Abstract
Sqstm1/p62 functions in the non-canonical activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). However, its physiological relevance is not certain. Here, we show that p62−/− mice exhibited an accelerated presentation of ageing phenotypes, and tissues from these mice created a pro-oxidative environment owing to compromised mitochondrial electron transport. Accordingly, mitochondrial function rapidly declined with age in p62−/− mice. In addition, p62 enhanced basal Nrf2 activity, conferring a higher steady-state expression of NAD(P)H dehydrogenase, quinone 1 (Nqo1) to maintain mitochondrial membrane potential and, thereby, restrict excess oxidant generation. Together, the p62–Nrf2–Nqo1 cascade functions to assure mammalian longevity by stabilizing mitochondrial integrity.
Keywords: mammalian longevity, mitochondria, nrf2, nqo1, p62
Introduction
Transcriptional induction of many cytoprotective enzymes in response to oxidative/electrophilic stress is regulated primarily by nuclear factor (erythroid-derived 2)-like 2 (Nrf2; Kaspar et al, 2009). Under non-stressed condition, Nrf2 is constitutively degraded through the ubiquitin–proteasome system by binding to Kelch-like ECH-associated protein 1 (Keap1), an adaptor of a ubiquitin ligase complex. Post-translational modification of Keap1 and/or Nrf2 by electrophiles and oxidants disrupts the Keap1–Nrf2 interaction, resulting in the stabilization and inducible activation of Nrf2. Recent studies have shown that expression of constitutively active Nrf2 or loss-of-function mutant Keap1 confers increased tolerance to oxidative stress and promotes longevity in worms and fruitflies (Sykiotis & Bohmann, 2008; Tullet et al, 2008). On the other hand, keap1 somatic mutation leads to carcinogenesis in humans through aberrant activation of Nrf2 (Hayes & McMahon, 2009). These observations implicate the regulation of the Keap1–Nrf2 pathway in higher organisms as a means of promoting longevity while concurrently risking carcinogenesis.
Through its ability to interact with ubiquitin and the LC3 component of autophagy, Sqstm1/p62 in vertebrates regulates autophagic removal of protein aggregates and damaged intracellular organelles, including mitochondria (Geisler et al, 2010; Komatsu & Ichimura, 2010). In addition, p62 interacts with Keap1 through the Keap1-binding region (KIR), and functions as an electrophile/oxidant-independent activator of Nrf2 by interfering with the Keap1 function and/or facilitating its degradation (Jain et al, 2010; Komatsu et al, 2010). A recent study showed that the accumulation of p62, which has been observed in a number of human cancers (Moscat & Diaz-Meco, 2009), persistently activates Nrf2 and contributes to the development of hepatocellular carcinoma (Inami et al, 2011). Thus, the functional inhibition of Keap1 either by somatic mutation or p62 accumulation appears to similarly mediate aberrant activation of Nrf2 and support carcinogenesis.
However, it is not clear whether p62 and its function to activate Nrf2 promote the longevity of higher organisms, although mature-onset obesity and features of neurodegeneration in p62−/− mice were reported previously (Rodriguez et al, 2006; Ramesh Babu et al, 2008). Importantly, mitochondrial dysfunction has long been considered a principal mechanism underlying the ageing process (Beckman & Ames, 1998). In the present study, we show that p62 has a significant role in assuring mammalian longevity by activating Nrf2 under non-stressed conditions and confers steady expression of NAD(P)H dehydrogenase, quinone 1 (nqo1) to maintain mitochondrial integrity. Furthermore, expression of p62 and Nqo1 declines with age in wild-type mice. Thus, p62 is presumed to be a gene that has evolved to delay mitochondrial dysfunction and thus attenuate the rate of ageing in vertebrates.
