Abstract
Background
Asthma prevalence varies widely among neighborhoods within New York City. Exposure to mouse and cockroach allergens has been suggested as a cause.
Objective
To test the hypotheses that children living in high asthma prevalence neighborhoods (HAPN) would have higher concentrations of cockroach and mouse allergens in their homes than children in low asthma prevalence neighborhoods (LAPN), and that these exposures would be related to sensitization and asthma.
Methods
In the NYC Neighborhood Asthma and Allergy Study, a case-control study of asthma, 7–8 year old children from HAPN (n=120) and LAPN (n=119) were recruited through the same middle-income health insurance plan. Children were classified as asthma cases (n=128) or non-asthma controls (n=111) based on reported symptoms or medication use. Allergens were measured in bed dust.
Results
HAPN homes had higher Bla g 2 (P=0.001), Mus m 1 (P=0.003) and Fel d 1 (P=0.003) and lower Der f 1 (P=0.001) than LAPN homes. Sensitization to indoor allergens was associated with asthma, but relevant allergens differed between LAPN and HAPN. Sensitization to cockroach was more common among HAPN than LAPN children (23.7% vs. 10.8%, P=0.011). Increasing allergen exposure was associated with increased probability of sensitization (IgE) to cockroach (P<0.001), dust mite (P=0.009) and cat (P=0.001), but not mouse (P=0.58) or dog (P=0.85).
Conclusions
These findings further demonstrate the relevance of exposure and sensitization to cockroach and mouse in an urban community and suggest that cockroach allergen exposure could contribute to the higher asthma prevalence observed in some compared with other NYC neighborhoods.
Keywords: Asthma, Urban, Cockroach, Mouse, Dust mite, Allergy
Introduction
The prevalence of asthma varies among communities in the US and is reported to be highest in low-income urban neighborhoods.1–3 In New York City (NYC), asthma prevalence among children entering school varies by neighborhood from 3% to 19%.4
In inner-city communities, exposure to cockroach and mouse and sensitization to these pests have been associated with asthma morbidity. Most of these studies were conducted in high asthma prevalence neighborhoods in inner-city US communities.5–11 The NYC Department of Health and Mental Hygiene (DOHMH) reported that cockroach and mouse sightings are more common in the lower socioeconomic status (SES) neighborhood, which also have a high pediatric asthma prevalence.12 Therefore, sensitization and exposure to these pests may, in part, explain the differences in asthma prevalence and morbidity between neighborhoods in NYC. We are unaware of any studies that have directly examined this by measuring allergens and allergic sensitization in both the high and low asthma prevalence urban neighborhoods.
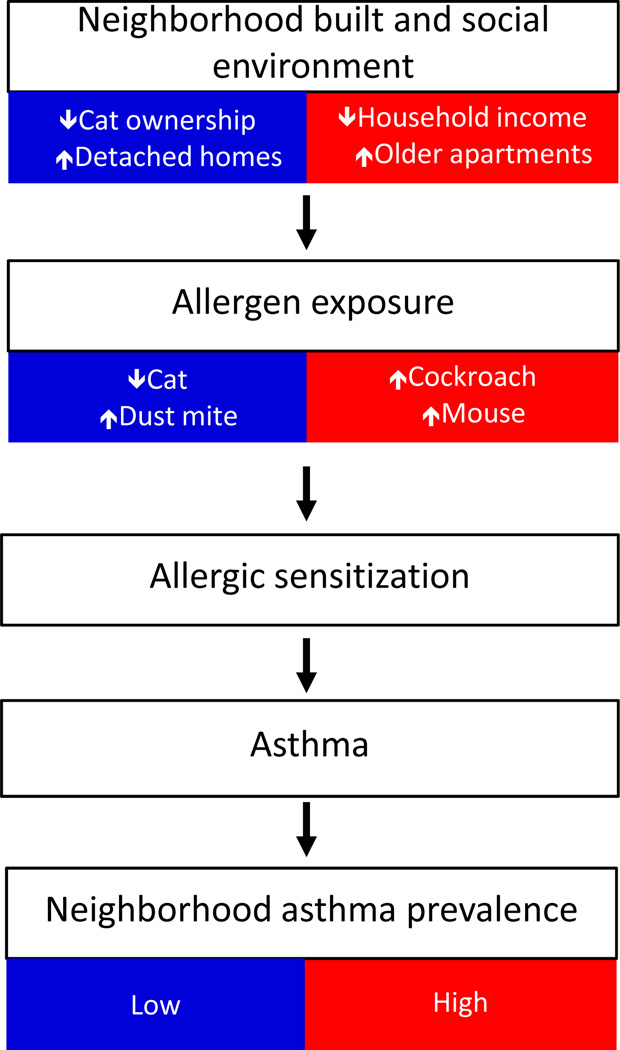
The NYC Neighborhood Asthma and Allergy Study (NAAS) is a case control study that is recruiting asthmatic (case) and non-asthmatic (control) 7–8 year old children who live in high (HAPN) and low (LAPN) asthma prevalence neighborhoods throughout NYC. To obtain a more homogeneous socio-demographic cohort, children were recruited through the same employer-based, middle-income health insurance plan. We hypothesized that despite being of similar SES, having similar access to health care and living in the same city, children living in the HAPN would have higher levels of cockroach and mouse allergens in their bed dust than children living in the LAPN, and that these difference would be related to features of the neighborhood built and social environment. Further, we hypothesized that allergen exposure would be associated with sensitization and that sensitization would be related to asthma case status (Figure 1).
Figure 1. Hypothesized mechanism of association between features of neighborhood and asthma prevalence through allergen exposure.
Methods
Study cohort
The NYC NAAS is a case-control study of asthmatic children. Parents of 7 and 8-year old children were recruited through the Health Insurance Plan of New York (HIP), a provider used primarily by a middle-income population. Neighborhoods were selected based on zip code level asthma prevalence among 5-year old children as reported by the NYC DOHMH.4 Asthma prevalence cut-points for low (LAPN) and high (HAPN) asthma prevalence neighborhoods were selected to yield an approximately equal number of eligible families in each neighborhood category. All NYC neighborhoods in the Bronx, Brooklyn, Queens and Manhattan below (<9%) and above (>11%) these cut-points were selected for recruitment.
