Abstract
The principal objective of this study was to determine whether visceral fat or liver fat is a more relevant risk factor for metabolic syndrome. A total of 98 subjects aged 18-65 yr, who visited a health promotion center in a university hospital, were enrolled in this study. Metabolic syndrome was diagnosed based on the modified National Cholesterol Education Program's Adult Treatment Panel III report (NCEP-ATPIII) criteria. We defined the visceral obesity as a visceral fat area of ≥ 100 cm2 which was acquired by CT at the L4-5 level. To evaluate fatty liver, we applied a liver-to-spleen attenuation ratio ≤ 1.1 as measured by CT at the T12 level. We employed binary logistic regression models that used the presence or absence of metabolic syndrome as a dependent variable and age, sex, and the presence or absence of visceral obesity and fatty liver as independent variables. Visceral obesity was not found to be an independent variable as a risk factor of metabolic syndrome (odds ratio 2.7; 95% confidence interval 0.55-13.30), but fatty liver was found to be significant in this model (odds ratio 71.3; 95% CI 13.04-389.53). Our study suggests that liver fat may be a more important risk factor than visceral fat in terms of its association with metabolic syndrome.
Keywords: Metabolic Syndrome, Visceral Fat, Liver Fat
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) has been recognized as a feature of insulin resistance. Recent evidence supports the notion that NAFLD is associated with a number of systemic diseases, including visceral obesity, cardiovascular disease, type 2 diabetes, and metabolic syndrome. This association has been attributed to increased cardiovascular mortality and morbidity (1-3). On the basis of liver biopsy results, Marchesini et al. (4) reported that nondiabetic patients with metabolic syndrome exhibited more severe fibrosis and inflammation in the liver, regardless of age, sex and body mass index (BMI), than those patients without metabolic syndrome. The diagnosis of NAFLD was rendered on the basis of liver biopsy, but computed tomography (CT) has been reported to provide a more objective evaluation, which is reproducible and correlated more closely with fat accumulation in the liver (5, 6).
Visceral fat is independently related to morbidity and mortality of the coronary heart disease and associated with metabolic syndrome, diabetes, and cardiovascular disease. Because of this, visceral fat has been employed as a clinical measure of risk for obesity (7, 8). Recently, Kantartzis et al. (9) reported that liver fat was a more relevant independent factor for glucose metabolism than visceral fat. Thus far, the impact of liver fat versus visceral fat in determining metabolic syndrome has not been investigated in any detail. Therefore, in this study we attempted to determine which of these fats as measured by CT was more closely associated with metabolic syndrome.
MATERIALS AND METHODS
Participants
We conducted a retrospective analysis using medical records. A total of 98 subjects (34 males, 64 females) aged 18-65 yr, who visited a health promotion center in a university hospital for regular health check-up and underwent visceral fat CT from September 2007 to June 2010 were enrolled in this study. According to the questionnaire responses, those with a history of consuming more than 40 g alcohol per week, or chronic liver disease were excluded. Additionally, those who were positive to the superficial antigen of hepatitis B (HBsAg) or hepatitis C antibody were also excluded.
Anthropometry
Height and weight were measured with the subject wearing only a gown, and having fasted for more than 12 hr. We measured up to 0.1 kg, 0.1 cm automatically (HM-170, Fanics, Korea) and conducted a body composition analysis using Inbody720 fat analyzer (Biospace Co., Seoul, Korea). Waist circumference was measured at the midline between the lowest rib and the upper part of the iliac crest, according to WHO guidelines (10).
The measurement of visceral fat area (VFA)
Visceral fat area was measured by single-slice CT (SOMATOM Plus4, Siemens, Erlangen, Germany) at the 4-5th level of lumbar vertebrae using an attenuation range of 30 to -190 Hounsfield units (HU). We defined visceral obesity as a visceral fat area of more than 100 cm2 (11).
