DSB repair (20). DNA-PKcs was found to be induced by HQ in HL60 cells in vitro at the mRNA and protein levels, along with DSBs and NHEJ, in the same study. Analysis of the interaction among benzene metabolites, DNA-PKcs induction and benzene toxicity in K562 cells revealed that that DNA-PKcs was induced by the benzene metabolite PH at both the protein and mRNA levels (144). Moreover, DNA-PKcs was found to translocate from the cytoplasm into the nucleus upon treatment with PH. Formation of γ-H2AX, which is a marker of DSB and NHEJ, was also increased by PH in a concentration-dependent manner. Together, the findings suggest that induction and activation of DNA-PKcs may contribute to benzene carcinogenesis by increasing NHEJ. Both HQ and BQ produced discrete foci of γ-H2AX within the nucleus of HL60 cells in a dose-dependent manner, apparently through the associated production of ROS, although the role of DNA-PKcs was not examined in that study (145).
Error-prone repair of DNA damage induced by benzene metabolites in HSCs, together with an intrinsic propensity of HSCs to survive rather than die by apoptosis, has been suggested to explain why these cells are susceptible to leukemic transformation by benzene (146). In the COMET assay, murine HSCs and myeloid progenitors were found to exhibit equal dose-dependent damage immediately after benzene treatment. However, HSCs showed evidence of DNA repair with lower levels of cell elimination after 24 h in culture as compared with myeloid progenitors. In follow-up studies, NHEJ was shown to be the preferential DNA repair mechanism in quiescent murine HSCs exposed to radiation and was found to be associated with the acquisition of genomic rearrangements that could persist in vivo (147). Thus, the enhanced short-term survival is achieved at the cost of an accumulation of deleterious mutations. However, a separate group found delayed DNA repair and an enhanced sensitivity to p53-dependent apoptosis induced by low-dose ionizing radiation in human HSCs derived from umbilical cord blood compared with more differentiated cells (148). While the exact nature of the response to DNA damage in human HSCs in vivo remains to be determined, mutations probably occur but their detection in this rare population of cells against a background of normal cells in the peripheral blood or bone marrow of exposed individuals is challenging. High-throughput single-cell genetic analysis techniques necessary to achieve the required sensitivity are being developed (149).
Epigenetic alterations
Benzene has been shown to alter the expression of many genes in the peripheral blood of exposed workers (17–19). Epigenetics is one of the major mechanisms by which gene expression is regulated, and epigenetic marks including histone modification, DNA methylation and microRNA expression, activate or repress expression of individual genes (150). The alteration of DNA methylation in cancer, including leukemia, is well known and involves genome-wide hypomethylation of non-coding regions which leads to genomic instability (151) and gene-specific promoter hypermethylation which silences critical genes such as those encoding tumor suppressors (151,152). A recent review suggested that epigenetic alterations may be involved in the toxicity of multiple environmental chemicals, including benzene (153). While evidence for some chemicals such as arsenic is relatively strong (154), the evidence remains somewhat limited for benzene because the results are either too preliminary or are in populations exposed to other factors in addition to benzene (Table I). Hypermethylation in p15 and hypomethylation in MAGE-1 were associated with very low benzene exposures (∼22 p.p.b.), in healthy subjects including gas station attendants and traffic police officers, although the degree of altered methylation was very low (24) and other potentially confounding exposures were present. Downregulation of p15 and p16 expression, potentially modulated by DNA promoter methylation, was reported more recently in benzene-poisoned workers (155). We reported altered DNA methylation and microRNA profiles in preliminary data from exposed workers (23). Global DNA hypomethylation, recently shown to be induced by HQ in TK6 cells in vitro, may be another mechanism underlying the leukemogenicity of benzene (25). Further study of the role epigenetics plays in the hematotoxicity and carcinogenicity of benzene is warranted, including studies of aberrant DNA methylation and altered microRNA expression.
Genomic instability
Benzene-induced leukemia probably begins as a mutagenic event in a stem or committed early progenitor cell and subsequent genomic instability allows for sufficient mutations to be acquired in a relatively short-time period. Studies have shown that HQ is similar to ionizing radiation in that it induces genomic instability in the bone marrow of susceptible mice (156). Recent findings showing the importance of DNA repair and maintenance genes, such as WRN, in human genetic susceptibility to benzene toxicity also support this mechanism (28,29,157,158). As discussed above, chromosomal aneuploidy was shown to be induced by benzene in exposed individuals in several studies (37,38,89,103–105), Table I. Two recent studies in yeast (159) and diverse human cancer cell lines (160) show that aneuploidy enhances genetic recombination and defective DNA damage repair, providing a mechanistic link between aneuploidy and genomic instability; thus, aneuploidy can cause a modest mutator phenotype and may be an initiating event in cancer.
Induction of HSCs from quiescence to cycling through dysregulation of the aryl hydrocarbon receptor
The transcription factor aryl hydrocarbon receptor (AhR), a cytosolic sensor of xenobiotics, notably dioxin and endogenous ligands, is thought to also function as a negative regulator of HSCs (161–163), controlling the balance between quiescence and proliferation (162). The AhR was shown to mediate benzene-induced hematotoxicity, in part through the induction of CYP2E1 (164), and mice engineered to lack AhR through knockout or through lethal irradiation followed by repopulation with AhR-null marrow cells, did not exhibit hematotoxicity following benzene exposure (164,165). Global gene–expression profiles of bone marrow cells from exposed AhR-knockout mice suggested the impairment of stem cell niches and consequent proliferation of hematopoietic progenitor cells (166). In addition, an increased amount of intracytoplasmic ROS was observed in the hematopoietic progenitor cells, particularly the Lin−c-kit+Sca-1+ fraction, compared with other blood cell fractions, after benzene exposure. While several possibilities have been proposed by which the altered presence and/or the activity of the AhR could modulate responses of hematopoietic stem/progenitor cells to benzene (167), additional research is needed to clarify these mechanisms.
Apoptosis and hematotoxicity
We hypothesize that the level or type of accumulated damage induced by benzene in HSCs leads to apoptosis which manifests as hematotoxicity. Induction of apoptosis has been demonstrated in vitro in human HL60 and CD34 cells (168) and in rat lymphoctyes (169) and an overrepresentation of genes involved in apoptosis was observed in the peripheral blood transcriptome of individuals occupationally exposed to a range of benzene exposures (18,19). Widespread hematotoxicity was observed in individuals exposed to <1 p.p.m. benzene in air (8,9). Bone marrow toxicity was suggested by the range of lineages affected and was further supported by the demonstration of highly significant dose-dependent decreases in myeloid progenitor cell colony formation in exposed individuals (8). Thus, hematotoxicity represents a useful quantitative biomarker of benzene effect even at levels under 1 p.p.m.
As well as causing hematotoxicity, the death by apoptosis of certain cell types in the stem cell niche, such as stromal cells, or the escape from the apoptotic process of stem cells that have acquired DNA or chromosomal damage, may result in the proliferation and clonal expansion of preleukemic stem cells.
Dysregulation of the HSC niche leads to increased proliferation
HSCs occupy an ordered environment in the bone marrow and interact with supportive stromal and endothelial cells and mature lymphocytes in the stem cell niche. Hematotoxic damage to this ordered stem cell microenvironment could result in abnormal hematopoiesis and allow for the clonal expansion of leukemic stem cells (LSCs).
Gap-junction intracellular communication with stromal cells in the bone marrow and thymus is thought to be necessary for normal hematopoietic regulation (170–173). Several benzene metabolites were found to interfere with gap-junction intercellular communication in vitro, the most potent being E,E-muconic acid (174,175). Many adhesion molecules (176) and chemokines (177) are important in niche function and play key roles in stem cell mobilization and homing. Genetic polymorphisms in specific cytokines, chemokines and cellular adhesion genes with known or potential roles in niche function have been associated with increased susceptibility to benzene toxicity in candidate gene association studies (30,32,178). Zhou et al. examined benzene-induced effects on human bone marrow endothelial cells using a transformed human bone marrow endothelial cell (TrHBMEC) line. In transformed human bone marrow endothelial cells in which NQO1 activity had been decreased by inhibition or knockdown, a marked decrease in tumor necrosis factor α-induced CD34+ hematopoietic cell adhesion, was observed (179). Since adhesion molecules have documented roles in hematopoietic cell homing and mobilization, impairment of the endothelial/vascular stem cell niche may play a role in the increased susceptibility to benzene toxicity of individuals lacking NQO1. In cellular studies, the levels of myeloperoxidase and NQO1 have been suggested to modulate the toxicity of phenolic metabolites of benzene particularly in stromal cells where multiple cell types exist with varying enzyme activities (180,181). Another key function of human bone marrow endothelial cell is tube formation leading to angiogenesis, a critical component of the HSC vascular microenvironment. HQ treatment of human bone marrow endothelial cell led to inhibition of tube formation in part through upregulation of chondromodulin 1, an anti-angiogenic gene expressed by endothelial cells (182). Thus, inhibition of tube formation is a potential mechanism by which benzene induces adverse effects in the bone marrow endothelial microenvironment critical to the development and differentiation of HSCs.