Each three months, all of the parents who 1) have HIP through an employer, 2) have child who will turn 7-years old in the subsequent 3 months (8-year olds were also recruited initially) and 3) reside in a selected zip code were contacted by HIP by mail, inviting them to participate in a brief screening questionnaire. With the initial recruitment between 2008 and 2009, potential participants were contacted by HIP by mail and telephone. Due to changes in research requirements at HIP in the summer of 2009, the recruitment procedure was modified to only include contact by mail. Families who were interested in participating in the study directly contacted the Columbia University research team.
Screening questionnaire and home visit
A brief telephone interview was administered to the parents during which the child’s eligibility was confirmed (age, insurance and residence). The screening questionnaire also included ascertainment of demographic information on the child and administration of the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire.13 Medication use for asthma was ascertained by the question, “Is your child currently taking any medication to treat or prevent wheezing, cough or other breathing problems, rhinitis or allergies”, followed by a question that queried on specific medications with a list of possibilities.14 Homes of willing families were visited during which a detailed questionnaire on the health history of the child, environmental exposures and socioeconomic and demographic information was administered.
Asthma case definition
Children were classified as asthmatic based on whether the parent reported at least one of the following for the child in the 12 months prior to administration of the questionnaire: 1) wheeze, 2) being woken at night by cough without having a cold, 3) wheeze with exercise or 4) report of medication use for asthma. Children who did not meet one of these criteria were classified as controls. For sensitivity analyses, controls also were compared with children with frequent symptoms, defined as in the past 12 months having any wheeze related symptom reported ≥4 times or sleep disturbed ≥1 time per week. Asthma cases with less frequent symptoms were excluded from these sensitivity analyses. The initial study design called for inviting all children with asthma and a matched number of randomly selected controls for a home visit. In practice, this recruitment method yielded an approximately equal number of children with and without asthma symptoms; therefore, all families were invited for the home visit.
Allergen measurements in the home
During the home visit a dust sample was collected from the child’s bed by vacuuming the fitted sheet on the upper-half of the bed and both sides of the pillows using a vacuum cleaner and Dustream® collector (Indoor Biotechnologies, Charlottesville, VA) for 3 minutes. The bed dust samples were extracted with PBS 0.05% Tween, pH 7.4 at a concentration of 50 mg/ml and stored at −20°C until analysis. Der f 1, Fel d 1, Can f 1, and Mus m 1 were measured by multiplex bead immunoassays.15 Bla g 2 was measured by ELISA (Indoor Biotechnologies, Charlottesville, VA).16 All results are based on the universal allergen standard curve.17 For results below the limit of detection (LOD), values of ½ LOD were used in analyses. Limits of detection and coefficients of variance for duplicates are described in the Online Supplement. Four of the samples assayed by multiplex lacked sufficient sample for Bla g 2 analyses.
Serum antibodies
IgE against German cockroach, mouse urine proteins, D. farinae, cat dander, dog dander, common ragweed, mixed tree pollen (Tx8) and mixed grass pollen (Gx2) were measured in serum by ImmunoCAP® (Phadia, Uppsala, Sweden). Children with specific IgE ≥0.35 IU/ml against any of the allergens tested were considered seroatopic. Sensitivity analyses were also conducted with the cut-point of 1.0 IU/ml (online repository Table E3, Figure E1).18
Local neighborhood level variables
Children’s home addresses were geocoded and linked to a comprehensive geospatial demographic database described previously.19 The median household income in the surrounding 500M radius were determined for each home. Neighborhood asthma prevalence was based on previously described data from NYC DOHMH.4
Statistics
As both IgE and allergen concentrations were log normally distributed, logarithmically transformed values were used in analyses. Geometric means with 95% confidence intervals are reported. To adjust for potential confounders and covariates, the association between allergic sensitization and asthma case status and allergen levels and sensitization were obtained using logistic regression. Interactions between allergic sensitization and neighborhood type were tested on a multiplicative scale. Linear regression models were used to predict variation in allergen exposure in the home with variables related to home characteristics, family behaviors and local neighborhood income. Variables were entered stepwise and removed from the model if they did not alter the β for the association between neighborhood asthma prevalence and allergen level or the overall regression coefficient by >10%. Data were analyzed in SPSS version 17 (SPSS, Chicago, IL.
Results
Four-hundred and three parents completed the screening questionnaire and of those, 248 had a home visit. There were no significant differences in the demographics of the families with and without a home visit (online Table E1). Among those with a home visit, nine children were missing allergen or data used to define case status, leaving 239 children for allergen exposure analyses. Among those, 225 donated serum for IgE analyses. Children recruited by the initial method that included telephone contact when compared with those recruited only by postal invitation were similar in all demographics except race and household income below $25k/year (Table E2).
Study population
There were approximately equal numbers of children from HAPN (n=119) and LAPN (n=120) (Figure 1). As compared with children living in the LAPN, those in the HAPN were more likely to be of African-American race or Hispanic ethnicity and to live in apartment buildings (Table I). Reporting a household income below the poverty line was rare. Among the 128 cases, 54 were classified as having frequent symptoms.
Table I.
