Abstract
The K562 cell line has been proposed as a useful experimental system to identify anti-tumor compounds acting by inducing terminal erythroid differentiation. K562 cells exhibit a low proportion of hemoglobin-synthesizing cells under standard cell growth conditions, but are able to undergo terminal erythroid differentiation when treated with a variety of anti-tumor compounds. In this paper we report a screening study on a set of different modified C(5) uracil derivatives for the evaluation of their antiproliferative effect in connection with erythroid differentiation pathways, and for defining a new class of drug candidates for the treatment of chronic myelogenous leukemia. Activity of the derivatives tested can be classified in two effect: an antiproliferative effect linked to a high level of erythroid differentiation activity and an antiproliferative effect without activation of gamma globin genes The highest antiproliferative effect and erythroid induction was shown by compound 9, a thymine derivative bearing a n-octyl chain on nitrogen N(1), whereas thymine did not show any effect, suggesting the importance of the linear alkyl chain in position N(1). To our knowledge this compound should be considered among the most efficient inducers of erythroid differentiation of K562 cells. This work is the starting point for the quest of more effective and specific drugs for the induction of terminal erythroid differentiation, for leading new insights in the treatment of neoplastic diseases with molecules acting by inducing differentiation rather than by simply exerting cytotoxic effects.
Keywords: Erythroid differentiation, Tumor growth, Isoorotic acid derivative, Chronic myelogenous leukemia, Beta-thalassemia
1. Introduction
The pyrimidine system is a very important pharmacophor core of naturally occurring and synthetic bioactive compounds, interacting with the synthesis and function of nucleic acids, interfering with biosynthetic pathways and competing for the same binding sites of naturally occurring pyrimidines (Lagoja, 2005). Modified pyrimidines are currently used as drugs; for example fluorouracil has cytostatic effects and is currently used in cancer therapeutics, azidothymidine (AZT) was the first applied drug for HIV treatment and bacimethrin (4-amino-5-hydroxymethyl-2-methoxypyrimidine) is known as the simplest pyrimidine antibiotics (Reddick et al., 2001).
The K562 cell line (Lozzio and Lozzio, 1975) has been proposed as a very useful experimental system to identify antitumor compounds (Lampronti et al., 2003a, b; Lampronti et al., 2006) acting by inducing terminal erythroid differentiation of this as well as other tumor cell lines (Bianchi et al., 1999, 2000, 2001; Chiarabelli et al., 2003; Gambari and Fibach, 2007; Olivieri, 1996; Osti et al., 1997). K562 cells exhibit a low proportion of hemoglobin-synthesizing cells under standard cell growth conditions, but are able to undergo terminal erythroid differentiation when treated with a variety of compounds, including short fatty acids (Gambari and Fibach, 2007), 5-azacytidine (Gambari and Fibach, 2007), mithramycin and chromomycin (Bianchi et al., 1999; Fibach et al., 2003), cisplatin and cisplatin analogs (Bianchi et al., 2000), tallimustine (Bianchi et al., 2001; Gambari and Fibach, 2007), rapamycin (Fibach et al., 2006), everolimus (Zuccato et al., 2007), psoralens (Lampronti et al., 2003a, b) and resveratrol (Bianchi et al., 2009). Following erythroid induction, increase of expression of ε and γ globin genes is observed, leading to accumulation of Hb Portland (ζ2γ2) and Hb Gower 1 (ζ2ε2) (Gambari and Fibach, 2007).
The design and production of antiproliferative molecules targeting the K562 cell system might be of great interest for the development of cocktails exhibiting applications in the treatment of chronic myelogenous leukemia. For instance smenospongine (Aoki et al., 2004a, b), crambescidin 800 (Aoki et al., 2004a, b) and doxorubicin derivatives (Szulawska et al., 2007) were reported as molecules of possible interest for inhibiting of chronic myelogenous leukemia cell growth, stimulating terminal differentiation along the erythroid program. Some molecules, such as Pivanex (an HDAC inhibitor) (Rabizadeh et al., 2007) and a morpholine derivative of doxorubicin (Jakubowska et al., 2008), are synergistic with the most common anti-chronic myelogenous leukemia agents, STI571 (Imatinib). In addition to synergistic effects, molecules inducing differentiation might be of great interest for treatment of Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines, as recently demonstrated for the phytoalexin resveratrol (Puissant et al., 2008).