Balanced regulation of ROS is critical for cell-fate determination and for stem cell development, function and survival (183). Dysregulation of ROS production has been implicated in abnormal hematopoiesis and potentially in functioning of the HSC niche (184). Recent evidence suggests that ROS play a key role in the development of in utero-initiated benzene toxicity potentially through disruption of hematopoietic cell signaling pathways (185) and inhibition of erythropoiesis (186).
HQ-induced dysregulation of external signals modulating the protein phosphorylation of the transcriptional regulator, PU.1, may promote the clonal proliferation of CD34+ cells (187). HQ was shown to alter the pattern of binding of PU.1 to DNA in a time- and dose-dependent manner and to induce hyperphosphorylation of the PU.1 protein, in cultured human CD34+ cells. These effects occurred in conjunction with a sustained immature CD34+ phenotype, cytokine-dependent enhanced clonogenic activity in cultured human HPC and decreased terminal differentiation.
Together these data show that the hematopoietic signaling functions of the stem cell niche, which comprises many potential target cells, could be disrupted by benzene and its metabolites through multiple potential mechanisms. Further research is needed to examine these effects, particularly in humans. This is challenging, however, because the niche is not readily accessible. Development of in vitro models of the bone marrow and stem cell niche is required and has been reported with some success (188–190). In such a model, it may be possible to measure genetic and epigenetic changes in the HSCs using microfluidic and other emerging technologies (191), along with determination of the rates of apoptosis, differentiation and proliferation.
Clonal evolution through decreased immunosurveillance
Ordinarily, clonal expansion of preleukemic stem cells would be monitored and prevented by immunosurveillance. However, as confirmed by multiple human studies, benzene exposure causes impaired immune function that could compromise immunosurveillance. Occupational benzene exposure to benzene, as well as causing hematotoxicity, was shown to alter ratios of immune cell subsets, even at low doses (8). The selective effect on CD4 T-lymphocytes could allow pre-leukemic or LSCs to escape detection and elimination, but it is unclear whether this reduction in numbers reflects reduced functional capacity. In a separate study, recent thymic output and T-cell immune function were apparently impaired in exposed workers (192). Microarray analysis revealed elevated cytochrome P450 4F3A (CYP4F3A), which encodes the leukotriene B4-hydroxylase, important for inactivation of leukotriene B4 in neutrophils, in the peripheral mononuclear blood cells of seven workers diagnosed with benzene poisoning, compared with seven matched controls (20). Our recent study of global gene expression in the blood of workers exposed to a range of benzene levels revealed a strong effect on immune response pathways (19). Together, these findings indicate that benzene induces immune suppression, a consequence of which is reduced immunosurveillance. Immune suppression was recently shown to increase the risk of developing AML, in recipients of kidney (N = 217219) and heart (N = 31005) transplants (193).
Mode of action approach to the risk assessment of benzene
Increasingly, a mode of action (MOA) approach is being used in risk assessment, although this approach is acknowledged to have general limitations (194). An MOA is a well-defined and biologically plausible series of critical key events that leads to an adverse effect and is usually determined in an animal test species and extrapolated to humans (195–197). Once the key events are determined, quantitative dose–response data on the measurable parameters associated with those key events are generated. As described above, the mechanism(s) by which benzene causes leukemia is not fully understood and many research questions remain. This limits the applicability of a MOA-based approach to the assessment of leukemia risk associated with benzene, as recently proposed (198). The hypothesized MOA for benzene comprises five key events: (i) metabolism of benzene to a benzene oxide metabolite; (ii) interaction of the benzene metabolite with target cells in the bone marrow; (ii) the formation of initiated, mutated bone marrow target cells; (iv) the selected clonal proliferation of these target cells and (v) the formation of the neoplasm (leukemia) (198).
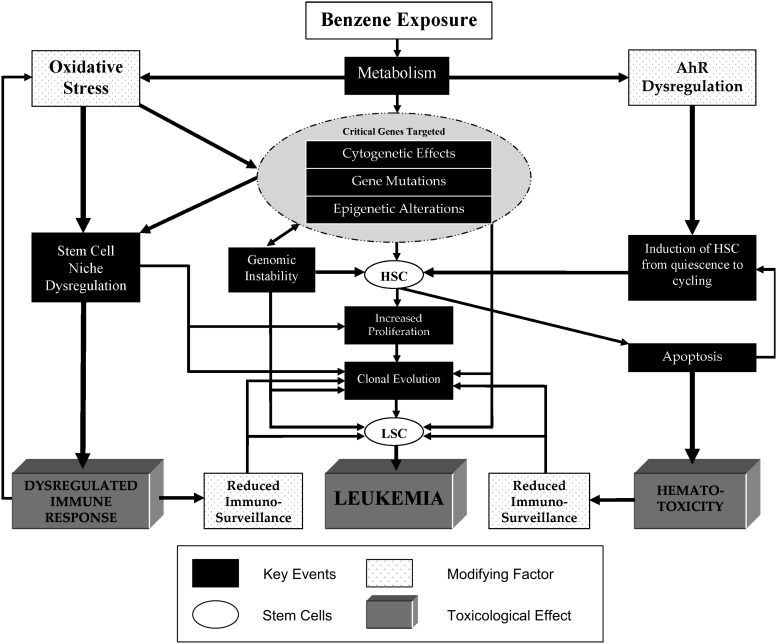
In the proposed MOA, apart from the first key event, which is specific but describes only one of the many potentially carcinogenic metabolites illustrated in Figure 1, the other key events proposed are vague, not well characterized and therefore difficult to quantitate and thus apply in risk assessment. Furthermore, this simplified MOA suggests that an ordered sequence of key events leads to leukemia. However, as multiple pathways are involved in the mechanism of action of benzene and the effects of benzene metabolites on the leukemogenic process are not singular and can occur throughout the process, it is difficult to define a simple stochastic MOA. This is illustrated in Figure 2, in which we have incorporated the updated mechanistic data discussed above to illustrate the range of potential key events and modifying factors that could contribute to the toxicological effects of benzene in an MOA approach. Key events in benzene-induced leukemogenesis include the targeting of critical genes through the induction of genetic, chromosomal or epigenetic abnormalities in HSCs; genomic instability; stromal cell dysregulation; apoptosis of HSCs and stromal cells and altered proliferation and differentiation, leading to the generation of LSCs. These key events modulated by oxidative stress, AhR dysregulation and reduced immunosurveillance, lead to dysregulated immune response, hematotoxicity and leukemia.
Fig. 2.
Multiple MOAs of benzene-induced leukemogenesis. Potential key events, modifying factors and toxicological effects are depicted in the following legend. Stem cells can be either HSCs or LSCs.
For a variety of reasons, it is challenging to identify relevant and quantifiable parameters for several of the key events detailed in Figure 2, for application in risk assessment. Metabolic differences among species, among genetic subtypes and lifestyles, at different concentrations and sites as well as incomplete knowledge regarding the relevance of individual metabolites to toxicity, make it challenging if not impossible to quantitate these effects. Immunosurveillance is a complex multifactorial mechanism that is difficult to conclusively detect or quantify. Benzene induces the dysregulation of critical genes through multiple mechanisms (cytogenetic alterations, aberrant mitotic recombination, gene mutations and/or epigenetic alterations) resulting in various end points, which makes it difficult to comprehensively quantitate these potentially relevant parameters. Currently, it is challenging to measure AhR dysregulation, an HSC-specific effect, or the number of non-cycling quiescent HSCs, in exposed individuals. As discussed earlier, additional studies are needed to examine the effects of benzene and its metabolites on the stem cell niche, particularly in humans.
Considerable differences among the responses of mouse, rat and human to benzene exposure limit the ability to extrapolate from animal effects to human in the MOA approach. For example, animal-to-human extrapolations using physiologically based pharmacokinetic models have been unable to accurately predict human metabolism as the proportions of benzene metabolites produced differ among mice, rats and humans (1). Furthermore, in humans, benzene exposure is predominantly associated with acute non-lymphocytic leukemia compared with mainly lymphocytic leukemia in mice (199,200), though benzene also induces many other types of tumor in rodents (201). Most animal studies to date have been performed at acute high-dose exposures that may not be relevant to typical human exposures even in occupational settings. Although hematopoietic neoplasms were induced at high-level exposures in mice, albeit with a non-linear increase with increasing dose, the incidence of hematopoietic neoplasms after low-level benzene exposure in wild-type mice has been equivocal and AML has not been detected in most mouse studies (202,203). Given these differences, measuring certain aspects of key events in animal models (cytotoxicity and genotoxicity) may be useful but other key events such as impact on specific lineages leading to leukemia might not be relevant. In their proposed benzene MOA, Meek and Klaunig performed a ‘concordance evaluation’ among rodents and humans and suggested that key events are qualitatively similar, but they neither specified which parameters were measured nor provided supporting references (198).