Study demographics
| LAPN% (n=119) |
HAPN% (n=120) |
P value |
||
|---|---|---|---|---|
| Case:Control (n) | 61:58 | 67:53 | - | |
| Male (%) | 58.0 | 50.0 | 0.22 | |
| Race (%)& | <0.001 | |||
| White | 20.2 | 7.5 | ||
| African-American | 36.1 | 53.3 | ||
| Asian | 21.0 | 1.7 | ||
| Other/mixed | 17.6 | 32.5 | ||
| Hispanic ethnicity (%)# | 23.5 | 47.5 | 0.001 | |
| Household income <$25K (%) | 8.4 | 11.7 | 0.40 | |
| Household family income (median) | $60–70K | $45–50K | 0.001 | |
| Household incomes for surrounding 500M (median)@ | $42K | $21K | <0.001 | |
| People in home per bedroom (median) | 1.8 | 2.0 | 0.14 | |
| Maternal education (%) | 0.083 | |||
| Not completed high school | 7.6 | 10.1 | ||
| Bachelor’s degree or higher | 49.6 | 35.3 | ||
| Paternal education (%) | 0.001 | |||
| Not completed high school | 6.0 | 8.9 | ||
| Bachelor’s degree or higher | 44.0 | 21.4 | ||
| Housing type (%) | <0.001 | |||
| Single family home | 32.8 | 5.0 | ||
| Multi-family home | 36.1 | 14.2 | ||
| Apartment building | 31.1 | 80.8 | ||
| Age of home (median) | 1939 | 1934 | 0.58 | |
| Cat in home (%) | 6.7% | 17.5% | 0.011 | |
| Lived in the same neighborhood for ≥7 years (%) | 68.7 | 70.1 | 0.85 | |
Children living in Low Asthma Prevalence Neighborhoods (LAPN) and High Asthma Prevalence Neighborhoods (HAPN).
There were 6 (5%) children in the LAPN and 6 (5%) in the HAPN that did not have a report for race.
There were 4 (3.4%) children in the LAPN and 7 (5.8%) in HAPN that did not have a report for Hispanic ethnicity.
Home address linked, census based variable of the median income of the household in the surrounding radial 500 meters was available on a subset (n=208) of the children.
Neighborhood features, asthma prevalence, and bed dust allergens
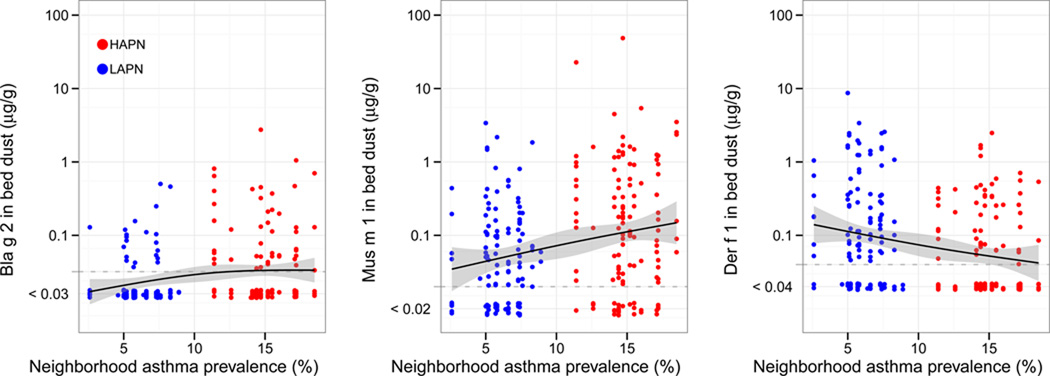
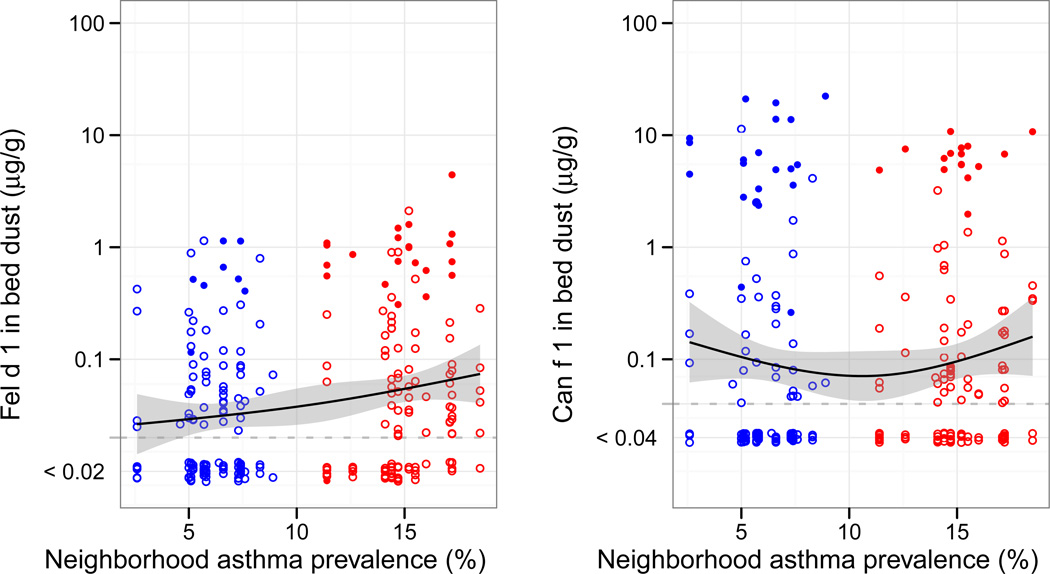
When compared with LAPN homes, HAPN homes had higher mean Bla g 2 (22 ng/g [19–25] vs. 37 ng/g [28–47], P=0.001), Mus m 1 (41 ng/g [30–56] vs. 93 ng/g [61–142], P=0.003) and Fel d 1 (30 ng/g [24–39] vs. 56 ng/g [41–76], P=0.003), lower Der f 1 (10 ng/g [7.7–14] vs. 5.3 ng/g [4.2–6.8], P=0.001) and similar Can f 1 (84 ng/g [57–124] vs. 99 ng/g [70–141], P=0.54) concentrations in bed dust (Figure 3). The difference in cat allergen between neighborhoods was driven by the greater frequency of cat ownership in HAPN vs. LAPN homes (17.5% vs. 6.7%, P=0.011) that was not observed for dog ownership (12.5% vs. 15.1%, P=0.56).
Figure 3. Cockroach (A.), Mouse (B.), Dust mite (C.), Cat (D.) and Dog (E.) allergen in the child’s bed dust by neighborhood asthma prevalence.