Results And Discussion
Male-biased accelerated ageing of p62−/− mice
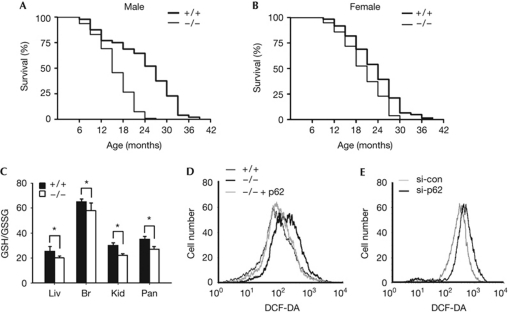
We deleted the sqstm1 gene in mice by replacing its first and second exons with a neomycin-resistance cassette (supplementary Fig S1 online). Although p62−/− mice at 7 weeks of age did not reveal any overt abnormalities, they exhibited a significantly reduced lifespan and accelerated ageing phenotypes. The mean and maximal lifespans of the p62−/− male mice were 68 and 115 weeks, respectively, whereas those of wild-type controls were 102 and 163 weeks, respectively (Fig 1A, P<0.0001). Thus, the mean lifespan of male p62−/− mice was 34% shorter than that of male wild-type controls. Reduced mean lifespan was also apparent in female p62−/− mice (13%, P<0.002), but less so than in males (Fig 1B). In addition, manifestations of premature tissue ageing were observed in male p62−/− mice; that is, early appearance of lordokyphosis, rough fur coat and thinning of the subcutaneous adipose layer in the dorsal skin (supplementary Fig S2 online). These data suggest that p62 promotes mouse longevity by delaying the ageing process.
Figure 1.
Lifespan reduction and pro-oxidative environment of p62−/− mice. (A,B) Kaplan–Meier survival curves for (A) male p62+/+ (n=48) and p62−/− (n=94) and (B) female p62+/+ (n=61) and p62−/− (n=78) mice. (C) GSH/GSSG ratio in the liver, brain, kidney and pancreas of 8-week-old p62+/+ and p62−/− male mice (n=5 per group, *P<0.01). (D,E) p62-dependent changes in DCF-sensitive oxidant levels in (D) MEFs and (E) HCT116 cells. For this, p62 level was manipulated by ectopic expression (p62) or knockdown of p62 (si-p62). Scrambled siRNA was used as a control (si-con). Br, brain; DCF-DA, dichlorofluorescein diacetate; GSH/GSSG, ratio of reduced to oxidized glutathione; Kid, kidney; Liv, liver; MEF, murine embryonic fibroblasts; Pan, pancreas.
The ratio of reduced to oxidized glutathione (GSH/GSSG) was significantly lower in the tissues of p62−/− mice than in those of wild-type controls (Fig 1C). This pro-oxidative shift in cellular redox is reflected in the accumulation of lipid peroxidation product in the brain of aged p62−/− mice and in the increased levels of oxidized proteins and nucleotides in p62−/− murine embryonic fibroblasts (MEFs; supplementary Fig S2 online). Furthermore, we observed (i) significantly elevated oxidant levels in p62−/− MEFs, (ii) decreased oxidant levels in p62−/− MEFs in response to the reintroduction of p62 and (iii) increased oxidant levels in p62-knockdown HCT116 cells (Fig 1D,E). Thus, p62 appears to decrease oxidant levels in the cells and tissues.
Rapid mitochondrial ageing in p62−/− mice
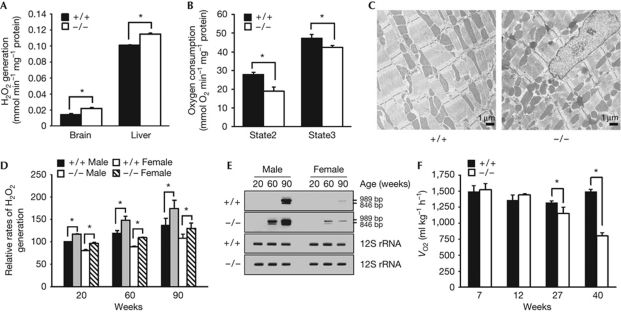
Mitochondria constitutively generate oxidants in the cell (Balaban et al, 2005). As compared with wild-type controls, mitochondria purified from the tissues of p62−/− mice produced increased amounts of hydrogen peroxide (H2O2; Fig 2A). Furthermore, the p62−/− mitochondria exhibited decreased rates of both state 2 and state 3 respiration, indicating a compromised mitochondrial electron transport in p62−/− tissues (Fig 2B). Morphological abnormalities of mitochondria were also apparent in the cardiac muscles of p62−/− mice, as exhibited by disturbed alignment, appearance of electron-dense matter and accumulation of distorted mitochondria despite the compatible expression of Lon protease (Fig 2C; supplementary Fig S3 online). Furthermore, p62 knockdown resulted in an increase of fragmented mitochondria within the HeLa cell population (supplementary Fig S3 online). These data together suggest that p62 stabilizes mitochondrial integrity and, thereby, limits oxidative stress.