Measurement of liver fat accumulation
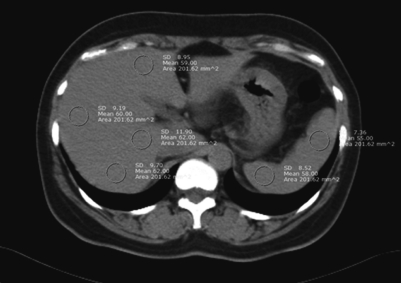
To quantify the accumulation of liver fat, we obtained the mean liver attenuation from an average of 4 selected areas of approximately 200 mm2 each, including the right-anterior lobe, right-posterior lobe, and left-interior lobe of the liver, at the T12 level. We also obtained the mean spleen attenuation from an average of 2 areas of the spleen (anterior and posterior pole) at the T12 level. Finally, we calculated the liver-to-spleen attenuation ratio (LSR), and fatty liver was defined as an LSR ≤ 1.1 (12, 13) (Fig. 1).
Fig. 1.
Abdominal fat CT for measurement of liver and spleen attenuation (in Hounsfield units).
Evaluation of metabolic risk factors
All subjects underwent blood sampling after fasting for 12 hr. Using an automatic blood pressure monitor (FT500-R, Jawon medical Co. LTD., Kyoungsan-City, Kyongsang Buk-do, Korea), we measured blood pressure in sitting position in the left arm, after the patient had rested for at least 5 min. Blood glucose, total cholesterol, triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol were determined using a chemistry immunoanalyzer (Olympus Au5400 Olympus Optical, Tokyo, Japan). Serum insulin was measured using a human insulin specific radioimmunoassay kit (Linco Research Inc., St. Charles, MO, USA). Insulin sensitivity was evaluated by calculating the Homeostasis model assessment for insulin resistance (HOMA-IR) index {(fasting insulin [µU/mL] × fasting blood glucose mM)/22.5}. Smoking status was defined as a current smoker or a non-smoker who had stopped smoking within the last 6 months.
Diagnosis of metabolic syndrome
Diagnosis of metabolic syndrome was rendered in accordance with the National Cholesterol Education Program's Adult Treatment Panel III report (NCEP-ATPIII) criteria with the exception of waist circumference (14). Diagnosis of metabolic syndrome required at least two of the following: triglyceride ≥ 150 mg/dL (1.7 mM), HDL-cholesterol < 40 mg/dL (1.03 mM) in men, < 50 mg/dL (1.29 mM) in women, blood pressure systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg, fasting blood glucose ≥ 100 mg/dL (5.6 mM).
Statistical analysis
Data were expressed as means ± standard deviation. Considering the characteristics of the variables, independent t-tests and Pearson chi-square tests were carried out in order to evaluate the differences between the metabolic syndrome group and the non-metabolic syndrome group. To determine the predictive effects of visceral fat and liver fat as risk factors for metabolic syndrome, binary logistic regression analyses were carried out. In this model, the presence of metabolic syndrome was a dependent variable, and age, sex, the presence of visceral obesity and the presence of fatty liver as measured by LSR were independent variables. P values of less than 0.05 were considered significant. PASW Statistics 18 statistical software (SPSS Inc., Chicago, IL, USA) was used for the analysis.
Ethics statement
This study protocol was reviewed and approved by institutional review board of Konyang University Hospital (research number: 11-32). Written informed consent was obtained from subjected patients.
RESULTS
Demographic and clinical characteristics according to the presence of metabolic syndrome
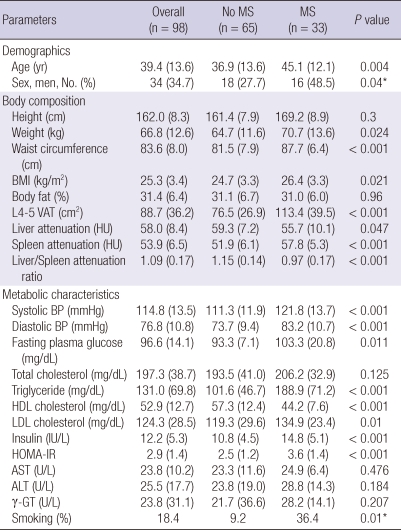
The 98 participants in this study, 34 men and 64 women, had a mean age of 39.4 ± 13.6 yr (men, 41 ± 13.1 yr; women, 38.4 ± 13.8 yr). The prevalence of metabolic syndrome was 33.7% overall. Average age of the subjects with metabolic syndrome (45.1 ± 12.1 yr) was significantly higher than that of the subjects without metabolic syndrome (36.9 ± 13.6 yr) (P = 0.004) (Table 1). BMI was significantly higher in the metabolic syndrome group (P = 0.021), but body fat percentage (body fat %) did not differ significantly between the two groups (31.0% ± 6.0%, 31.4% ± 6.7%, respectively). Visceral adipose tissue (VAT) was significantly higher in the metabolic syndrome group (113.4 ± 39.5 cm2) than the non-metabolic syndrome group (76.5 ± 26.9 cm2) (P < 0.001). CT attenuations values of each liver and spleen varied significantly, and LSR also showed significant difference between the two groups (P < 0.001). The two groups differed significantly in terms of blood pressure, fasting blood glucose, triglyceride, HDL-cholesterol, and LDL-cholesterol, but not in total cholesterol (P = 0.125). HOMA-IR was 3.6 ± 1.4 in the metabolic syndrome group, and 2.5 ± 1.2 in the non-metabolic syndrome group, and this difference reached significance (P < 0.001). No differences in liver enzymes (aspartate aminotransferase, alanine aminotransferase and γ-glutamyltransferase) were detected between the two groups. The smoking rate was significantly higher in the subjects with metabolic syndrome (36.4%, 12/33) than in the subjects without metabolic syndrome (9.2%, 6/65) (P = 0.01) (Table 1).