Lines represent natural spline linear models smoothed with 3 degrees of freedom with 95% confidence intervals (gray). For figures 3D and 3E, full circles represent homes with cat or dogs, respectively, and empty circles represent those without.
In multivariable models, Bla g 2 concentrations were higher among homes reporting cat ownership and inversely associated with local neighborhood income. Mus m 1 was higher for children who ate in their bedroom, lower for homes on ≥8th floor and inversely associated with local neighborhood income. Der f 1 concentrations were higher in detached homes and homes with cats, lower in beds of children whose parent reported that they had ever encased bedding because of their child’s asthma or allergy and inversely associated with the age of the building. Fel d 1 was only associated with pet ownership (data not shown).
Allergic sensitization and asthma
Sensitization to cockroach allergen was more common among children (cases and controls) living in the HAPN than LAPN (23.7% vs. 10.8%, P=0.011). There were no significant differences by neighborhood in prevalence of sensitization to any of the other individual allergens and overall sensitization to any allergen was equally common among children living in LAPN and HAPN (53.2% vs. 50.0%, respectively, P=0.64).
Sensitization to inhalant allergens was more common among asthmatics than controls for children living in both the LAPN (P<0.001) and HAPN (P=0.038) (Table III). While the adjusted ORs for case status with sensitization were higher for cockroach, ragweed and tree among HAPN vs. LAPN children and vice versa for mouse allergen, the effect modification by neighborhood was only statistically significant for ragweed sensitization (Table III, Pinteraction=0.009). When children with frequent symptoms (defined in methods) were compared with controls, the ORs with cockroach, ragweed and tree sensitization was higher for HAPN vs. LAPN children and the opposite pattern was observed for mouse and dust mite (data not shown). However, only the interaction term for ragweed approached statistical significance (P=0.055).
Table III.
Association between allergen specific sensitization and case vs. control with stratification by neighborhood asthma prevalence.
| Overall (n=225) |
LAPN (n=111) |
HAPN (n=114) |
Pinteraction between LAPN and HAPN& |
|||||
|---|---|---|---|---|---|---|---|---|
| Sensitization% | (%) | Adjusted OR [95% CI]# |
(%) | Adjusted OR [95% CI] |
(%) | Adjusted OR [95% CI] |
||
| Cockroach | Non-asthma | 9.6 | 3.8 | 15.7 | ||||
| Asthma | 24.0 | 2.85 [1.23–6.59]* | 17.2 | 7.34 [1.28–42.0]* | 30.2 | 2.34 [0.84–6.50] | 0.27 | |
| Mouse | Non-asthma | 5.8 | 7.5 | 3.9 | ||||
| Asthma | 14.9 | 2.46 [0.91–6.67] | 10.3 | 1.39 [0.35–5.59] | 19.0 | 5.64 [1.08–29.4]* | 0.29 | |
| Dust mite | Non-asthma | 15.4 | 18.9 | 11.8 | ||||
| Asthma | 29.8 | 2.55 [1.27–5.13]** | 34.4 | 2.66 [1.00–7.07] | 25.4 | 3.11 [1.03–9.37]* | 0.91 | |
| Cat | Non-asthma | 17.8 | 13.5 | 21.6 | ||||
| Asthma | 28.1 | 1.67 [0.83–3.37] | 27.6 | 2.48 [0.79–7.83] | 28.6 | 1.47 [0.55–3.96] | 0.34 | |
| Dog | Non-asthma | 11.5 | 9.4 | 13.7 | ||||
| Asthma | 27.3 | 2.78 [1.29–5.99]** | 25.9 | 3.14 [0.99–10.0] | 28.6 | 2.91 [0.98–8.65] | 0.61 | |
| Any Indoor | Non-asthma | 29.8 | 30.2 | 29.4 | ||||
| Asthma | 52.1 | 2.44 [1.37–4.34]** | 55.2 | 3.12 [1.31–7.44]* | 49.2 | 2.39 [1.01–5.65]* | 0.70 | |
| Ragweed | Non-asthma | 13.5 | 7.5 | 19.6 | ||||
| Asthma | 27.3 | 2.15 [1.04–4.44]* | 34.5 | 6.08 [1.80–20.5]** | 20.6 | 0.80 [0.29–2.22] | 0.009 | |
| Tree | Non-asthma | 16.5 | 13.5 | 19.6 | ||||
| Asthma | 38.0 | 3.14 [1.60–6.18]** | 43.1 | 4.77 [1.67–13.6]** | 33.3 | 2.37 [0.89–6.28] | 0.23 | |
| Grass | Non-asthma | 12.5 | 13.2 | 11.8 | ||||
| Asthma | 19.0 | 1.52 [0.71–3.26] | 24.1 | 1.87 [0.65–5.38] | 14.3 | 1.04 [0.31–3.49] | 0.40 | |
| Non-asthma | 21.2 | 17.0 | 25.5 | |||||
| Any outdoor | Asthma | 41.3 | 2.59 [1.37–4.86]** | 43.1 | 3.47 [1.30–9.29]* | 39.7 | 2.03 [0.83–4.97] | 0.30 |
| Non-asthma | 36.5 | 34.0 | 39.2 | |||||
| Any allergen | Asthma | 64.5 | 3.14 [1.76–5.62]*** | 70.7 | 5.36 [2.12–13.5]*** | 57.7 | 2.31 [1.02–5.21]* | 0.14 |
Children were considered sensitized to an allergen if then had >0.35 IU/ml IgE against that allergen.
Odds ratios were adjusted for race, Hispanic ethnicity, sex, maternal asthma and cockroach, mouse, dust mite and cat allergen concentrations in bed dust in logistic regression.
Effect modification by neighborhood asthma prevalence was tested using a multiplicative interaction term of allergen sensitization and neighborhood asthma prevalence group in logistic regression models.