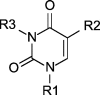
Recently we reported a novel class of C-5 linked N-1 alkylated uracil dimers rationally designed to potentially interact with adenine in biological systems. These pyrimidine derivatives have shown antiproliferative and erythroid differentiation activities toward human chronic myelogenous leukemia K562 cells (Accetta et al., 2009). In this paper we report a screening study on a set of different modified C(5) uracil derivatives (Table 1) for the evaluation of their antiproliferative effect in connection with erythroid differentiation pathways, and for defining a new class of drugs candidates for the treatment of chronic myelogenous leukemia.
Table 1.
Schematic description of compounds screened in the present study.
| Compound | R1 | R2 | R3 | |
|---|---|---|---|---|
 |
1 | –H | –H | –H |
| 2 | –H | –CH3 | –H | |
| 3 | –H | –CH2N3 | –H | |
| 4 | –CH2COOH | CH3 | –H | |
| 5 | –H | –COOCH3 | –H | |
| 6 | –H | –H | –COPh | |
| 7 | –CH3 | –H | –COPh | |
| 8 | –CH3 | –CONHnC4H9 | –H | |
| 9 | –nC8H17 | –CH3 | –H | |
| 10 | –nC8H17 | –COOH | –H | |
| 11 | –nC8H17 | –CONHCH2Ph | –H | |
| 12 | –nC8H17 | –CH3 | –nC8H17 | |
| 13 | –CH2COOtBu | –I | –H | |
| 14 | –CH2COOH | –I | –H | |
| 15 | –CH2COOtBu | –CH2N3 | –H | |
| 16 | –CH2COOEt | –CH2N3 | –H | |
| 17 | –CH2COOEt | –CH2NH2 | –H | |
| 18 | –CH2COOEt | –CH2NHCO(2-naphthyl) | –H |
Name (alternative name): 1, uracil; 2, thymine (5-methyluracil); 3, 5-azidomethyluracil; 4, (thymin-1-yl)acetic acid (1-(carboxymethyl)thymine); 5, methyl 5-uracilcarboxylate (methyl isoorotate); 6, 3-benzoyluracil; 7, 3-benzoyl-1-methyluracil; 8N-butyl-1-methyl-5-uracilcarboxamide; 9, 1-octylthymine; 10, 1-octyl-5-uracilcarboxylic acid (1-octylisoorotic acid); 11, N-benzyl-1-octyl-5-uracilcarboxamide; 12, 1,3-dioctylthymine; 13, t-butyl (5-iodouracil-1-yl)acetate; 14, (5-iodouracil-1-yl)acetic acid; 15, t-butyl (5-azidomethyluracil-1-yl)acetate; 16, ethyl (5-azidomehtyluracil-1-yl)acetate; 17, ethyl (5-aminomethyluracil-1-yl)acetate; 18, ethyl [5-(N-(2-naphthylcarboxyl)aminomethyl)uracil-1-yl]acetate.
2. Materials and methods
2.1. Chemical synthesis of uracil compounds
Compounds 1, 2 and 4 were purchased from Sigma-Aldrich and tested without further purification. Synthesis of compounds 5, 8, 9, 10 and 11 was previously reported in Accetta et al. (2009). All syntheses started from uracil, thymine or 5-carboxyuracil (isoorotic acid) as described schematically in the Results section. Full synthetic procedures and characterization of compounds 3, 12, 13, 14, 15, 16, 17 and 18 are reported elsewhere (Accetta et al., 2010).
2.2. Cell culture conditions, proliferation assay and cell differentiation
The human K562 cells (Lozzio and Lozzio, 1975), obtained from the American Type Culture Collection (Rockville, Md., USA), were maintained in a humidified atmosphere of 5% CO2 at 37 °C in suspension culture using RPMI 1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Analitical de Mori, Milan, Italy), 50 units/ml penicillin and 50 mg/ml streptomycin (Bianchi et al., 2000). For the experiments, cell were seeded at an initial concentration of 3 × 104 cells/ml and cultured in the presence of increasing concentrations of compounds. Non-treated cells were considered as control. Cell growth, according to cell number/ml, was usually determined after 3, 4 and 5 days of culture, using a cell counter (Coulter Electronics, Hialeah, FL, USA). These time points were selected because between days 3 and 5 untreated control K562 cells are on the log phase of cell growth (Bianchi et al., 2000). All experiments were repeated at least 3 times. In order to determine the effects on the erythroid differentiation, cells were seeded at an initial concentration of 3 × 104 cells/ml and the proportion of benzidine-positive cells was determined after 4–7 days of cell culture using a solution containing 0.2% benzidine in 0.5 M glacial acetic acid (10% H2O2) as previously described (Bianchi et al., 2001; Chiarabelli et al., 2003). Benzidine positivity indicates the presence of intracellular hemoglobin.