Systems biology approach to the risk assessment of benzene
Toxicogenomic studies of benzene’s toxic effects in exposed humans and in relevant in vitro and animal models can inform the mechanisms of benzene toxicity and identify biomarkers relevant to risk assessment. Toxicogenomic studies of exposed human populations can identify potential biomarkers of early effect and susceptibility of relevance to risk assessment and lead to a better understanding of the mechanisms of toxicity, as discussed recently by us (204) and others (205). In order to increase the relevance of toxicogenomic data to disease risk, true effects need to be distinguished from adaptive responses in the context of phenotypic anchoring. Integration of multiple types of toxicogenomic data in a systems biology approach can identify more robust biomarkers. We are applying such an approach to assess the effects of benzene exposure (23) and have generated various toxicogenomic datasets including transcriptomics, proteomics and epigenomics, as listed in Table I. Our recent study of global gene expression in 125 workers occupationally exposed to benzene, identified a signature of genes expression altered at a range of exposure levels and found altered immune and AML pathways, even in individuals exposed at <1 p.p.m., suggesting relevance of the observed changes to leukemogenesis (19). Genes involved in susceptibility to benzene toxicity have been identified through functional genomics and candidate gene association studies, Table I, and approaches such as genome-wide association studies or next-generation sequencing could identify additional genes.
Toxicogenomic studies have also been conducted on hematopoietic cells of mice exposed to high levels of benzene. Microarray analysis revealed altered mRNA expression of various apoptosis, cell cycle and growth control genes in HSCs from mice exposed to 100 p.p.m. inhaled benzene for 6 h/day, 5 days/week for 2 weeks, compared with control mice (206). In a study of wild-type and p53-knockout mice exposed to 300 p.p.m. benzene for 6 h/ day, 5 days/week for 2 weeks (207), the p53 tumor suppressor gene was shown to be central to the mechanism of benzene action in bone marrow cells, by strictly regulating specific genes involved in the pathways of cell cycle arrest, apoptosis and DNA repair. It was proposed that dysfunction of the p53 gene, possibly through effects of repeated benzene exposure, could lead to problems in these critical functions and lead to hematopoietic malignancies. In a more recent study, heterozygous p53-deficient mouse models (C3H/He and C57BL/6 strains) were found to produce a higher incidence of hematopoietic neoplasms and a higher than threshold incidence of hematopoietic neoplasms at lower doses, compared with the corresponding wild-type strains, and AML was induced in the p53-deficient C3H/He mice exposed to benzene 300 p.p.m. (208).
The p53 response pathway was not identified in our human toxicogenomic studies and the relevance of gene expression changes induced by high-dose animal studies to typical human exposure levels is unclear; therefore, there is a need to conduct toxicogenomic studies of animals chronically exposed to lower levels of benzene. As well as dose, other factors such as differences in tissues examined, microarray technologies/platforms, co-exposures (toluene is a potential confounder for some peripheral blood effects in exposed workers (12)) and other uncontrolled confounding in the human study may have contributed to the lack of concordance between the human and animal findings and should be considered in future studies. Studies in transgenic mouse models engineered to express factors known or suspected to be involved in human susceptibility to benzene toxicity would also be informative.
Global gene expression has been analyzed in vitro in human HL-60 cells (209) and peripheral blood mononuclear cells (210) exposed to benzene metabolites. However, as the target cell for leukemia induction is probably the HSC and benzene disrupts hematopoietic signaling functions of the bone marrow stem cell niche, characterization of benzene toxicity in in vitro models of the bone marrow stem cell niche is probably more relevant to understanding benzene-induced leukemogenesis than investigation of effects in mature hematopoietic cells. The stem cell niche is a dynamic environment and the ‘functiotype’ of stem cells is the result of molecular, cellular, extracellular, biomechanical and spatio-dimensional effects, as summarized (211). These cues, together with niche context (osteoclast or vascular niche), lead to a ‘signaling network state’ of stem cells and determine whether the cells remain quiescent or proliferate. Despite its inherent complexity, the stem cell niche has been bioengineered through various approaches including microfluidic devices such as interconnected micro-channels or systems that generate gradients of effector molecules (212); micrometer-sized silicone cavities coated with extracellular matrix proteins and a 96-well platform in which a microcontact-printing technology is used to control niche size, shape and density of cells and effector molecules (213,214). Advances have also been made in understanding systemic niche dynamics using molecular measurements and in tracking single-cell fates using high-content imaging as well as in modeling the niche using various mathematical modeling techniques (211). These models and modeling methods can be applied to study the toxic effects of benzene on the stem cell niche.
For application in risk assessment, it is necessary to model dose–response relationships of particular biomarkers at different levels of benzene exposure in humans or experimental animals. Relevant biomarkers identified to date that could be used in the dose–response modeling of benzene-induced leukemia include lymphocyte counts, leukemia-relevant genetic and epigenetic damage, proliferation rates of blood stem and progenitor cells, levels of HSC and LSC apoptosis and expression of AML pathway genes. Several of these biomarkers have been measured in human populations exposed to a broad range of benzene concentrations. Linear or supra-linear dose-dependent effects on lymphocyte counts, colony formation from myeloid stem and progenitor cells and gene expression have been shown to occur at low levels of occupational exposure (≤1 p.p.m. to >10 p.p.m.) (8,9,19,215). Studies measuring leukemia-relevant biomarkers in individuals or animals exposed to benzene at environmental levels of exposure in the 1–250 p.p.b. range are needed to help model risk at these levels. Currently, we are statistically modeling the dose–response of AML pathway gene expression in our benzene-exposed population but toxicogenomic studies of populations exposed at lower environmental levels are needed for comparison.
In conclusion, dose–response modeling of existing biomarkers in the context of a phenotypic anchor such as hematotoxicity and identification of additional biomarkers of early effect on the causal pathway to leukemia at environmental exposures, by toxicogenomic and systems biology approaches in exposed human populations, exposed animals and in vitro bone marrow niche models are useful approaches for the advancement of benzene risk assessment.
Funding
This work was supported in part by the U.S. Environmental Protection Agency under order number EP09H000461. Additional support was provided by National Institutes of Health grant (P42ES004705 to M.T.S)
Acknowledgments
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Conflict of Interest Statement: M.T.S. has received consulting and expert testimony fees from law firms representing both plaintiffs and defendants in cases involving exposure to benzene.
Glossary
Abbreviations
- AhR
aryl hydrocarbon receptor
- AML
acute myeloid leukemia
- 1,4-BQ
benzoquinone
- CYP
cytochrome-P450
- CYP2E1
cytochrome P450 2E1
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- DSB
double-strand break
- HQ
hydroquinone
- HSC
hematopoietic stem cell
- LSC
leukemic stem cell
- MOA
mode of action
- mRNA
messenger RNA
- NHEJ
non-homologous end joining
- NQO1
NAD(P)H:quinone oxidoreductase 1
- PH
phenol
- p.p.m.