Figure 2.
Mitochondrial dysfunction in p62−/− mice. (A) The rate of oxidant generation in mitochondria purified from brains and livers of 10-week-old p62+/+ and p62−/− male mice (n=4 for group, *P<0.01). (B) The state 2 and state 3 respiration rates of mitochondria isolated from the livers of p62+/+ and p62−/− male mice (n=5 per group, *P<0.05). (C) Electron microscopical analysis of the cardiac muscles of 32-week-old p62+/+ and p62−/− mice. (D) Relative rates of oxidant generation in liver mitochondria of p62+/+ and p62−/− mice at three different ages. Data are expressed as percentage of the rate in 20-week-old male wild-type mice (n=4–6 per group, *P<0.05). (E) Appearance of two PCR products of mitochondrial DNA, 989 and 846 bp, corresponding to age-associated deletions at 9089–12956 and 9553–13279, respectively. As a control, PCR products of an ageing-resistant mitochondrial DNA segment (12S rRNA) are also presented. (F) Oxygen consumptions during the light cycle of the 7-, 12-, 27- and 40-week-old p62+/+ and p62−/− mice (n=5 per group, *P<0.01). H2O2, hydrogen peroxide; VO2, oxygen consumption.
We then compared age-associated rates of mitochondrial dysfunction. At each of the three ages examined, male p62−/− mitochondria always had a greater rate of oxidant generation (RH2O2) than that observed in wild-type controls (Fig 2D). This difference was especially substantial at later ages. Compared with 20-week-old wild-type controls, 90-week-old wild-type mitochondria generate 57% more H2O2, whereas 90-week-old p62−/− mitochondria generate fully twice the amount of H2O2. Accordingly, amplification of mitochondrial DNA revealed the largely increased appearance of two major fragments corresponding to age-associated deletions (Wang et al, 1997) in p62−/− samples from 60- and 90-week-old mice (Fig 2E). Deletions in this region provide a robust measure of age-dependent mitochondrial mutation. In contrast, these fragments were not detected in wild-type mice aged less than 90 weeks. Furthermore, whole-body oxygen consumption (VO2) of 27- and 40-week-old p62−/− mice was only 84% and 53% of that in age-matched wild-type mice, respectively (Fig 2F). Thus, protracted loss of p62 function accelerated the rate of mitochondrial dysfunction. The rate of mitochondrial dysfunction was also faster in female p62−/− mice than in female wild-type controls, but relatively slower than that of male p62−/− mice (Fig 2D,E). The correlation between the rate of mitochondrial dysfunction and the magnitude of lifespan reduction in both sexes (Fig 1) further suggests that loss of p62 functionality contributed to the accelerated ageing of p62−/− mice.
Attenuated basal expression of Nqo1 in p62−/− mice
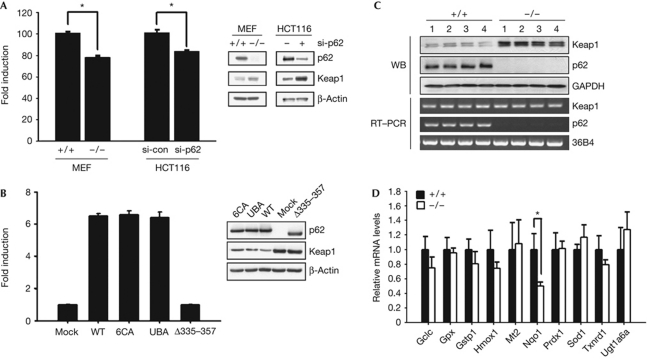
Consistent with previous reports (Jain et al, 2010), Nrf2-dependent antioxidant response was directly related to the p62 expression level in MEFs, whereas antioxidant response on exposure to an electrophile, tertiary butylhydroquinone, was similarly induced in p62+/+ and p62−/− MEFs (supplementary Figs S4A,B online). Furthermore, p62 activated Nrf2 even in the presence of an antioxidant, N-acetylcysteine, under which tertiary butylhydroquinone-induced antioxidant response was largely suppressed (supplementary Fig S4C online). Thus, p62-mediated antioxidant response is expected to have a role separate from that induced by electrophiles or oxidants. Interestingly, as compared with control cells, p62−/− MEFs and p62-knockdown HCT116 cells demonstrated a 20–30% lower basal antioxidant response along with increased Keap1 protein levels (Fig 3A). Consistent with previous reports (Komatsu et al, 2010), deletion of a region encompassing the KIR (Δ335–357), but not mutation of cysteines in the ZZ domain (6CA) or two leucines in the ubiquitin association domain (UBA), resulted in the loss of p62 function for both basal antioxidant response and Keap1 stability (Fig 3B). In accordance with this, protein levels of Keap1 were significantly higher in the skeletal muscles of p62−/− mice, despite their comparable message levels (Fig 3C). Furthermore, p62−/− tissues exhibited attenuated expression of Nqo1 but not other Nrf2 target genes (Fig 3D). Thus, p62 seems to support the basal activation of Nrf2 through modulating Keap1 stability, conferring a higher steady-state expression of Nqo1.