2.3. Transfection of K562 cells with fluorescence protein genes under the γ-globin and the β-globin gene promoters
K562 cells were stably transfected with the pCCL.Promβ.HcRed1.Promγ.EGFP, containing the green and red fluorescence protein (FP) genes under the control of the γ-globin and β-globin gene promoters, respectively (Guerrini et al., 2009; Lampronti et al., 2009). In this system, increases in the green and red signals are consistent with γ-globin and β-globin gene promoter driven activity, respectively. To determine the activity of chemical compounds in inducing the expression of γ-globin and β-globin genes, cells were seeded at 8000 cells/ml and treated with the appropriate concentration of the chemical inducer. After 5 days of culture, cells were assayed for fluorescent proteins expression. First of all they were analyzed under a fluorescence inverted microscope, using filters suitable for both green and red FPs. The fluorescence intensity was then determined by fluorescence-activated cell sorting (FACS) analysis.
2.4. FACS analysis
For the determination of fluorescence intensity by FACScan (Becton Dickinson, Franklin Lakes, NJ, USA), cells were harvested and washed. Then 1 × 104 cells were analyzed by the CellQuest™ version 3.3 software (Becton Dickinson, Franklin Lakes, NJ, USA), using the fl1 channel to detect green fluorescence and fl3 channel to detect red fluorescence. The results were expressed as median fold, i.e. the ratio between the median fluorescence intensity values obtained in the presence and absence of treatment, respectively. A graphic presentation of data was finally obtained by histograms, showing the number of cells versus the expressed fluorescence intensity (Salvatori et al., 2009).
2.5. mRNA measurements
RNA was isolated from K562 cells and measured by reverse transcription quantitative real-time polymerase chain reaction (qRT-PCR) as described (Chiarabelli et al., 2003) using gene-specific double fluorescence labeled probes in an ABI Prism 7700 Sequence Detection System version 1.7.3 (Applied Biosystems, Monza, Italy). The following primer and probe sequences were used: α-globin forward primer, 5′-CAC GCG CAC AAG CTT CG-3′; α-globin reverse primer, 5′-AGG GTC ACC AGC AGG CAG T-3′; α-globin probe, 5′-FAM-TGG ACC CGG TCA ACT TCA AGC TCC T-TAMRA-3′; γ-globin forward primer, 5′-TGG CAA GAA GGT GCT GAC TTC-3′; γ-globin reverse primer, 5′-TCA CTC AGC TGG GCA AAG G-3′; γ-globin probe, 5′-FAM-TGG GAG ATG CCA TAA AGC ACC TGG-TAMRA-3′; glycophorin A forward primer: 5′-CGG TAT TCG CCG ACT GAT AAA-3′; glycophorin A reverse primer: 5′-AAA GGC AGT CTG TGT CAG GT-3′; glycophorin A probe: 5′-FAM-AAA GCC CAT CTG ATG TAA AAC CTC TTC CCC T-TAMRA-3′; transferrin receptor forward primer: 5′-TCA GAGCGTCGGGATATCG-3′; transferrin receptor reverse primer: 5′-TGA ACT GCC ACA CAG AAG AAC A-3′, transferrin receptor probe: 5′-FAM-TGG CGG CTC GGG ACG GA-TAMRA-3′. The kit for quantitative qRT-PCR for ζ-globin mRNA and ε-globin mRNA was from Applied Biosystems (ζ-globin mRNA: Hs00923579_m1; ε-globin mRNA: Hs00362216_m1). The fluorescent reporter and the quencher were 6-carboxyfluorescein (FAM) and 6-carboxy-N,N,N′,N′-tetramethylrhodamine (TAMRA), respectively. For qRT-PCR, the reference gene was 18S; this probe was fluorescent-labeled with VIC (Applied Biosystems, Monza, Italy) (Bianchi et al., 1999; Chiarabelli et al., 2003).