parts per million
- ROS
reactive oxygen species
- t-AML
therapy-related acute myeloid leukemia
- t-MDS
therapy-related myelodysplastic syndrome
- topo II
topoisomerase II
References
- 1.ATSDR. Toxicological Profile For Benzene. 2007. http://www.atsdr.cdc.gov/toxprofiles/tp3.html. U.S. Department Of Health And Human Services. Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- 2.NIOSH. National Occupational Exposure Survey (1981-83) 1990. http://www.cdc.gov/noes/noes3/empl0003.html. National Institute for Occupational Safety and Health Cincinnati, OH. [Google Scholar]
- 3.Weisel CP. Benzene exposure: an overview of monitoring methods and their findings. Chem. Biol. Interact. 2010;184:58–66. doi: 10.1016/j.cbi.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. Benzene exposure in the shoemaking industry in China, a literature survey, 1978-2004. Regul. Toxicol. Pharmacol. 2006;46:149–156. doi: 10.1016/j.yrtph.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Rappaport SM, et al. Evidence that humans metabolize benzene via two pathways. Environ. Health Perspect. 2009;117:946–952. doi: 10.1289/ehp.0800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace L. Environmental exposure to benzene: an update. Environ. Health Perspect. 1996;104(suppl. 6):1129–1136. doi: 10.1289/ehp.961041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schettgen T, et al. A biomarker approach to estimate the daily intake of benzene in non-smoking and smoking individuals in Germany. J. Expo. Sci. Environ. Epidemiol. 2009;20:427–433. doi: 10.1038/jes.2009.32. [DOI] [PubMed] [Google Scholar]
- 8.Lan Q, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Q, et al. Hematological changes among Chinese workers with a broad range of benzene exposures. Am. J. Ind. Med. 2002;42:275–285. doi: 10.1002/ajim.10121. [DOI] [PubMed] [Google Scholar]
- 10.Ward E, et al. Risk of low red or white blood cell count related to estimated benzene exposure in a rubberworker cohort (1940-1975) Am. J. Ind. Med. 1996;29:247–257. doi: 10.1002/(SICI)1097-0274(199603)29:3<247::AID-AJIM4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B. [Investigation of health status in workers exposed to low-level benzene] Zhonghua Yu Fang Yi Xue Za Zhi. 1996;30:164–166. [PubMed] [Google Scholar]
- 12.Schnatter RA, et al. Peripheral blood effects in benzene-exposed workers. Chem. Biol. Interact. 2010;184:174–181. doi: 10.1016/j.cbi.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Swaen GM, et al. Low level occupational benzene exposure and hematological parameters. Chem. Biol. Interact. 2010;184:94–100. doi: 10.1016/j.cbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Collins JJ, et al. Evaluation of lymphopenia among workers with low-level benzene exposure and the utility of routine data collection. J. Occup. Environ. Med. 1997;39:232–237. doi: 10.1097/00043764-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SP, et al. A hematology surveillance study of petrochemical workers exposed to benzene. Regul. Toxicol. Pharmacol. 2004;40:67–73. doi: 10.1016/j.yrtph.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Pesatori AC, et al. Early effects of low benzene exposure on blood cell counts in Bulgarian petrochemical workers. Med. Lav. 2009;100:83–90. [PubMed] [Google Scholar]
- 17.Forrest MS, et al. Discovery of novel biomarkers by microarray analysis of peripheral blood mononuclear cell gene expression in benzene-exposed workers. Environ. Health Perspect. 2005;113:801–807. doi: 10.1289/ehp.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHale CM, et al. Changes in the peripheral blood transcriptome associated with occupational benzene exposure identified by cross-comparison on two microarray platforms. Genomics. 2009;93:343–349. doi: 10.1016/j.ygeno.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHale CM, et al. Global gene expression profiling of a population exposed to a range of benzene levels. Environ. Health Perspect. 2010;119:628–634. doi: 10.1289/ehp.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi Y, et al. Gene expression in benzene-exposed workers by microarray analysis of peripheral mononuclear blood cells: induction and silencing of CYP4F3A and regulation of DNA-dependent protein kinase catalytic subunit in DNA double strand break repair. Chem. Biol. Interact. 2009;184:207–211. doi: 10.1016/j.cbi.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Vermeulen R, et al. Decreased levels of CXC-chemokines in serum of benzene-exposed workers identified by array-based proteomics. Proc. Natl. Acad. Sci. USA. 2005;102:17041–17046. doi: 10.1073/pnas.0508573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo WA, et al. Proteomic analysis of plasma proteins of workers exposed to benzene. Mutat. Res. 2004;558:35–44. doi: 10.1016/j.mrgentox.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, et al. Systems biology of human benzene exposure. Chem. Biol. Interact. 2010;184:86–93. doi: 10.1016/j.cbi.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollati V, et al. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67:876–880. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 25.Ji Z, et al. A comparison of the cytogenetic alterations and global DNA hypomethylation induced by the benzene metabolite, hydroquinone, with those induced by melphalan and etoposide. Leukemia. 2010;24:986–991. doi: 10.1038/leu.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North M, et al. Utilizing functional genomics in yeast to discover novel biomarkers of benzene toxicity in humans. Toxicol. Lett. 2009;189 Abstracts of the 46th Congress of the European Societies of Toxicology, S93. [Google Scholar]
- 27.Galvan N, et al. Depletion of WRN enhances DNA damage in HeLa cells exposed to the benzene metabolite, hydroquinone. Mutat. Res. 2008;649:54–61. doi: 10.1016/j.mrgentox.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X, et al. Werner syndrome protein, WRN, protects cells from DNA damage induced by the benzene metabolite hydroquinone. Toxicol. Sci. 2009;107:367–375. doi: 10.1093/toxsci/kfn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan Q, et al. Large-scale evaluation of candidate genes identifies associations between DNA repair and genomic maintenance and development of benzene hematotoxicity. Carcinogenesis. 2009;30:50–58. doi: 10.1093/carcin/bgn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan Q, et al. Polymorphisms in cytokine and cellular adhesion molecule genes and susceptibility to hematotoxicity among workers exposed to benzene. Cancer Res. 2005;65:9574–9581. doi: 10.1158/0008-5472.CAN-05-1419. [DOI] [PubMed] [Google Scholar]
- 31.Shen M, et al. Polymorphisms in DNA repair genes and risk of non-Hodgkin lymphoma among women in Connecticut. Hum. Genet. 2006;119:659–668. doi: 10.1007/s00439-006-0177-2. [DOI] [PubMed] [Google Scholar]
- 32.Hosgood HD, III, et al. Association between genetic variants in VEGF, ERCC3 and occupational benzene haematotoxicity. Occup. Environ. Med. 2009;66:848–853. doi: 10.1136/oem.2008.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun P, et al. Association of genetic polymorphisms, mRNA Expression of p53 and p21 with chronic benzene Poisoning in a Chinese occupational population. Cancer Epidemiol. Biomarkers Prev. 2009;18:1821–1828. doi: 10.1158/1055-9965.EPI-09-0140. [DOI] [PubMed] [Google Scholar]
- 34.Wu F, et al. Genetic polymorphisms in hMTH1, hOGG1 and hMYH and risk of chronic benzene poisoning in a Chinese occupational population. Toxicol. Appl. Pharmacol. 2008;233:447–453. doi: 10.1016/j.taap.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Sun P, et al. Polymorphisms in phase I and phase II metabolism genes and risk of chronic benzene poisoning in a Chinese occupational population. Carcinogenesis. 2008;29:2325–2329. doi: 10.1093/carcin/bgn208. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, et al. Genetic polymorphisms in XRCC1, APE1, ADPRT, XRCC2, and XRCC3 and risk of chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol. Biomarkers Prev. 2005;14:2614–2619. doi: 10.1158/1055-9965.EPI-05-0143. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, et al. Use of OctoChrome fluorescence in situ hybridization to detect specific aneuploidy among all 24 chromosomes in benzene-exposed workers. Chem. Biol. Interact. 2005;153–154:117–122. doi: 10.1016/j.cbi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, et al. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis. 2011;32:605–612. doi: 10.1093/carcin/bgq286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khalade A, et al. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environ. Health. 2010;9:31. doi: 10.1186/1476-069X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MT. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health. 2010;31:133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinmaus C, et al. Meta-analysis of benzene exposure and non-Hodgkin lymphoma: biases could mask an important association. Occup. Environ. Med. 2008;65:371–378. doi: 10.1136/oem.2007.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eden T. Aetiology of childhood leukaemia. Cancer Treat. Rev. 2010;36:286–297. doi: 10.1016/j.ctrv.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Galbraith D, et al. Benzene and human health: a historical review and appraisal of associations with various diseases. Crit. Rev. Toxicol. 2010;40(suppl. 2):1–46. doi: 10.3109/10408444.2010.508162. [DOI] [PubMed] [Google Scholar]