Figure 3.
p62–Keap1–Nrf2 axis for basal Nqo1 expression. (A) Reduced basal antioxidant response and increased Keap1 protein levels in p62−/− MEFs and p62-knockdown HCT116 cells (si-p62; *P<0.01). (B) Effects of p62 mutant expression on anti-oxidant response and Keap1 stability in p62−/− MEFs. Three p62 mutants either deleted the KIR-containing region (Δ335–357) or mutated six cysteines in the ZZ domain (6CA) or Leu416/417 in the UBA domain to alanines (UBA) were used. These mutants p62 lost their binding activities for Keap1, zinc or ubiquitin, respectively (Shin J, unpublished results). (C) Keap1 protein and message levels in the p62+/+ and p62−/− skeletal muscles. (D) Message levels of Nrf2 target genes and other primary oxidant scavenging enzymes in the p62+/+ and p62−/− skeletal muscles (n=4, *P<0.05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Keap; Kelch-like ECH-associated protein 1; KIR, Keap1-binding region; MEF, murine embryonic fibroblasts; nrf2; nuclear factor (erythroid-derived 2)-like 2; nqo1, NAD(P)H dehydrogenase, quinone 1; UBA, ubiquitin association domain; WB, western blot; WT, wild type.
p62–Nrf2–Nqo1 cascade for mitochondrial integrity
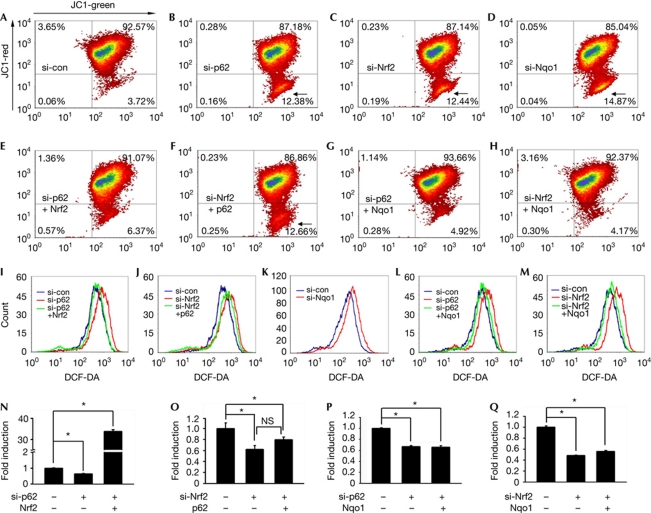
We then examined whether mitochondrial dysfunction in p62-deficient cells is a consequence of an attenuated basal antioxidant response. Knockdown of either p62 or Nrf2 in HCT116 cells similarly decreased the mitochondrial membrane potential (Δψm; Fig 4B,C) and antioxidant response (Fig 4N,O) but increased cellular oxidant levels (Fig 4I,J). Furthermore, the number of cells exhibiting a largely reduced Δψm increased under these conditions (arrows). Interestingly, Δψm, antioxidant response and oxidant concentration in p62-knockdown HCT116 cells were restored by ectopic expression of Nrf2 (Fig 4E,I,N). In contrast, ectopic expression of p62 induced neither effect in the Nrf2-knockdown HCT116 cells (Fig 4F,J,O). Thus, the positive role of p62 in the maintenance of mitochondrial integrity reflects enhanced basal antioxidant response. As a result, p62−/− MEFs exhibited a lower Δψm and a higher intracellular oxidant level, which were restored by the expression of wild-type p62 but not p62Δ335–357 (supplementary Fig S5 online). Accordingly, overexpression of Keap1 induced the opposite effects in HCT116 cells.