P<0.05,
P<0.01,
P<0.001
Allergen exposure and sensitization
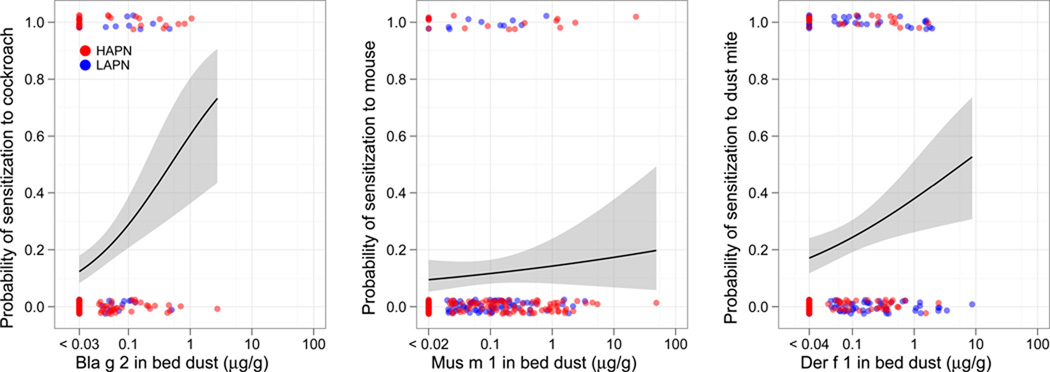
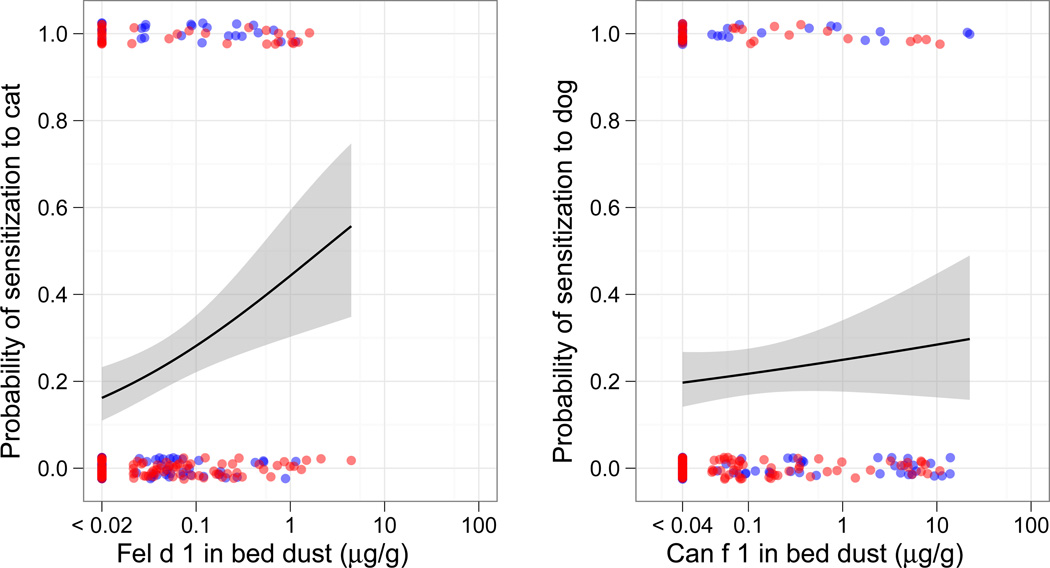
There was a significant association between bed dust allergen concentrations and sensitization for cockroach, dust mite and cat, but not mouse or dog allergens (Figure 4). These associations held with adjustment for race, Hispanic ethnicity, sex, LAPN/HAPN, case/control, and maternal asthma (cockroach OR 1.9 [1.4–2.6], P<0.001; dust mite OR 1.3 [1.07–1.63], P=0.009; cat 1.43 [1.16–1.75], P=0.001). Among children who had ever lived with a cat (21%), sensitization to cat was more common than for those who had not (40.4% vs. 18.6%, P=0.002). Among children who had ever lived with a dog (14%), the prevalence of sensitization to dog was similar to that of children who had not (18.8% vs. 20.2%, P=0.85)
Figure 4. Probability of sensitization to Cockroach (A.), Mouse (B.) Dust mite (C.), Cat (D.) and Dog (E.) allergens with exposure to the relevant major allergen.
Points represent allergen concentration in the bed dust on the x-axis and the absence (0) or presence (1) of IgE ≥0.35 IU/ml to the relevant allergen on the y-axis. Lines represent logistic regression models with 95% confidence intervals (gray).
Mean cockroach, mouse, dust mite, cat or dog concentrations were not significantly different for children with or without a case definition of asthma or frequent symptoms (data not shown). Neither current nor ever ownership of a cat or dog were associated with case or frequent symptom status (P values=0.16–0.78). We did not have a sufficient sample size to examine the associations between allergen exposure and symptoms among the asthmatic children sensitized to cockroach (n=29), mouse (n=18), dust mite (n=36), cat (n=34) or dog (n=33).
Discussion
Among a middle-income population of asthmatic and non-asthmatic 7–8 year old children living throughout neighborhoods in NYC with large variations in asthma prevalence, mean cockroach, mouse and cat allergen concentrations were higher and dust mite allergen concentrations were lower in the bed dust from the HAPN when compared with LAPN homes. These associations between neighborhood asthma prevalence and allergen concentrations were partially explained by home characteristics, living habits and local neighborhood economic variables. Sensitization to indoor allergens was associated with asthma in general, but relevant allergens differed between LAPN and HAPN. Exposures to cockroach, dust mite and cat allergens were significantly associated with sensitization. These findings further demonstrate the relevance of exposure and sensitization to cockroach, mouse, dust mite and cat in an urban community and suggest that cockroach allergen exposure could contribute to the higher asthma prevalence observed in some NYC neighborhoods.