- 44.Pyatt D. Benzene and hematopoietic malignancies. Clin. Occup. Environ. Med. 2004;4:529–555. doi: 10.1016/j.coem.2004.03.014. , vii. [DOI] [PubMed] [Google Scholar]
- 45.Natelson EA. Benzene-induced acute myeloid leukemia: a clinician's perspective. Am. J. Hematol. 2007;82:826–830. doi: 10.1002/ajh.20934. [DOI] [PubMed] [Google Scholar]
- 46.Godley LA, et al. Therapy-related myeloid leukemia. Semin. Oncol. 2008;35:418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowley JD, et al. Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood. 1981;58:759–767. [PubMed] [Google Scholar]
- 48.Smith SM, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 49.Pedersen-Bjergaard J, et al. Cytogenetic characteristics of therapy-related acute nonlymphocytic leukaemia, preleukaemia and acute myeloproliferative syndrome: correlation with clinical data for 61 consecutive cases. Br. J. Haematol. 1987;66:199–207. doi: 10.1111/j.1365-2141.1987.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst.) 2006;5:1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen-Bjergaard J, et al. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–248. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen-Bjergaard J, et al. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 53.Nishikawa T, et al. Benzene induces cytotoxicity without metabolic activation. J. Occup. Health. 2011;11:11. doi: 10.1539/joh.10-002-oa. [DOI] [PubMed] [Google Scholar]
- 54.Ross D. The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J. Toxicol. Environ. Health. 2000;61:357–372. doi: 10.1080/00984100050166361. [DOI] [PubMed] [Google Scholar]
- 55.Sammett D, et al. Partial hepatectomy reduces both metabolism and toxicity of benzene. J. Toxicol. Environ. Health. 1979;5:785–792. doi: 10.1080/15287397909529789. [DOI] [PubMed] [Google Scholar]
- 56.Valentine JL, et al. Reduction of benzene metabolism and toxicity in mice that lack CYP2E1 expression. Toxicol. Appl. Pharmacol. 1996;141:205–213. doi: 10.1006/taap.1996.0277. [DOI] [PubMed] [Google Scholar]
- 57.Andrews LS, et al. Effects of toluene on the metabolism, disposition and hemopoietic toxicity of [3H]benzene. Biochem. Pharmacol. 1977;26:293–300. doi: 10.1016/0006-2952(77)90180-0. [DOI] [PubMed] [Google Scholar]
- 58.Wetmore BA, et al. Genotoxicity of intermittent co-exposure to benzene and toluene in male CD-1 mice. Chem. Biol. Interact. 2008;173:166–178. doi: 10.1016/j.cbi.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Bird MG, et al. Influence of toluene co-exposure on the metabolism and genotoxicity of benzene in mice using continuous and intermittent exposures. Chem. Biol. Interact. 2010;184:233–239. doi: 10.1016/j.cbi.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Snyder R, et al. An overview of benzene metabolism. Environ. Health Perspect. 1996;104(suppl. 6):1165–1171. doi: 10.1289/ehp.961041165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powley MW, et al. Benzene metabolism by the isolated perfused lung. Inhal. Toxicol. 2002;14:569–584. doi: 10.1080/08958370290084502. [DOI] [PubMed] [Google Scholar]
- 62.Sheets P, et al. Kinetic factors involved in the metabolism of benzene in mouse lung and liver. J. Toxicol. Environ. Health A. 2004;67:421–430. doi: 10.1080/15287390490273488. [DOI] [PubMed] [Google Scholar]
- 63.Andrews LS, et al. 3H-Benzene metabolism in rabbit bone marrow. Life Sci. 1979;25:567–572. doi: 10.1016/0024-3205(79)90550-2. [DOI] [PubMed] [Google Scholar]
- 64.Irons RD, et al. Benzene is metabolized and covalently bound in bone marrow in situ. Chem. Biol. Interact. 1980;30:241–245. doi: 10.1016/0009-2797(80)90130-1. [DOI] [PubMed] [Google Scholar]
- 65.Subrahmanyam VV, et al. Phenol-induced stimulation of hydroquinone bioactivation in mouse bone marrow in vivo: possible implications in benzene myelotoxicity. Toxicology. 1990;62:107–116. doi: 10.1016/0300-483x(90)90035-f. [DOI] [PubMed] [Google Scholar]
- 66.Subrahmanyam VV, et al. Hydroxylation of phenol to hydroquinone catalyzed by a human myeloperoxidase-superoxide complex: possible implications in benzene-induced myelotoxicity. Free Radic. Res. Commun. 1991;15:285–296. doi: 10.3109/10715769109105224. [DOI] [PubMed] [Google Scholar]
- 67.Nedelcheva V, et al. Metabolism of benzene in human liver microsomes: individual variations in relation to CYP2E1 expression. Arch. Toxicol. 1999;73:33–40. doi: 10.1007/s002040050583. [DOI] [PubMed] [Google Scholar]
- 68.Powley MW, et al. Cytochromes P450 involved with benzene metabolism in hepatic and pulmonary microsomes. J. Biochem. Mol. Toxicol. 2000;14:303–309. doi: 10.1002/1099-0461(2000)14:6<303::AID-JBT2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 69.Koskela S, et al. Expression of CYP2A genes in human liver and extrahepatic tissues. Biochem. Pharmacol. 1999;57:1407–1413. doi: 10.1016/s0006-2952(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 70.Su T, et al. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- 71.Rappaport SM, et al. Human benzene metabolism following occupational and environmental exposures. Chem. Biol. Interact. 2009;184:189–195. doi: 10.1016/j.cbi.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue O, et al. Excretion of 1,2,4-benzenetriol in the urine of workers exposed to benzene. Br. J. Ind. Med. 1989;46:559–565. doi: 10.1136/oem.46.8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Witz G, et al. Reactive ring-opened aldehyde metabolites in benzene hematotoxicity. Environ. Health Perspect. 1996;104(suppl. 6):1195–1199. doi: 10.1289/ehp.961041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith MT. The mechanism of benzene-induced leukemia: a hypothesis and speculations on the causes of leukemia. Environ. Health Perspect. 1996;104(suppl. 6):1219–1225. doi: 10.1289/ehp.961041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Subrahmanyam VV, et al. Potential role of free radicals in benzene-induced myelotoxicity and leukemia. Free Radic. Biol. Med. 1991;11:495–515. doi: 10.1016/0891-5849(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 76.Iskander K, et al. Quinone oxidoreductases in protection against myelogenous hyperplasia and benzene toxicity. Chem. Biol. Interact. 2005;153–154:147–157. doi: 10.1016/j.cbi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 77.Long DJ, II, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–3036. [PubMed] [Google Scholar]
- 78.Rothman N, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997;57:2839–2842. [PubMed] [Google Scholar]
- 79.Irons RD, et al. Cell proliferation and differentiation in chemical leukemogenesis. Stem Cells. 1993;11:235–242. doi: 10.1002/stem.5530110311. [DOI] [PubMed] [Google Scholar]
- 80.Bratton SB, et al. Identification of quinol thioethers in bone marrow of hydroquinone/phenol-treated rats and mice and their potential role in benzene-mediated hematotoxicity. Chem. Res. Toxicol. 1997;10:859–865. doi: 10.1021/tx960208r. [DOI] [PubMed] [Google Scholar]
- 81.Monks TJ, et al. The fate of benzene-oxide. Chem. Biol. Interact. 2010;184:201–206. doi: 10.1016/j.cbi.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross D, et al. Relationships between metabolic and non-metabolic susceptibility factors in benzene toxicity. Chem. Biol. Interact. 2010;184:222–228. doi: 10.1016/j.cbi.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mansi A, et al. Low occupational exposure to benzene in a petrochemical plant: modulating effect of genetic polymorphisms and smoking habit on the urinary t, t-MA/SPMA ratio. Toxicol. Lett. 2011;15:15. doi: 10.1016/j.toxlet.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Bogadi-Soare A, et al. Genotoxic effects in workers exposed to benzene: with special reference to exposure biomarkers and confounding factors. Ind. Health. 1997;35:367–373. doi: 10.2486/indhealth.35.367. [DOI] [PubMed] [Google Scholar]
- 85.Ding XJ, et al. Chromosome changes in patients with chronic benzene poisoning. Chin. Med. J. (Engl.) 1983;96:681–685. [PubMed] [Google Scholar]
- 86.Forni AM, et al. Chromosome changes and their evolution in subjects with past exposure to benzene. Arch. Environ. Health. 1971;23:385–391. doi: 10.1080/00039896.1971.10666024. [DOI] [PubMed] [Google Scholar]
- 87.Kasuba V, et al. Cytogenetic changes in subjects occupationally exposed to benzene. Chemosphere. 2000;40:307–310. doi: 10.1016/s0045-6535(99)00265-9. [DOI] [PubMed] [Google Scholar]