3. Results
3.1. Synthetic scheme of the compounds tested
The C-5 position of pyrimidine nucleosides can be easily modified with a variety of different residues. Substitution at this position is often used for introducing substituents on DNA oligomers with reporter groups. In a general project, aimed at molecular engineering of peptide nucleic acid (PNA) derivatives (A. Accetta, 2010), we prepared a set of N(1) and/or N(3) alkyl-C(5) modified uracil derivatives. Since these compounds are structurally analogs of modified nucleosides, they could be considered as potential drugs per se.
The list of compounds tested is reported in Table 1. This set of compounds was chosen for screening since: i) it contains compounds alkylated at N1 and N3 positions or both; ii) it contains a series of C5 derivatives with different functional groups; iii) it has lipophilic groups in both the N1, N3, or C5 region of uracil; iv) it contains derivatives with intact uracil (and thymine) hydrogen bond pattern or lacking of the N3 hydrogen.
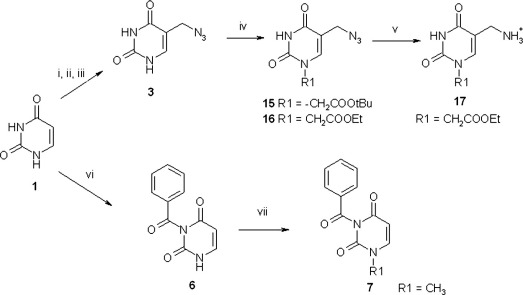
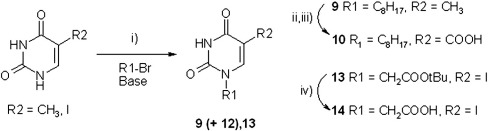
The synthetic pathways used for different compounds are reported in Figs. 1 and 2 (Accetta et al., 2009; Accetta et al., 2010).
Fig. 1.
Synthetic pathways used for derivatives obtained from uracil. i) CH2O, Et3N, 60 °C, overnight; ii) HCl, 37 °C, 4 h, r.t.; iii) NaN3, DMF, r.t., 2 h; iv) BrCH2COOR; K2CO3, 0 °C to r.t., overnight; v) PPh3, THF, rt, overnight, then TFA; vi) a) BzCl acetonitrile/pyridine, r.t. 24 then b) K2CO3 , water/dioxane, r.t., 40 min; and c) NaHCO3 sat r.t. 1 h; vii) CH3I, DMF, r.t., 3 h.
Fig. 2.
Synthetic pathways used for derivatives obtained from thymine and 5-iodouracil. i) For 9 Br-n-C8H17 , NaH as base, DMF, 80 °C, 4 h; for 13 BrCH2COOtBu and K2CO3 as base, DMF; ii) 2,6-lutidine, K2S2O8, CuSO4, H2O/AcCN, 80 °C, 1,5 h; iii) NaClO2, NaH2PO4; t-BuOH/THF, room temp, 24 h).; iv) TFA, DCM, 0 °C, 1h.
Briefly, modification of uracil at both N1 and C5 positions was obtained in several ways. For the more reactive uracil and isoorotic acid, and in the presence of small electrophiles such as methyl iodide, the regioselective alkylation at N1 can be obtained by temporary protection of the N3 with trimethylsilyl (Accetta et al., 2009) or benzoyl (Frieden et al., 1998) groups. The latter method was used in the synthesis of 7 from 6. Azidomethyl derivative 3 was obtained by reaction of uracil with formaldehyde, thus introducing a 5-hydroxymethyl derivative which could be further elaborated to azide by nucleophilic substitution (via a chloromethyl intermediate). Alkylation of 3 with α-bromoacetic acid derivatives led to compounds 15 and 16. The latter was converted to 17, bearing an amino group through Staudinger reduction. The amide derivative 18 was then obtained by reaction of the amino compounds with 2-naphthalenecarboxylic acid after activation of the latter with HBTU. Regioselective alkylation at N1 was obtained also by exploiting the higher reactivity of the N1 position in thymine and iodouracil toward sterically hindered electrophiles (Fig. 2), which allowed direct synthesis of compounds 9 (according to Coutouli-Argyropoulou and Zachariadou, 2005) and 13. The N1, N3-doubly alkylated compound 12 was obtained as a side product of thymine alkylation. Oxidation of thymine methyl group led to the N1-alkylated-5-carboxylic derivative 10 (Accetta et al., 2009), which was then converted into the carboxyamide derivative 11 via HBTU activation and reaction with benzylamine. Hydrolysis of 13 with TFA provided derivative 14, bearing a polar substituent in the N1-position.