While many studies have focused on environmental exposures among inner-city asthmatics,9 to our knowledge none have included a comprehensive study of environmental exposures in geographically adjacent low asthma prevalence communities. In NYC and other US cities, asthma prevalence is higher in communities with lower SES and a higher proportion of racial and ethnic minorities.3, 4, 20 We designed the NYC NAAS to minimize heterogeneity in SES, race and ethnicity by recruiting through a middle-income health insurance plan. While families represented a range of incomes, they were primary of middle-income, living among higher (LAPN) and lower (HAPN) socioeconomic communities. Also, while there were more African-Americans among our HAPN participants, one-third of the children in the LAPN were African-American. Despite living in or near what would be considered inner-city neighborhoods, HAPN families in our cohort differed on demographics from previous inner-city cohort studies. For example, mothers in our HAPN as compared with the National Cooperative Inner City Asthma study populations were more likely to have a household income ≥$30K (73% vs. 22%), be married (55% vs. 24%) and have a high school degree (90% vs. 66%).21
While the relationships between allergen levels and housing and household demographics have been examined in the US on a national level, an advantage to focusing on a single city is decreasing the likelihood of confounding by regional differences (e.g. building types, weather). For example, the National Survey of Lead and Allergens in the Home (NSLAH) reported that homes in high rise apartments had higher mouse allergen, but within NYC we observed significantly lower mouse allergens in homes on the 8th floor or higher, which we also previously observed in a low-income, HAPN cohort.22, 23 The NSLAH also reported that mouse and cockroach levels were higher in low than in higher income homes.24, 25 In this NYC study of middle-income families, however, a child’s neighborhood income was more important in predicting the likelihood of exposure to pests in the home than his or her family income. It is important to point out the allergen analyses were conducted using the new allergen standards and thus direct comparisons to previously published concentrations should be performed after applying published correction factors.17 Bla g 2 concentrations were similar to those reported from two previous studies in Northeastern US cities (see Online Supplement).23, 26 Fel d 1 allergen concentrations were lower than those reported for the US in general, but similar to those reported in US Inner-city homes.27, 28 Mus m 1 concentrations were similar to those reported from Inner-city homes.28
Higher concentrations of dust mite allergens in the LAPN as compared with HAPN homes also were observed. This finding is not particularly surprising because it has been reported previously that apartment buildings in the Northeast are over heated in the winter leading to a dryer, less-dust mite conducive environment.29 A principal difference between the HAPN and LAPN homes is housing type, with apartments much more common in the HAPN and detached homes more common in the LAPN. Dust mite allergen concentrations were significantly higher among single-family and newer homes, which presumably are less likely to be overheated.
In the study sample overall, sensitizations to cockroach, mouse, dust mite and cat were important risks for being classified as an asthma case, reinforcing the importance of examining domestic exposure to these allergens. A novel finding was that ragweed sensitization appeared to be a greater risk for asthma in the LAPN than in the HAPN. This study is not a prevalence study, so while we have approximately equal numbers of asthmatics from the HAPN and LAPN, they do not represent an equal proportion of the population in their neighborhoods. As such, the HAPN asthmatics may be a more heterogeneous population of asthmatics with and without allergic triggers, while the LAPN children who may be a more homogeneous allergy-triggered asthma population. Also, given that the LAPN children live in less densely populated environments, their exposure to ragweed may be greater.
A significant association between allergen exposure and sensitization for cockroach and dust mite was observed; however, we did not observe an association for mouse. We may have been underpowered to detect such an association given the low prevalence of sensitization to mouse (11%). However, mouse allergens typically travel on smaller particles that are more likely to become and remain airborne than the larger particles that carry dust mite and cockroach allergens. This may contribute to higher exposure to mouse than dust mite and cockroach allergens outside of the home (e.g., subway stations, schools). We have previously observed that mouse allergen was detectable in 81% of air samples in schools, while cockroach was only detectable in 22% of samples.30
As we have reported from a study of low-income children living in HAPN previously, children in homes with cats and higher levels of cat allergen were more likely to be sensitized to cats.31 There has been much debate in the literature as to the association between cats in the home and development of allergic disease with increased, decreased and no associations reported.32–36 Urban NYC children seem to fall into an exposure paradigm similar to those in other communities with relatively moderate or low cat ownership.31, 34 In these communities passively transferred cat allergens are less common due to lower community cat ownership. Thus, at least early in childhood, having a cat at home may be necessary for many atopic individuals to be exposed to sufficient allergen to become allergic. However, we found no association between cat ownership or allergen levels and asthma status, further suggesting a complicated relationship between exposure to cats and development of allergic disease.
There are several limitations of our study. While there did not appear to be a bias in home visits among those who completed the telephone survey (Table E1), there was a bias in who chose to participate in the telephone survey (based on the similar number of cases and controls, when controls should have been more common in the target population). A wide range of variables to control for potential confounding was assessed, but there is a possibility of unrecognized confounders. The symptom and medication based definition we have used is more sensitive and less specific, and thus may result in non-asthmatic children being classified as asthmatic. However, sensitivity analyses using the more specific definition based on frequent symptoms yielded similar results. Lower levels of IgE, such as those used in this study, may be transient in early childhood.37 However, sensitivity analyses using the IgE cut-point of 1.0 IU./ml, which Matricardi et al. found to be less subject to transience in children between the ages of 7 and 10,18 yielded similar results to those using the 0.35 IU/ml cut-point.
With this unique study cohort, significant differences in allergen exposure in homes throughout NYC have been demonstrated. Cockroach 1) allergen was higher in the HAPN homes, 2) sensitization was higher among children living in the HAPN, 3) allergen exposure was associated with sensitization and 4) sensitization was associated with increased risk for asthma. These findings combined point to cockroach allergen exposure potentially leading to a higher prevalence of asthma in some urban neighborhoods.
Clinical Implications.
Sensitizations to cockroach, mouse, cat and dust mite allergens are important predictors of asthma morbidity among children in the urban Northeast, but exposures may vary by neighborhood within a city.