- 88.Picciano D. Cytogenetic study of workers exposed to benzene. Environ. Res. 1979;19:33–38. doi: 10.1016/0013-9351(79)90031-8. [DOI] [PubMed] [Google Scholar]
- 89.Sasiadek M. Nonrandom distribution of breakpoints in the karyotypes of workers occupationally exposed to benzene. Environ. Health Perspect. 1992;97:255–257. doi: 10.1289/ehp.9297255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sasiadek M, et al. Genotoxic effects observed in workers occupationally exposed to organic solvents. Pol. J. Occup. Med. 1990;3:103–108. [PubMed] [Google Scholar]
- 91.Sasiadek M, et al. Localization of breakpoints in the karyotype of workers professionally exposed to benzene. Mutat. Res. 1989;224:235–240. doi: 10.1016/0165-1218(89)90161-4. [DOI] [PubMed] [Google Scholar]
- 92.Tompa A, et al. Monitoring of benzene-exposed workers for genotoxic effects of benzene: improved-working-condition-related decrease in the frequencies of chromosomal aberrations in peripheral blood lymphocytes. Mutat. Res. 1994;304:159–165. doi: 10.1016/0027-5107(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 93.Tough IM, et al. Chromosome aberrations and exposure to ambient benzene. Lancet. 1965;1:684. doi: 10.1016/s0140-6736(65)91835-0. [DOI] [PubMed] [Google Scholar]
- 94.Tough IM, et al. Chromosome studies on workers exposed to atmospheric benzene. The possible influence of age. Eur. J. Cancer. 1970;6:49–55. doi: 10.1016/0014-2964(70)90053-8. [DOI] [PubMed] [Google Scholar]
- 95.Yardley-Jones A, et al. Analysis of chromosomal aberrations in workers exposed to low level benzene. Br. J. Ind. Med. 1990;47:48–51. doi: 10.1136/oem.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, et al. Increased aneusomy and long arm deletion of chromosomes 5 and 7 in the lymphocytes of Chinese workers exposed to benzene. Carcinogenesis. 1998;19:1955–1961. doi: 10.1093/carcin/19.11.1955. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L, et al. Aberrations in chromosomes associated with lymphoma and therapy-related leukemia in benzene-exposed workers. Environ. Mol. Mutagen. 2007;48:467–474. doi: 10.1002/em.20306. [DOI] [PubMed] [Google Scholar]
- 98.Zhang L, et al. The nature of chromosomal aberrations detected in humans exposed to benzene. Crit. Rev. Toxicol. 2002;32:1–42. doi: 10.1080/20024091064165. [DOI] [PubMed] [Google Scholar]
- 99.Smith MT, et al. Increased translocations and aneusomy in chromosomes 8 and 21 among workers exposed to benzene. Cancer Res. 1998;58:2176–2181. [PubMed] [Google Scholar]
- 100.Zhang L, et al. Benzene increases aneuploidy in the lymphocytes of exposed workers: a comparison of data obtained by fluorescence in situ hybridization in interphase and metaphase cells. Environ. Mol. Mutagen. 1999;34:260–268. [PubMed] [Google Scholar]
- 101.Smith MT, et al. Hydroquinone, a benzene metabolite, increases the level of aneusomy of chromosomes 7 and 8 in human CD34-positive blood progenitor cells. Carcinogenesis. 2000;21:1485–1490. [PubMed] [Google Scholar]
- 102.Stillman WS, et al. The benzene metabolite, hydroquinone, selectively induces 5q31- and -7 in human CD34+CD19- bone marrow cells. Exp. Hematol. 2000;28:169–176. doi: 10.1016/s0301-472x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 103.Forni A. Chromosome changes due to chronic exposure to benzene. Vienna, Australia: International Congress on Occupational Health; 1966. pp. 437–439. [Google Scholar]
- 104.Li Y, et al. Chromosome changes by G-banding in patients with chronic benzene poisoning. Chinese J. Ind. Med. 1990;3:29–31. [Google Scholar]
- 105.Kim YJ, et al. Micronucleus-centromere assay in workers occupationally exposed to low level of benzene. Hum. Exp. Toxicol. 2010;29:343–350. doi: 10.1177/0960327110361500. [DOI] [PubMed] [Google Scholar]
- 106.Irons RD. Quinones as toxic metabolites of benzene. J. Toxicol. Environ. Health. 1985;16:673–678. doi: 10.1080/15287398509530777. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L, et al. Detection of 1,2,4-benzenetriol induced aneuploidy and microtubule disruption by fluorescence in situ hybridization and immunocytochemistry. Mutat. Res. 1994;320:315–327. doi: 10.1016/0165-1218(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 108.Eastmond DA, et al. Characterization and mechanisms of chromosomal alterations induced by benzene in mice and humans. Res. Rep. Health Eff. Inst. 2001;103:1–68. ; discussion, 69–80. [PubMed] [Google Scholar]
- 109.Lindsey RH, Jr, et al. Effects of benzene metabolites on DNA cleavage mediated by human topoisomerase II alpha: 1,4-hydroquinone is a topoisomerase II poison. Chem. Res. Toxicol. 2005;18:761–770. doi: 10.1021/tx049659z. [DOI] [PubMed] [Google Scholar]
- 110.Chen H, et al. Topoisomerase inhibition by phenolic metabolites: a potential mechanism for benzene's clastogenic effects. Carcinogenesis. 1995;16:2301–2307. doi: 10.1093/carcin/16.10.2301. [DOI] [PubMed] [Google Scholar]
- 111.Frantz CE, et al. Inhibition of human topoisomerase II in vitro by bioactive benzene metabolites. Environ. Health Perspect. 1996;104(suppl. 6):1319–1323. doi: 10.1289/ehp.961041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hutt AM, et al. Inhibition of human DNA topoisomerase II by hydroquinone and p-benzoquinone, reactive metabolites of benzene. Environ. Health Perspect. 1996;104(suppl. 6):1265–1269. doi: 10.1289/ehp.961041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fung J, et al. Inhibition of topoisomerase II in 32D.3(G) cells by hydroquinone is associated with cell death. J. Appl. Toxicol. 2004;24:183–188. doi: 10.1002/jat.960. [DOI] [PubMed] [Google Scholar]
- 114.Lindsey RH, Jr., et al. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry. 2004;43:7563–7574. doi: 10.1021/bi049756r. [DOI] [PubMed] [Google Scholar]
- 115.Whysner J, et al. Genotoxicity of benzene and its metabolites. Mutat. Res. 2004;566:99–130. doi: 10.1016/s1383-5742(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 116.Eastmond DA, et al. Topoisomerase II inhibition by myeloperoxidase-activated hydroquinone: a potential mechanism underlying the genotoxic and carcinogenic effects of benzene. Chem. Biol. Interact. 2005;153–154:207–216. doi: 10.1016/j.cbi.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 117.Baker RK, et al. Benzene metabolites antagonize etoposide-stabilized cleavable complexes of DNA topoisomerase IIalpha. Blood. 2001;98:830–833. doi: 10.1182/blood.v98.3.830. [DOI] [PubMed] [Google Scholar]
- 118.Pandey AK, et al. In silico studies with human DNA topoisomerase-II alpha to unravel the mechanism of in vitro genotoxicity of benzene and its metabolites. Mutat. Res. 2009;661:57–70. doi: 10.1016/j.mrfmmm.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Ji Z, et al. The benzene metabolite, hydroquinone and etoposide both induce endoreduplication in human lymphoblastoid TK6 cells. Mutagenesis. 2009;24:367–372. doi: 10.1093/mutage/gep018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rothman N, et al. Benzene induces gene-duplicating but not gene-inactivating mutations at the glycophorin A locus in exposed humans. Proc. Natl Acad. Sci. USA. 1995;92:4069–4073. doi: 10.1073/pnas.92.9.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rothman N, et al. An epidemiologic study of early biologic effects of benzene in Chinese workers. Environ. Health Perspect. 1996;104(suppl. 6):1365–1370. doi: 10.1289/ehp.961041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boyse J, et al. Glycophorin A mutations and risk of secondary leukaemia in patients treated for childhood acute lymphoblastic leukaemia. Br. J. Haematol. 1996;93:117–124. doi: 10.1046/j.1365-2141.1996.4621001.x. [DOI] [PubMed] [Google Scholar]
- 123.Yu K, et al. Decreased topoisomerase IIalpha expression and altered histone and regulatory factors of topoisomerase IIalpha promoter in patients with chronic benzene poisoning. Toxicol. Lett. 2011;5:5. doi: 10.1016/j.toxlet.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Andersen MK, et al. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33:395–400. doi: 10.1002/gcc.10043. [DOI] [PubMed] [Google Scholar]
- 125.Mistry AR, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N. Engl. J. Med. 2005;352:1529–1538. doi: 10.1056/NEJMoa042715. [DOI] [PubMed] [Google Scholar]
- 126.Pedersen-Bjergaard J, et al. Genetic pathways in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Hematology Am. Soc. Hematol. Educ. Program. 2007:392–397. doi: 10.1182/asheducation-2007.1.392. [DOI] [PubMed] [Google Scholar]
- 127.Voltz R, et al. Mitoxantrone therapy in multiple sclerosis and acute leukaemia: a case report out of 644 treated patients. Mult. Scler. 2004;10:472–474. doi: 10.1191/1352458504ms1047cr. [DOI] [PubMed] [Google Scholar]
- 128.Schoch C, et al. Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia. 2005;19:1224–1228. doi: 10.1038/sj.leu.2403810. [DOI] [PubMed] [Google Scholar]