Table 1.
Demographic and clinical characteristics according to presence of metabolic syndrome
Data are expressed as means (SD; standard deviation), independent t-test or chi-square test*. BMI, body mass index; L4-5 VAT, visceral adipose tissue area at the 4th-5th lumbar vertebral level; HU, Hounsfield unit; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyltransferase.
Prevalence of metabolic risk factors
The prevalence of hypertriglyceridemia was 27.6% (men 44.1%, women 18.8%). 30.6% of total subjects (men, 20.6%; women, 35.9%) had low HDL-cholesterol. High blood pressure and hyperglycemia were noted in 26.5% (men, 50.0%; women, 14.1%) and 27.6% (men, 41.2%; females, 20.3%) respectively. The prevalence of metabolic syndrome defined as a cluster of at least two metabolic risk factors was 33.7% of the total subjects (men, 47.1%; women, 26.6%) (Table 2).
Table 2.
Prevalence of metabolic risk factors
*Hypertriglyceridemia defined as triglycerides ≥ 150 mg/dL; †Low HDL defined as HDL cholesterol < 40 mg/dL in men, < 50 mg/dL in women; ‡High BP defined as systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg; §Hyperglycemia defined as fasting plasma glucose ≥ 100 mg/dL or previously diagnosed type 2 diabetes. HDL, high-density lipoprotein cholesterol; BP, blood pressure.
Comparison of the metabolic risk factors according to the liver to spleen ratio and the visceral fat area
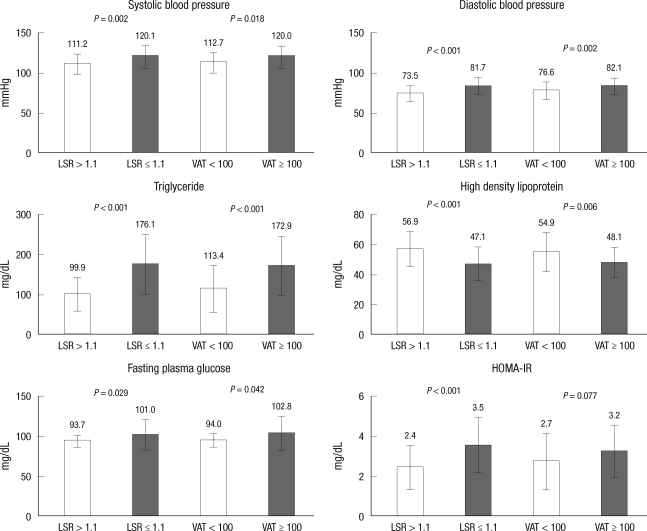
Subjects with fatty liver (defined as LSR ≤ 1.1) exhibited significantly higher waist circumference (P < 0.001), systolic blood pressure (P = 0.002), diastolic blood pressure (P < 0.001), fasting blood glucose (P = 0.029), triglycerides (P < 0.001) and HOMA-IR (P < 0.001) than subjects without fatty liver. HDL-cholesterol levels were significantly lower in the subjects with fatty liver (P < 0.001) (Fig. 2). Subjects with visceral abdominal obesity (defined as VFA ≥ 100 cm2) also had significantly higher waist circumference (P < 0.001), systolic blood pressure (P = 0.018), diastolic blood pressure (P = 0.002), fasting blood glucose (P = 0.042), and triglycerides (P < 0.001) than subjects without visceral obesity. HDL-cholesterol levels were lower in the group with visceral obesity (P = 0.006). However, HOMA-IR did not differ significantly regardless of presence of visceral obesity (P = 0.077) (Fig. 2).