Supplementary Material
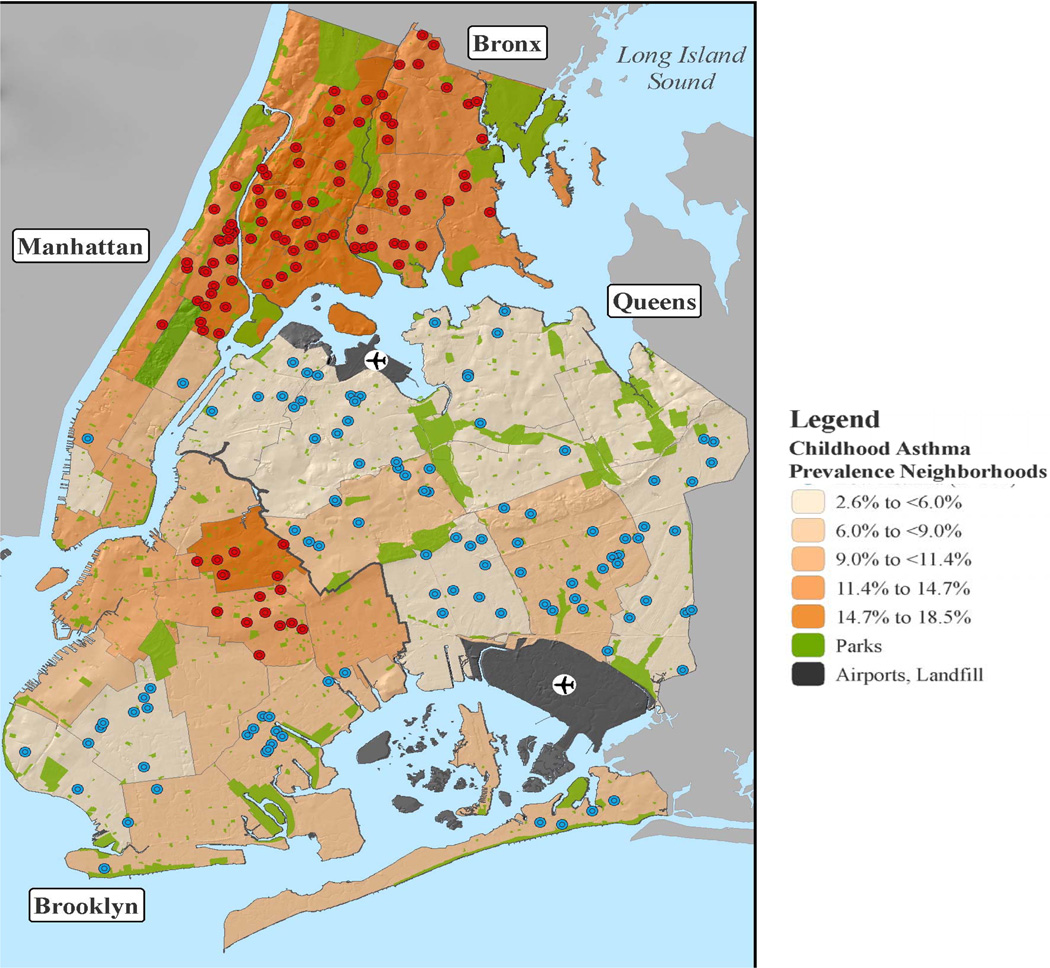
Figure 2. Location of study participants.
Each dot represents a study subject’s home and is color coded according to whether they have been classified as living in low (blue) or high (red) asthma prevalence neighborhoods. Neighborhoods are color coded according to the legend by NYC DOHMH reported asthma prevalence among 5 year-old children in 2000.
Table II.
Association between domestic environmental exposures and neighborhood asthma prevalence in multivariable% regression models (n=189).
| Bla g 2# | Mus m 1 | Der f 1 | |
|---|---|---|---|
| Neighborhood asthma prevalence& | β= −0.007 [−0.058–0.044] | β=0.061 [−0.025–0.15] | β= −0.051 [−0.11–0.008] |
| Age of building | B<0.001 [−0.007–0.007] | - | β= −0.008 [−0.015–0.0001] |
| Home is detached | β=0.19 [−0.34–0.72] | β=0.76 [−0.13–1.6] | β=1.0 [0.42–1.64]** |
| Median household income (500 meters) in $10K@ | β= −0.024 [−0.043– −0.006]* | β= −0.047 [−0.078– − 0.016]** | β=0.005 [−0.016 – 0.027] |
| Home is on 8th or higher floor | - | β= −1.2 [−2.06–0.30]** | - |
| Number of people per bedroom | - | β=0.30 [−0.037–0.64] | - |
| Cat in home | β=0.59 [0.036–1.2]* | - | β=2.1 [1.4–2.7]*** |
| Ever changed or encased mattress or pillow because of child’s asthma or allergy symptoms | - | - | β= −0.74 [−1.4– −0.065]* |
| Child snacks on food in bedroom | β=0.11 [−0.25–0.46] | β=0.94 [0.35–1.53]** | - |
| Overall model | R=0.39, P=0.002 | R=0.48, P<0.001 | R=0.57, P<0.001 |
Multivarable regression models were built and variables were removed stepwise if they did not alter the β for the association between neighborhood asthma prevalence and allergen level or the overall regression coefficient by 10% or more. The variables case/control status, race of child, Hispanic ethnicity, and reported household family income were used in all models, although, none of these were statistically significant in any of the models. β values with 95% confidence intervals are reported.
Allergen levels were log transformed in regression models.
School based prevalence of asthma among 5 year old children for the child’s United Hospital Fund Neighborhood (several zip codes).
GIS census based variable of the median income of the household in the surrounding radian 500 meters.
P<0.05,
P<0.01,
P<0.001
Acknowledgments
This project would not have been possible without our collaborators at HIP Health Plan. Beatriz Jaramillo, DrPH was instrumental in the design and initial implementation of the study and Michael Byrne, MA, has ensured the continued success of the recruitment for the study. We would like to thank the NAAS field team for their hard work. We would like to thank Stephanie Soucier for artistic design of recruitment material. We would also like to thank the families who have participated on the study.