Figure 4.
Assurance of mitochondrial integrity by p62–Keap1–Nrf2–Nqo1 cascade. Δψm (A–H), oxidant level (I–M) and relative ARE reporter activity (N–Q, *P<0.05 and NS, non-significant) in HCT116 cells were measured after p62 knockdown (si-p62; B,I,N), p62 knockdown and Nrf2 overexpression (si-p62+Nrf2; E,I,N), Nrf2 knockdown (si-Nrf2; C,J,O), Nrf2 knockdown and p62 overexpression (si-Nrf2+p62; F,J,O), Nqo1 knockdown (D,K), p62 knockdown and Nqo1 overexpression (si-p62+Nqo1; G,L,P) and Nrf2 knockdown and Nqo1 overexpression (si-Nrf2+Nqo1; H,M,Q). Cells exhibiting a largely reduced low Δψm are marked by arrows. ARE, antioxidant response element; DCF-DA, dichlorofluorescein diacetate; Keap1, Kelch-like ECH-associated protein 1; nrf2; nuclear factor (erythroid-derived 2)-like 2; nqo1, NAD(P)H dehydrogenase, quinone 1; si-con, scrambled siRNA.
Interestingly, both decreased Δψm and increased cellular oxidant level were also observed in Nqo1-knockdown HCT116 cells (Fig 4D,K). Furthermore, ectopic expression of Nqo1 completely restored Δψm and oxidant concentration in the p62- or Nrf2-knockdown HCT116 cells (Fig 4G,H,L,M), despite the fact that it could not increase the antioxidant response (Fig 4P,Q). Thus, Nqo1 has a role in stabilizing mitochondrial integrity as a downstream effector of p62-induced basal Nrf2 activation.
Role of p62 for mammalian longevity
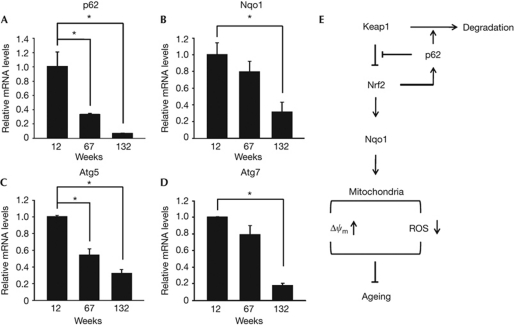
Previous observations of the ageing-related function of Nrf2 in worms (Tullet et al, 2008) and male-biased lifespan extension in the fruitfly by loss-of-function mutation of keap1 (Sykiotis & Bohmann, 2008) indicated the pro-longevity role of Keap1–Nrf2 pathway also in mammalian species. Importantly, expression of p62 and Nqo1 rapidly declined during normal ageing of mice, and message levels of p62 and Nqo1 in the livers of 132-week-old wild-type mice were only about 5% and 30%, respectively, of those in 12-week-old mice (Fig 5A,B). Conversely, enhanced expression of Nrf2-dependent genes including Nqo1 has been observed in the tissues and cultured cells derived from long-lived Ames and Snell dwarf mice (Leiser & Miller, 2010; Sun et al, 2011). As p62 is also a target of Nrf2 transcriptional activity (Jain et al, 2010), our studies together imply that a ‘vicious loop’ within the p62–Keap1–Nrf2–Nqo1 cascade underlies progressive mitochondrial dysfunction and mammalian ageing (Fig 5E).
Figure 5.
Age-dependent changes in expression of p62, Nqo1 and autophagy components. (A–D) Relative message levels of p62, Nqo1, Atg5 and Atg7 in livers of wild-type mice at three different ages were measured by quantitative RT–PCR. All data are normalized to 18S rRNA (n=4 per group, *P<0.02). (E) Schematic presentation of the role of p62 in mammalian longevity. Nqo1, NAD(P)H dehydrogenase, quinone 1; ROS, reactive oxygen species.