- 129.Kerkel K, et al. Altered DNA methylation in leukocytes with trisomy 21. PLoS Genet. 2010;6:e1001212. doi: 10.1371/journal.pgen.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Falini B, et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–885. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 131.Mardis ER, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Albertini RJ, et al. Hprt mutant frequency and p53 gene status in mice chronically exposed by inhalation to benzene. Chem. Biol. Interact. 2010;184:77–85. doi: 10.1016/j.cbi.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 133.Mullin AH, et al. Inhalation of benzene leads to an increase in the mutant frequencies of a lacI transgene in lung and spleen tissues of mice. Mutat. Res. 1995;327:121–129. doi: 10.1016/0027-5107(94)00181-4. [DOI] [PubMed] [Google Scholar]
- 134.Mullin AH, et al. Inhaled benzene increases the frequency and length of lacI deletion mutations in lung tissues of mice. Carcinogenesis. 1998;19:1723–1733. doi: 10.1093/carcin/19.10.1723. [DOI] [PubMed] [Google Scholar]
- 135.Billet S, et al. Benzene-induced mutational pattern in the tumour suppressor gene TP53 analysed by use of a functional assay, the functional analysis of separated alleles in yeast, in human lung cells. Arch. Toxicol. 2010;84:99–107. doi: 10.1007/s00204-009-0478-z. [DOI] [PubMed] [Google Scholar]
- 136.Petitjean A, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum. Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 137.Ho TY, et al. Increased gene expression in human promyeloid leukemia cells exposed to trans, trans-muconaldehyde, a hematotoxic benzene metabolite. Carcinogenesis. 1997;18:739–744. doi: 10.1093/carcin/18.4.739. [DOI] [PubMed] [Google Scholar]
- 138.Kolachana P, et al. Benzene and its phenolic metabolites produce oxidative DNA damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 1993;53:1023–1026. [PubMed] [Google Scholar]
- 139.Wiemels J, et al. Enhancement of myeloid cell growth by benzene metabolites via the production of active oxygen species. Free Radic. Res. 1999;30:93–103. doi: 10.1080/10715769900300101. [DOI] [PubMed] [Google Scholar]
- 140.Winn LM. Homologous recombination initiated by benzene metabolites: a potential role of oxidative stress. Toxicol. Sci. 2003;72:143–149. doi: 10.1093/toxsci/kfg008. [DOI] [PubMed] [Google Scholar]
- 141.Shen M, et al. Association between mitochondrial DNA copy number, blood cell counts, and occupational benzene exposure. Environ. Mol. Mutagen. 2008;49:453–457. doi: 10.1002/em.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siegel D, et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol. Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 143.Hartwig A. The role of DNA repair in benzene-induced carcinogenesis. Chem. Biol. Interact. 2010;184:269–272. doi: 10.1016/j.cbi.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 144.Xiao X, et al. Role of DNA-PKcs in the biological effect of a benzene metabolite: phenol toxicity to human K562 cells in vitro. Chem. Biol. Interact. 2010;184:302–305. doi: 10.1016/j.cbi.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 145.Ishihama M, et al. Generation of phosphorylated histone H2AX by benzene metabolites. Toxicol. In Vitro. 2008;22:1861–1868. doi: 10.1016/j.tiv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 146.Alexander D, et al. Differential DNA repair in hematopoietic stem and progenitor cells following genotoxic damage by benzene metabolites. Toxicol. Sci. 2010;114:29. [Google Scholar]
- 147.Mohrin M, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Milyavsky M, et al. A distinctive DNA damage response in human hematopoietic stem cells reveals an apoptosis-independent role for p53 in self-renewal. Cell Stem Cell. 2010;7:186–197. doi: 10.1016/j.stem.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 149.Zeng Y, et al. High-performance single cell genetic analysis using microfluidic emulsion generator arrays. Anal. Chem. 2010;82:3183–3190. doi: 10.1021/ac902683t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guil S, et al. DNA methylomes, histone codes and miRNAs: tying it all together. Int. J. Biochem. Cell Biol. 2009;41:87–95. doi: 10.1016/j.biocel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 151.Shames DS, et al. DNA methylation in health, disease, and cancer. Curr. Mol. Med. 2007;7:85–102. doi: 10.2174/156652407779940413. [DOI] [PubMed] [Google Scholar]
- 152.Esteller M, et al. Cancer as an epigenetic disease: dNA methylation and chromatin alterations in human tumours. J. Pathol. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- 153.Baccarelli A, et al. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ren X, et al. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ. Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xing C, et al. Methylation and expression analysis of tumor suppressor genes p15 and p16 in benzene poisoning. Chem. Biol. Interact. 2010;184:306–309. doi: 10.1016/j.cbi.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 156.Gowans ID, et al. Genotype-dependent induction of transmissible chromosomal instability by gamma-radiation and the benzene metabolite hydroquinone. Cancer Res. 2005;65:3527–3530. doi: 10.1158/0008-5472.CAN-04-4242. [DOI] [PubMed] [Google Scholar]
- 157.Ren X, et al. Comparison of proliferation and genomic instability responses to WRN silencing in hematopoietic HL60 and TK6 cells. PLoS One. 2011;6:e14546. doi: 10.1371/journal.pone.0014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen M, et al. Polymorphisms in genes involved in DNA double-strand break repair pathway and susceptibility to benzene-induced hematotoxicity. Carcinogenesis. 2006;27:2083–2089. doi: 10.1093/carcin/bgl061. [DOI] [PubMed] [Google Scholar]
- 159.Sheltzer JM, et al. Aneuploidy drives genomic instability in yeast. Science. 2011;333:1026–1030. doi: 10.1126/science.1206412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solomon DA, et al. Mutational inactivation of STAG2 causes aneuploidy in human cancer. Science. 2011;333:1039–1043. doi: 10.1126/science.1203619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hirabayashi Y, et al. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem. Pharmacol. 2009;77:521–535. doi: 10.1016/j.bcp.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 162.Singh KP, et al. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem. Pharmacol. 2009;77:577–587. doi: 10.1016/j.bcp.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Westphal G, et al. The benzene metabolite para -benzoquinone is genotoxic in human, phorbol-12-acetate-13-myristate induced, peripheral blood mononuclear cells at low concentrations. Arch. Toxicol. 2009;83:721–729. doi: 10.1007/s00204-009-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yoon BI, et al. Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol. Sci. 2002;70:150–156. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 165.Hirabayashi Y, et al. Benzene-induced hematopoietic toxicity transmitted by AhR in wild-type mouse and nullified by repopulation with AhR-deficient bone marrow cells: time after benzene treatment and recovery. Chemosphere. 2008;73:S290–S294. doi: 10.1016/j.chemosphere.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 166.Hirabayashi Y, et al. Benzene-induced bone-marrow toxicity: a hematopoietic stem-cell-specific, aryl hydrocarbon receptor-mediated adverse effect. Chem. Biol. Interact. 2010;184:252–258. doi: 10.1016/j.cbi.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 167.Gasiewicz TA, et al. The aryl hydrocarbon receptor has an important role in the regulation of hematopoiesis: implications for benzene-induced hematopoietic toxicity. Chem. Biol. Interact. 2010;184:246–251. doi: 10.1016/j.cbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Moran JL, et al. Induction of apoptosis by benzene metabolites in HL60 and CD34+ human bone marrow progenitor cells. Mol. Pharmacol. 1996;50:610–615. [PubMed] [Google Scholar]
- 169.Martínez-Velázquez M, et al. Benzene metabolites induce apoptosis in lymphocytes. Exp. Toxicol. Pathol. 2006;58:65–70. doi: 10.1016/j.etp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 170.Montecino-Rodriguez E, et al. Regulation of hematopoiesis by gap junction-mediated intercellular communication. J Leukoc Biol. 2001;70:341–347. [PubMed] [Google Scholar]
- 171.Ploemacher RE, et al. Hematopoiesis: gap junction intercellular communication is likely to be involved in regulation of stroma-dependent proliferation of hemopoietic stem cells. Hematology. 2000;5:133–147. doi: 10.1080/10245332.2000.11746498. [DOI] [PubMed] [Google Scholar]
- 172.Montecino-Rodriguez E, et al. Expression of connexin 43 (Cx43) is critical for normal hematopoiesis. Blood. 2000;96:917–924. [PubMed] [Google Scholar]
- 173.Cancelas JA, et al. Connexin-43 gap junctions are involved in multiconnexin-expressing stromal support of hemopoietic progenitors and stem cells. Blood. 2000;96:498–505. [PubMed] [Google Scholar]