3.2. Antiproliferative and erythroid differentiating activities of the uracil monomers
We first determined for all the synthesized molecules the effects on cell proliferation. To this aim, K562 cells were cultured in the presence of increasing concentrations of compounds and cell number/ml was determined after 3, 4 and 5 days of treatment. The results are shown in Table 2, where it appears evident that the most active compounds with respect to antiproliferative effects (IC50 values lower that 150 μM) are compounds 3, 9, 11, 12 and 18, compound 9 being the most active molecule. However, among these molecules, only compound 9 is a very effective inducer of erythroid differentiation of K562 cells (Table 2). When analysis of the proportion of benzidine-positive cell is performed, K562 cells cultured in the presence of 50 μM compound 9 display over 90% hemoglobin-expressing (benzidine-positive) cells. As far as the other compounds, only 8 and 18 are able to induce differentiation, but at very high concentrations (400 μM and 200 μM, respectively). When the same compounds were added at higher concentrations, no further increase in the proportion of benzidine-positive cells was obtained (data not shown). According with these results, compound 9 was analyzed in more detail.
Table 2.
Antiproliferative activity (IC50)a and induction of erythroid differentiationb (% B+ cells) of uracil derived-compounds on erythroleukemia K562 cells line.
| Compound | IC50 | % B+ cells | Concentration |
|---|---|---|---|
| (μM) | |||
| 1 | > 400 μM | 1.1 ± 0.8 | 400 |
| 2 | > 400 μM | 1.3 ± 0.6 | 400 |
| 3 | 120.05 ± 4.7 μM | 4.1 ± 1.2 | 100 |
| 4 | > 400 μM | 2.0 ± 0.9 | 400 |
| 5 | > 800 μM | 7.3 ± 1.9 | 800 |
| 6 | 350.7 ± 15.9 μM | 1.1 ± 0.5 | 400 |
| 7 | 650.6 ± 12.4 μM | 1.5 ± 0.8 | 800 |
| 8 | 488 ± 68 μM | 12 ± 1.9 | 400 |
| 9 | 29.4 ± 1.2 μM | 98 ± 3.6 | 50 |
| 10 | 268 ± 15.5 μM | 25 ± 2.9 | 200 |
| 11 | 66.54 ± 13 μM | 2.2 ± 1.0 | 100 |
| 12 | 29.7 ± 1.4 μM | 2.1 ± 0.9 | 50 |
| 13 | > 400 μM | 1.2 ± 0.2 | 400 |
| 14 | > 400 μM | 1.6 ± 0.8 | 400 |
| 15 | 721.3 ± 20.9 μM | 2.3 ± 0.6 | 800 |
| 16 | 764.2 ± 25.6 μM | 2.1 ± 0.8 | 800 |
| 17 | > 800 μM | 2.0 ± 0.9 | 800 |
| 18 | 115 ± 16 μM | 30 ± 4.9 | 200 |
Results are presented as average ± S.D. (three independent experiments performed).
Values are expressed as average ± S.D. (three independent experiments performed) after 6 days of treatment at concentrations close to those needed to obtain 50% inhibition of cell growth after 3 day culture period (and reported of the far right column).