Supported by: The National Institute of Environmental Health Sciences (NIEHS) (grant #’s ES 014400, P30 ES009089, P01 ES09600, P50 ES015905, RO1 ES08977)
Abbreviations
- ELISA
Enzyme Linked Immunosorbant Assay
- ETS
Environmental tobacco smoke
- ISAAC
International Study of Asthma and Allergy in Childhood
- HAPN
High Asthma Prevalence Neighborhoods
- HIP
Health Insurance Plan of New York
- LAPN
Low Asthma Prevalence Neighborhoods
- LOD
Limit of Detection
- NAAS
Neighborhood Asthma and Allergy Study
- NYC
New York City
- NYC DOHMH
New York City Department of Health and Mental Hygiene
- OR
Odds Ratio
- SES
Socioeconomic Status
References
- 1.Lin S, Fitzgerald E, Hwang SA, Munsie JP, Stark A. Asthma hospitalization rates and socioeconomic status in New York State (1987–1993) J Asthma. 1999;36:239–251. doi: 10.3109/02770909909075407. [DOI] [PubMed] [Google Scholar]
- 2.Crain EF, Weiss KB, Bijur PE, Hersh M, Westbrook L, Stein RE. An estimate of the prevalence of asthma and wheezing among inner-city children. Pediatrics. 1994;94:356–362. [PubMed] [Google Scholar]
- 3.Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. The protective effect of community factors on childhood asthma. J Allergy Clin Immunol. 2009;123:1297–1304. doi: 10.1016/j.jaci.2009.03.039. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asthma Facts. Second Edition. New York: The City of New York Department of Health and Mental Hygiene; 2003. [Google Scholar]
- 5.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 6.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 7.Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner LA, et al. Cockroach allergen levels and associations with cockroach-specific IgE. J Allergy Clin Immunol. 2008;121:240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunol. 2010;125:540–544. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Busse WW. The National Institutes of Allergy and Infectious Diseases networks on asthma in inner-city children: an approach to improved care. J Allergy Clin Immunol. 2010;125:529–537. doi: 10.1016/j.jaci.2010.01.036. quiz 38-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donohue KM, Al-alem U, Perzanowski MS, Chew GL, Johnson A, Divjan A, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008;122:914–920. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass D, McKelvey W, Van Wye G, Kerker B, Mostashari F, Eisenhower D, et al. Pests Can Be Controlled...Safely. NYC Vital Signs. 2005;4:1–4. [Google Scholar]
- 13.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson JS, Mellins RB, Garfinkel R, Rundle AG, Perzanowski MS, Chew GL, et al. Asthma, body mass, gender, and Hispanic national origin among 517 preschool children in New York City. Allergy. 2008;63:87–94. doi: 10.1111/j.1398-9995.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 15.Earle CD, King EM, Tsay A, Pittman K, Saric B, Vailes L, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119:428–433. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87:505–510. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 17.Chapman MD, Filep S, Tsay A, Vailes LD, Gadermaier G, Ferreira F, et al. Allergen standardization: CREATE principles applied to other purified allergens. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M. 2009;96:21–24. discussion 5. [PubMed] [Google Scholar]
- 18.Matricardi PM, Bockelbrink A, Keil T, Gruber C, Niggemann B, Hamelmann E, et al. Dynamic evolution of serum immunoglobulin E to airborne allergens throughout childhood: results from the Multi-Centre Allergy Study birth cohort. Clin Exp Allergy. 2009;39:1551–1557. doi: 10.1111/j.1365-2222.2009.03348.x. [DOI] [PubMed] [Google Scholar]
- 19.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. Geographic variability in childhood asthma prevalence in Chicago. J Allergy Clin Immunol. 2008;121:639–645. doi: 10.1016/j.jaci.2007.11.036. e1. [DOI] [PubMed] [Google Scholar]
- 21.Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24:253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Cohn RD, Arbes SJ, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J. Allergy Clin. Immunol. 2004;113:1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 23.Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J Allergy Clin Immunol. 2004;113:1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 25.Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in U.S. households. Environ Health Perspect. 2006;114:522–526. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 27.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2004;114:111–117. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R, et al. Results of a home-based environmental intervention among urban children with asthma. N. Engl.J. Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 29.Chew GL, Higgins KM, Gold DR, Muilenberg ML, Burge HA. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy. 1999;54:1058–1066. doi: 10.1034/j.1398-9995.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 30.Chew GL, Correa JC, Perzanowski MS. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor Air. 2005;15:228–234. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 31.Perzanowski MS, Chew GL, Divjan A, Johnson A, Goldstein IF, Garfinkel RS, et al. Cat ownership is a risk factor for the development of anti-cat IgE but not current wheeze at age 5 years in an inner-city cohort. J Allergy Clin Immunol. 2008;121:1047–1052. doi: 10.1016/j.jaci.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ownby D, Johnson CC, Peterson EL. Exposure to Dogs and Cats in the First Year of Life and Risk of Allergic Sensitization at 6 and 7 Years of Age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 33.Perzanowski MS, Ronmark E, Platts-Mills TA, Lundback B. Effect of Cat and Dog Ownership on Sensitization and Development of Asthma among Preteenage Children. Am J Respir Crit Care Med. 2002;166:696–702. doi: 10.1164/rccm.2201035. [DOI] [PubMed] [Google Scholar]
- 34.Al-Mousawi MS, Lovel H, Behbehani N, Arifhodzic N, Woodcock A, Custovic A. Asthma and sensitization in a community with low indoor allergen levels and low pet-keeping frequency. J Allergy Clin Immunol. 2004;114:1389–1394. doi: 10.1016/j.jaci.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Melen E, Wickman M, Nordvall SL, van Hage-Hamsten M, Lindfors A. Influence of early and current environmental exposure factors on sensitization and outcome of asthma in pre-school children. Allergy. 2001;56:646–652. doi: 10.1034/j.1398-9995.2001.00387.x. [DOI] [PubMed] [Google Scholar]
- 36.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med. 2002;165:176–180. doi: 10.1164/ajrccm.165.2.2104032. [DOI] [PubMed] [Google Scholar]
- 37.Holt PG, Strickland DH, Bosco A, Jahnsen FL. Pathogenic mechanisms of allergic inflammation: atopic asthma as a paradigm. Adv Immunol. 2009;104:51–113. doi: 10.1016/S0065-2776(08)04003-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.