- 174.Rivedal E, et al. Metabolites of benzene are potent inhibitors of gap-junction intercellular communication. Arch. Toxicol. 2005;79:303–311. doi: 10.1007/s00204-004-0638-0. [DOI] [PubMed] [Google Scholar]
- 175.Rivedal E, et al. Gap junction intercellular communication and benzene toxicity. Chem. Biol. Interact. 2009;184:229–232. doi: 10.1016/j.cbi.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 176.Yin T, et al. The stem cell niches in bone. J. Clin. Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang J, et al. Stem cell niche: microenvironment and beyond. J. Biol. Chem. 2008;283:9499–9503. doi: 10.1074/jbc.R700043200. [DOI] [PubMed] [Google Scholar]
- 178.Lv L, et al. The TNF-α 238A polymorphism is associated with susceptibility to persistent bone marrow dysplasia following chronic exposure to benzene. Leuk. Res. 2007;31:1479–1485. doi: 10.1016/j.leukres.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 179.Zhou H, et al. NAD(P)H:quinone oxidoreductase 1-compromised human bone marrow endothelial cells exhibit decreased adhesion molecule expression and CD34+ hematopoietic cell adhesion. J. Pharmacol. Exp. Ther. 2010;334:260–268. doi: 10.1124/jpet.110.167841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smart RC, et al. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol. Pharmacol. 1984;26:105–111. [PubMed] [Google Scholar]
- 181.Thomas DJ, et al. Bone marrow stromal cell bioactivation and detoxification of the benzene metabolite hydroquinone: comparison of macrophages and fibroblastoid cells. Mol. Pharmacol. 1990;37:255–262. [PubMed] [Google Scholar]
- 182.Zhou H, et al. Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol. Pharmacol. 2009;76:579–587. doi: 10.1124/mol.109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pervaiz S, et al. Oxidative stress regulation of stem and progenitor cells. Antioxid. Redox Signal. 2009;11:2777–2789. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- 184.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal. 2008;10:1923–1940. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Badham HJ, et al. In utero exposure to benzene disrupts fetal hematopoietic progenitor cell growth via reactive oxygen species. Toxicol. Sci. 2010;113:207–215. doi: 10.1093/toxsci/kfp242. [DOI] [PubMed] [Google Scholar]
- 186.Badham HJ, et al. In utero and in vitro effects of benzene and its metabolites on erythroid differentiation and the role of reactive oxygen species. Toxicol. Appl. Pharmacol. 2010;244:273–279. doi: 10.1016/j.taap.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 187.Gross SA, et al. PU.1 phosphorylation correlates with hydroquinone-induced alterations in myeloid differentiation and cytokine-dependent clonogenic response in human CD34(+) hematopoietic progenitor cells. Cell Biol. Toxicol. 2006;22:229–241. doi: 10.1007/s10565-006-0128-7. [DOI] [PubMed] [Google Scholar]
- 188.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Di Maggio N, et al. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. Biomaterials. 2011;32:321–329. doi: 10.1016/j.biomaterials.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 190.Au WW, et al. Challenge assay: a functional biomarker for exposure-induced DNA repair deficiency and for risk of cancer. Int. J. Hyg. Environ. Health. 2010;213:32–39. doi: 10.1016/j.ijheh.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 191.Shuga J, et al. Selected technologies for measuring acquired genetic damage in humans. Environ. Mol. Mutagen. 2010;51:851–870. doi: 10.1002/em.20630. [DOI] [PubMed] [Google Scholar]
- 192.Li B, et al. Decreased T-cell receptor excision DNA circles in peripheral blood mononuclear cells among benzene-exposed workers. Int. J. Immunogenet. 2009;36:107–111. doi: 10.1111/j.1744-313X.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- 193.Gale RP, et al. Commentary: does immune suppression increase risk of developing acute myeloid leukemia? Leukemia. 2011 doi: 10.1038/leu.2011.224. doi:10.1038/leu.2011.224. [DOI] [PubMed] [Google Scholar]
- 194.Guyton KZ, et al. A reexamination of the PPAR-alpha activation mode of action as a basis for assessing human cancer risks of environmental contaminants. Environ. Health Perspect. 2009;117:1664–1672. doi: 10.1289/ehp.0900758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.US Environmental Protection Agency. Guidelines for carcinogen risk assessment and supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. Fed. Regist. 2005;vol. 70:17765–17817. [Google Scholar]
- 196.Meek ME, et al. A framework for human relevance analysis of information on carcinogenic modes of action. Crit. Rev. Toxicol. 2003;33:591–653. doi: 10.1080/713608373. [DOI] [PubMed] [Google Scholar]
- 197.Boobis AR, et al. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit. Rev. Toxicol. 2006;36:781–792. doi: 10.1080/10408440600977677. [DOI] [PubMed] [Google Scholar]
- 198.Meek ME, et al. Proposed mode of action of benzene-induced leukemia: interpreting available data and identifying critical data gaps for risk assessment. Chem. Biol. Interact. 2010;184:279–285. doi: 10.1016/j.cbi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Snyder CA, et al. The inhalation toxicology of benzene: incidence of hematopoietic neoplasms and hematotoxicity in ARK/J and C57BL/6J mice. Toxicol. Appl. Pharmacol. 1980;54:323–331. doi: 10.1016/0041-008x(80)90202-1. [DOI] [PubMed] [Google Scholar]
- 200.Cronkite EP, et al. Hematotoxicity and carcinogenicity of inhaled benzene. Environ. Health Perspect. 1989;82:97–108. doi: 10.1289/ehp.898297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.NTP. Report on Carcinogens. 2011. ntp.niehs.nih.gov/ntp/roc/twelfth/profiles/Benzene.pdf, Department of Health and Human Services, Research Triangle Park, NC. [Google Scholar]
- 202.Huff JE, et al. Multiple-site carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice. Environ. Health Perspect. 1989;82:125–163. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.NTP. NTP Toxicology and Carcinogenesis Studies of Benzene (CAS No. 71-43-2) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Technical Report Series. 1986. U.S. Department Of Health And Human Services, Public Health Service, National Institutes of Health Bethesda, MD, Vol. 289, pp. 1–277. [PubMed] [Google Scholar]
- 204.McHale CM, et al. Toxicogenomic profiling of chemically exposed humans in risk assessment. Mutat. Res. 2010;705:172–183. doi: 10.1016/j.mrrev.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wilson VS, et al. Utilizing toxicogenomic data to understand chemical mechanism of action in risk assessment. Toxicol. Appl. Pharmacol. 2011;2:2. doi: 10.1016/j.taap.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 206.Faiola B, et al. Gene expression profile in bone marrow and hematopoietic stem cells in mice exposed to inhaled benzene. Mutat. Res. 2004;549:195–212. doi: 10.1016/j.mrfmmm.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 207.Yoon BI, et al. Mechanisms of benzene-induced hematotoxicity and leukemogenicity: cDNA microarray analyses using mouse bone marrow tissue. Environ. Health Perspect. 2003;111:1411–1420. doi: 10.1289/ehp.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kawasaki Y, et al. Benzene-induced hematopoietic neoplasms including myeloid leukemia in Trp53-deficient C57BL/6 and C3H/He mice. Toxicol. Sci. 2009;110:293–306. doi: 10.1093/toxsci/kfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sarma SN, et al. Differential gene expression profiles of human leukemia cell lines exposed to benzene and its metabolites. Environ. Toxicol. Pharmacol. 2011;32:285–295. doi: 10.1016/j.etap.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 210.Gillis B, et al. Identification of human cell responses to benzene and benzene metabolites. Genomics. 2007;90:324–333. doi: 10.1016/j.ygeno.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 211.Roeder I, et al. Towards a quantitative understanding of stem cell-niche interaction: experiments, models, and technologies. Blood Cells Mol. Dis. 2011;46:308–317. doi: 10.1016/j.bcmd.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 212.Kobel S, et al. High-throughput methods to define complex stem cell niches. Biotechniques. 2010;48:ix–xxii. doi: 10.2144/000113401. [DOI] [PubMed] [Google Scholar]
- 213.Peerani R, et al. Patterning mouse and human embryonic stem cells using micro-contact printing. Methods Mol. Biol. 2009;482:21–33. doi: 10.1007/978-1-59745-060-7_2. [DOI] [PubMed] [Google Scholar]
- 214.Peerani R, et al. Manipulation of signaling thresholds in “engineered stem cell niches” identifies design criteria for pluripotent stem cell screens. PLoS One. 2009;4:e6438. doi: 10.1371/journal.pone.0006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lan Q, et al. Benzene exposure and hematotoxicity: response. Science. 2006;312:998–998. doi: 10.1126/science.312.5776.998b. [DOI] [PubMed] [Google Scholar]