3.3. Effects of compound 9 on proliferation and erythroid differentiation of K562 cells
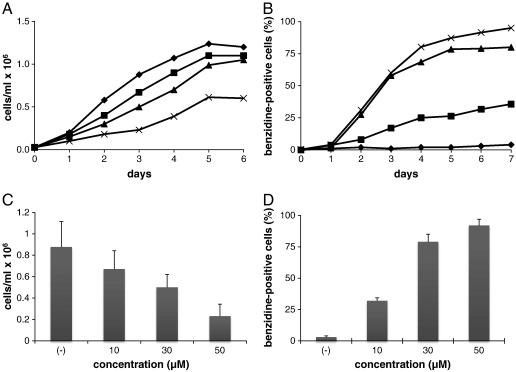
The analysis of the effects of compound 9 on the K562 cellular system is shown in Fig. 3. In the experiment shown in Fig. 3A, K562 cells were cultured for 6–7 days in the absence or in the presence of increasing concentrations (10, 30, 50 μM) of compound 9. As it is clearly evident, a concentration-dependent inhibition of cell growth was observed. Fig. 3C reports the effect on the proliferation of K562 cells after 3 days of treatment with compound 9 at different concentrations. IC50 was obtained at about 30 μM. Fig. 3B shows the percentages of differentiation induced by compound 9 at different concentrations (10, 30, 50 μM). We like to underline that more than 60% benzidine positive cells are detectable after only 3 days of induction, demonstrating that the inducing effects of compound 9 was very high, and it can be considered among the most powerful inducer of K562 differentiation (see also Table 3). Despite the fact that there is no linear relationship between proportion of differentiated cells and cells with lower proliferation efficiency, the data summarized in Fig. 3C and D demonstrate that 30 μM compound 9 is a concentration sufficient to (a) inhibit cell growth and (b) induce differentiation of the majority of treated K562 cells. Further experiments are required to verify whether decrease of cell growth is a pre-requisite for differentiation or is the cause of this feature. Supplementary Fig. 1 shows a microscopic analysis of K562 cells cultured taken after 6 days of K562 cell cultured in the absence or in the presence of 30 μM compound 9. In these assays, the blue-color of benzidine-stained cells is compatible with high accumulation of hemoglobin (Bianchi et al., 2001).
Fig. 3.
Effects of compound 9 on the proliferation (A) and erythroid differentiation of K562 cells (B). Cells were cultured for 6–7 days in the absence ( ) or in the presence of increasing concentrations 10 μM (
) or in the presence of increasing concentrations 10 μM ( ), 30 μM (
), 30 μM ( ), and 50 μM (
), and 50 μM ( ) of compound 9. The effect on the proliferation of K562 after 3 days of treatment with compound 9 at different concentrations (C) and the percentage of benzidine-positive cells after 6 days of treatment (D). The values in treated cultures represented in C) were compared with untreated control cultures (taken as 100%). The data represents the mean ± S.D. of three independent experiments.
) of compound 9. The effect on the proliferation of K562 after 3 days of treatment with compound 9 at different concentrations (C) and the percentage of benzidine-positive cells after 6 days of treatment (D). The values in treated cultures represented in C) were compared with untreated control cultures (taken as 100%). The data represents the mean ± S.D. of three independent experiments.
Table 3.
Antiproliferative effects (IC50)a and induction of erythroid differentiationb (% B+ cells) of uracil, compound 9 and 5-fluorouracil on K562 cell line.
| Compound | IC50 | % B+ cells |
|---|---|---|
| (30 μM) | ||
| Uracil | > 500 μM | 1.2 ± 0.2 |
| Compound 9 | 17.52 ± 2.88 μM | 92.3 ± 2.1 |
| 5-Fluorouracil | 17.73 ± 2.23 μM | 3.1 ± 0.6 |
Results are presented as average ± S.D. (three independent experiments performed) of concentration needed to obtain 50% inhibition of cell growth after 3 days culture period.
Values are expressed as average ± S.D. (three independent experiments performed) after 6 days of treatment.
In a second set of experiments, the biological activity of compound 9 was compared to that of 5-fluorouracil (5-FU), extensively employed as anti-tumor agent in leukemias (Mini et al., 1990; Schilsky, 1996). Interestingly, as far as the effects on cell proliferation, similar IC50 values were obtained when compound 9 and 5-FU were employed. On the other hand the effects of compound 9 and 5-FU on erythroid differentiation were found to be very different, being only compound 9 able to activate the full program of differentiation. As expected, uracil, in agreement with the first set of results depicted in Table 2, did not show any effects on K562 with respect to inhibition of cell proliferation and induction of erythroid differentiation (Table 3).