Fig. 2.
Comparison of the risk factor of metabolic syndrome according to the liver to spleen ratio (LSR) and the L4-5 visceral adipose tissue (VAT). P value from t-test. VAT, visceral adipose tissue area at the 4-5th lumbar vertebral level (cm2); LSR, Liver to spleen attenuation ratio.
Comparison between visceral fat and liver fat as risk factors of metabolic syndrome
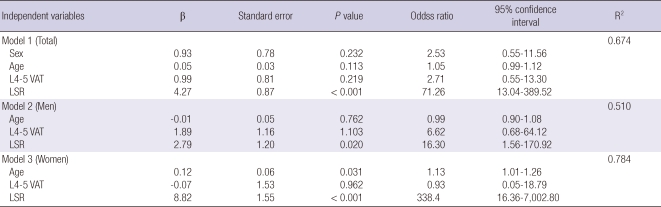
Binary logistic regression analyses were carried out to compare visceral obesity (VFA ≥ 100 cm2) and fatty liver (LSR ≤ 1.1) as risk factors of metabolic syndrome. In these models, the presence or absence of metabolic syndrome was used as a dependent variable and age, sex, and the presence or absence of visceral obesity and fatty liver as independent variables. There was no multi-collinearity problem among these explanatory variables (Table 3).
Table 3.
Binary logistic regression analysis for the presence of metabolic syndrome in the study subjects
Dependent variable: presence or absence of metabolic syndrome. Independent variables: sex, age, presence of visceral obesity with visceral adipose tissue area (VAT) ≥ 100 cm2, presence of fatty liver (LSR ≤ 1.1). L4-5 VAT, visceral adipose tissue area at the 4-5th lumbar vertebral level (cm2); LSR, Liver to spleen attenuation ratio.
In the total subjects, fatty liver (defined as LSR ≤ 1.1) adjusted for age and sex was found to be a significant independent variable as a risk factor for metabolic syndrome (odds ratio 71.3; 95% confidence interval 13.04-389.53; P < 0.001), but visceral obesity was not (odds ratio 2.7; 95% CI 0.55-13.30; P = 0.219) (Model 1). We observed the same results in the sex-sorted data: fatty liver was the only significant independent variable to explain metabolic syndrome (Model 2 and 3).
DISCUSSION
Recent studies have shown that although obesity is strongly associated with metabolic disorder, the site of fat accumulation is a more important risk factor for the metabolic disorder than obesity itself. In this study, using the data of 98 participants who visited the health promotion center in a university hospital, we attempted to determine whether visceral fat or liver fat was a more relevant risk factor for metabolic syndrome. The number of women participant in our study was almost double to that of men participants because much more number of men had history of alcohol which was one of the exclusion criteria. With regard to the smoking history, the group with metabolic syndrome contained a significantly higher proportion of smokers than the group without metabolic syndrome (36.4% vs 9.2%, respectively; P = 0.01). The effects of smoking on metabolic syndrome have been previously reported that smoking is the one of risk factors for insulin resistance (15-17). However, in our total study population, significant independent variables to metabolic syndrome did not change after adjustment of smoking history during the logistic regression analysis.
It has been shown that the prevalence of cardiovascular disease increases after the age of 40 in men and 50 in women, but the average age of the participants in this study was 39.4; this may be the reason that the men in this study had a higher prevalence of metabolic risk factors than the women in this study (Table 2).
In the present study, fatty liver (defined as LSR ≤ 1.1) adjusted for age and sex was found to be a significant independent variable as a risk factor for metabolic syndrome, but visceral obesity (defined as VFA ≥ 100 cm2) was not. We obtained the same results in the sex-sorted data: fatty liver was the only significant independent variable. These findings are consistent with the recently reported data of Fan et al. (18), who determined that NAFLD was more relevant than obesity itself in determining metabolic syndrome. Despite the fact that liver fat or the presence of fatty liver was found to be closely associated with metabolic disorders (prediabetes, diabetes, and hypertension) in many studies (9, 19-21), there have been no studies conducted specifically to compare the relevance of visceral fat and liver fat to metabolic syndrome as far as we know. Thus, our study may provide new insights into this issue.