In addition, p62 might also promote mammalian longevity through its adaptor function for selective autophagy, particularly for autophagic removal of depolarized mitochondria; that is, mitophagy (Geisler et al, 2010; Komatsu & Ichimura, 2010). Thus, we do not exclude the possibility that the accelerated ageing in p62−/− mice might also be attributable to inefficient mitophagy. However, as controversial data suggesting a dispensable role for p62 during mitophagy are also available (Narendra et al, 2010), further work under in vivo conditions will be required to evaluate the influence of p62 on the clearance of dysfunctional mitochondria generated during senescence.
The present study showed that p62 suppresses excessive oxidant generation. Paradoxically, however, accumulation of p62 protein in autophagy-deficient mice has been correlated with increased oxidative stress and tumour promotion (Inami et al, 2011). As the autophagy activity declines with age (Fig 5C,D), cells in older animals might face an environment similar to that observed in autophagy-deficient mice. However, as p62 expression also declines with age (Fig 5A), animals might benefit from the longevity-promoting nature of p62 during relatively earlier life stages and the reduced adverse effect of p62 protein accumulation in old age.
Another interesting observation from our study is the sexually dimorphic effect of the p62 gene deletion on the mitochondrial and organismal ageing (Figs 1, 2). Although previous reports suggest enhanced activity of superoxide dismutase 2 (SOD2) or glutathione peroxidase (GPX) in the mitochondria of female animals (Borras et al, 2003), expression of these enzymes was insignificantly different between males and females, as well as between p62−/− and p62+/+ mice (J.K. et al, unpublished results). Thus, females might have an advantage over males in longevity assurance because of additional means of supporting mitochondrial integrity independently of p62. Interestingly, mutation of genes such as IGF1 receptor and S6K1 more profoundly extended the lifespan of female mice (Holzenberger et al, 2003; Selman et al, 2009), which is opposite to the phenotypes that arose in p62−/− mice with regard to lifespan modulation and sexual dimorphism. However, it is not clear whether these contrary effects are a result of the separate, converse or compensatory activities of these genes. Thus, careful examination of these systems in combination might provide a way to define the nature of an increased longevity in females.
Altered metabolic homeostasis, cognitive impairment and ageing are correlated with each other (Craft, 2005). Thus, mature-onset obesity and features of neurodegeneration observed previously in p62−/− mice (Rodriguez et al, 2006; Ramesh Babu et al, 2008) seem to have an inseparable relationship with the rapid ageing phenotype described in the present study. However, obesity and insulin resistance developed similarly in both male and female p62−/− mice (J.K. et al, unpublished results), whereas senescence was less severely affected in females (Fig 1). In addition, loss of working memory was observed in p62−/− mice from 6 months of age, whereas anxiety and depression was observed as early as 2 months of age (Ramesh Babu et al, 2008). These results indicate that some phenotypes that arose in p62−/− mice are associated with each other, but some are intrinsic defects due to the loss of tissue-specific p62 function.
Sqstm1/p62 and its homologues are found in vertebrates, and the amino-acid sequences of these homologues are highly conserved (>90%) among mammals. In contrast, Drosophila expresses a gene, ref(2)p, which shares only limited local homology with p62 within the ZZ and UBA domain (Avila et al, 2002). At present, it is not clear whether ref(2)p also preserves the longevity assurance function of p62 in Drosophila. However, unlike the KIR that is not conserved in ref(2)p, either the ZZ domain or UBA domain in p62 was inessential for inducing Keap1 degradation (Fig 3B). Thus, ref(2)p might contribute to only a limited extent to the longevity assurance mechanism, unless it contains a specific but not yet characterized motif for Drosophila Keap1 regulation. Nevertheless, except for ref(2)p, the lack of p62 homologue in invertebrates or lower organisms suggests that p62 evolved in concert with the extended lifespans of higher metazoans. Therefore, ageing animal models carrying mutations in genes specific to higher organisms, such as p62−/− mice, will provide a unique advantage in future studies on the ageing of mammalian subjects.
Methods
Mice. Mice (p62−/−) were generated by standard gene targeting methods based on previously published protocols (Hogan et al, 1994), and cumulative survival of wild-type and p62−/− mice was determined using the Kaplan–Meier method. Whole-body oxygen consumption was determined by indirect calorimetry (Butler et al, 2001).