Ryysy et al. (22) reported previously that variations in hepatic fat content may affect insulin requirements via an effect on the sensitivity of endogenous glucose production to insulin in type 2 diabetic patients with insulin therapy. Moreover, they demonstrated that as more liver fat accumulates, it becomes more closely associated with the insulin-resistance conditions, including hyperinsulinemia, hypertriglyceridemia, low HDL-cholesterol, and high systolic blood pressure. Seppälä-Lindroos et al. (23) reported that the accumulation of fat in the liver is, independent of body mass index and visceral obesity, characterized by several features of insulin resistance in moderately overweight and normal-weight subjects. Although abdominal visceral fat was a correlate of increased systemic and splanchnic rates of lipolysis, upper body nonsplanchnic tissue was definitively shown to be the principal contributor to whole body lipolysis in a previous study examining the relationship among deep abdominal subcutaneous fat, visceral fat, and glucose disappearance using the portal vein catheterization technique of Basu et al. (24). Despite the known association between visceral fat and insulin resistance and metabolic disorders, our findings demonstrate that fat accumulation in insulin-sensitive tissues may be a more important determinant of insulin sensitivity than visceral fat itself. On the other hand, visceral fat levels tend to be lower in women than in men, and lower in young people than in older individuals. In this regard, because the proportion of women in the total subject population was high, the average patient age was 39.4 yr, and the average VFA was 88.7 cm2, we are unable to exclude the possibility that our dataset had some effect on this result.
This study suffered from several limitations. First of all, the cross-sectional nature of the study did not allow definitive conclusions about causal relationships to be drawn. Second, as mentioned earlier, because we enrolled only a total of 98 subjects and there was a sex-ratio imbalance in the subject population, these may have been influenced the outcome. Third, the results of the present study cannot be directly applied to general populations, because the study was designed for, and performed in, a limited population. Finally, the diagnostic criterion of fatty liver which was used in this study has its own limitation as a reference of standard.
In conclusion, we found that liver fat was more associated with metabolic syndrome than visceral fat. Thus, NAFLD may be a more relevant risk factor for metabolic syndrome than is visceral obesity.
References
- 1.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 5.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 6.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 8.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 9.Kantartzis K, Machann J, Schick F, Fritsche A, Häring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia. 2010;53:882–889. doi: 10.1007/s00125-010-1663-6. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 11.Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, Fujii K, Kiuchi T, Tanaka K. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 13.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, Lindstrom MJ, Hinshaw JL. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194:623–628. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 14.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Chen CC, Li TC, Chang PC, Liu CS, Lin WY, Wu MT, Li CI, Lai MM, Lin CC. Association among cigarette smoking, metabolic syndrome, and its individual components: the metabolic syndrome study in Taiwan. Metabolism. 2008;57:544–548. doi: 10.1016/j.metabol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Yatsuya H, Iso H, Tamakoshi K, Toyoshima H. Incidence of metabolic syndrome according to combinations of lifestyle factors among middle-aged Japanese male workers. Prev Med. 2010;51:118–122. doi: 10.1016/j.ypmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–1130. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 18.Fan JG, Li F, Cai XB, Peng YD, Ao QH, Gao Y. Effects of nonalcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol. 2007;22:1086–1091. doi: 10.1111/j.1440-1746.2006.04781.x. [DOI] [PubMed] [Google Scholar]
- 19.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–2944. doi: 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- 20.Friis-Liby I, Aldenborg F, Jerlstad P, Rundström K, Björnsson E. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;39:864–869. doi: 10.1080/00365520410006431. [DOI] [PubMed] [Google Scholar]
- 21.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O'Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryysy L, Häkkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Järvinen H. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49:749–758. doi: 10.2337/diabetes.49.5.749. [DOI] [PubMed] [Google Scholar]
- 23.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab. 2001;280:E1000–E1006. doi: 10.1152/ajpendo.2001.280.6.E1000. [DOI] [PubMed] [Google Scholar]