Analysis of tissue mitochondria. The rate of mitochondrial H2O2 generation was determined by linear increase in fluorescence of oxidized homovanillic acid in the presence of horseradish peroxidase (Barja, 2002) using mitochondria isolated from tissues by differential centrifugation and iodixanol density gradient centrifugation (Sharer et al, 2002). The respiration rate was measured using a Clark-type oxygen electrode. Mouse cardiac ventricular ultrastructure was observed under a transmission electron microscope after tissue sections were fixed and embedded in osmium tetroxide and epoxy resin, respectively.
Flow cytometry and reporter assay. Intracellular oxidant level and mitochondrial membrane potential (Δψm) were assessed by measuring the fluorescence of dichlorofluorescein (DCF) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylben-zimidazolyl-carbocyanine iodide (JC1, Molecular Probes), respectively (Bass et al, 1983; Royall & Ischiropoulos, 1993). The relative luciferase reporter activity (human Nqo1-ARE-luciferase versus pRL-TK Renilla luciferase) was measured 24 h after transfection of plasmids.
Details of protocols and additional methods are available as supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We are grateful to Dr Sue Goo Rhee for comments on the manuscript, Ms Hyunjin Shin for manuscript preparation and to Dr Seok Hyun Hong for organizing the research programme. This work was supported partly by the Samsung Biomedical Research Institute and STC Life Inc.
Author contributions: A.L., S.K.P., Y.J.S., G.T.O. and H.L. contributed to the p62−/− mice generation. J.K., J.L., Y.C., S.L. and K.L. contributed to the phenotype analyses of p62-deficient mice. E.H., J.K., C.B. and W.S. performed biochemical and molecular cell biological analyses. J.S. designed the research and wrote the paper with the input of C.P. and M.S.O.
Footnotes
The funding agency (STC Life Inc.) was not involved in any process of the research design, data collection, analysis and interpretation, and preparation of the manuscript.
References
- Avila A, Silverman N, Diaz-Meco MT, Moscat J (2002) The Drosophila atypical protein kinase C-ref(2)p complex constitutes a conserved module for signaling in the Toll pathway. Mol Cell Biol 22: 8787–8795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120: 483–495 [DOI] [PubMed] [Google Scholar]
- Barja G (2002) The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg Biomembr 34: 227–233 [DOI] [PubMed] [Google Scholar]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M (1983) Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol 130: 1910–1917 [PubMed] [Google Scholar]
- Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78: 547–581 [DOI] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J (2003) Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34: 546–552 [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD (2001) Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4: 605–611 [DOI] [PubMed] [Google Scholar]
- Craft S (2005) Insulin resistance syndrome and Alzheimer′s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging 26(Suppl 1): 65–69 [DOI] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131 [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M (2009) NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci 34: 176–188 [DOI] [PubMed] [Google Scholar]
- Hogan BH, Beddington R, Costantini F, Lacy E (1994) Manipulating the Mouse Embryo. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182–187 [DOI] [PubMed] [Google Scholar]
- Inami Y et al. (2011) Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 193: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T (2010) p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285: 22576–22591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar JW, Niture SK, Jaiswal AK (2009) Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47: 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y (2010) Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 584: 1374–1378 [DOI] [PubMed] [Google Scholar]
- Komatsu M et al. (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12: 213–223 [DOI] [PubMed] [Google Scholar]
- Leiser SF, Miller RA (2010) Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol 30: 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT (2009) p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 137: 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ (2010) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6: 1090–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh Babu J et al. (2008) Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem 106: 107–120 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J (2006) Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 3: 211–222 [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H (1993) Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys 302: 348–355 [DOI] [PubMed] [Google Scholar]
- Selman C et al. (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326: 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer JD, Shern JF, Van Valkenburgh H, Wallace DC, Kahn RA (2002) ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter. Mol Biol Cell 13: 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Bokov AF, Richardson A, Miller RA (2011) Hepatic response to oxidative injury in long-lived Ames dwarf mice. FASEB J 25: 398–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14: 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK (2008) Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Wong A, Cortopassi G (1997) The rate of mitochondrial mutagenesis is faster in mice than humans. Mutat Res 377: 157–166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.