3.4. Effects of compound 9 on the transcriptional activity of the γ-globin and the β-globin gene promoters of K562 cells
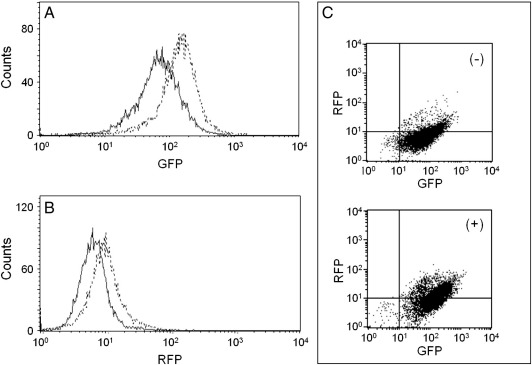
To determine the effects of compound 9 on the transcription of the globin genes, we used a clonal K562 cell population stably transfected with a reporter construct, carrying the genes for the green (EGFP) and red (RFP) fluorescent proteins (FP), under the control of the γ-globin and the β-globin promoters, respectively (Guerrini et al., 2009; Lampronti et al., 2009). To compare the relative effect of compound 9, the experiment depicted in Fig. 4 was performed. Cells were treated with 30 μM compound 9, isolated after 5 days and employed in FACS analysis; this assay demonstrated that compound 9 enhanced EGFP by 2.10 ± 0.63 fold, and the increase in RFP was 1.39 ± 0.10 fold, indicating an effect of compound 9 on the transcription directed by both the γ-globin and β-globin gene promoters. On the contrary, as recently published by our research group, other erythroid differentiation inducers have a stimulatory selectivity only for the γ-globin gene promoter (Guerrini et al., 2009 and data not shown).
Fig. 4.
Representative example of FACS analysis of a K562 cell clone containing the reporter construct pCCL.Promβ.HcRed1.Promγ.EGFP, untreated or treated with compound 9 30 μM for 5 days. (A,B) Histogram plots obtained from untreated (solid lines) or treated (dotted lines) cells, showing the relationship between number of cells and intensity of expressed fluorescence, green (A) or red (B), respectively. (C) Dot plots obtained from untreated (−, upper panel) or treated (+, lower panel) cells, showing the cell population distribution as a function of the two different fluorescences.
3.5. Effects of compound 9 on biochemical parameters associated to the activation of the K562 erythroid phenotype
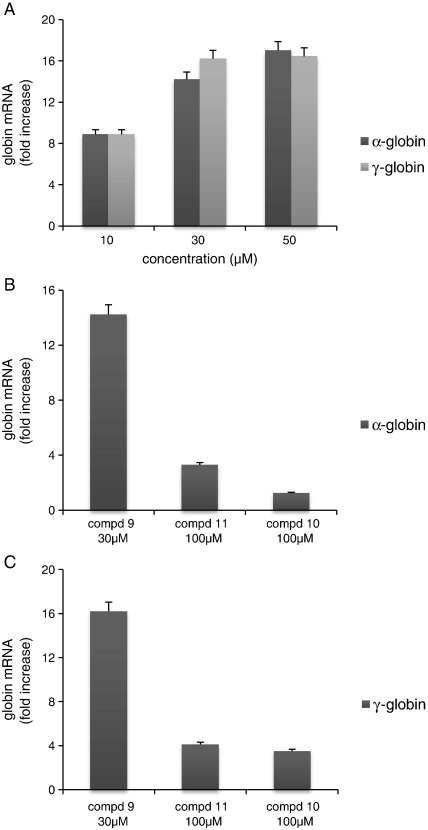
As we can see in Fig. 5, exposure for 6 days of K562 cells to 30 μM compound 9 stimulates a sharp accumulation of α-globin mRNA (black symbols) and γ-globin mRNA (gray symbols). The effects of compound 9 on the transcription of α-globin mRNA and γ-globin mRNA were analyzed by qRT-PCR amplifications and found to be clearly associated with the ability of compound 9 to induce erythroid differentiation of K562 cells. Compounds 10 and 11, which induce erythroid differentiation to a much lower extent (Table 3), induce only slight increase of accumulation of α-globin (Fig. 5B) and γ-globin (Fig. 5C) mRNAs. The qRT-PCR data concerning the effects of compound 9 are fully in agreement with HPLC analyses, showing that this molecule induces a sharp increase of embryonic and fetal hemoglobins (Supplementary Fig. 2).
Fig. 5.
Accumulation of α-globin mRNA (A, black columns) and γ-globin mRNA (A, gray columns) in K562 cells treated for 6 days with 30 μM compound 9. qRT-PCR amplifications were performed on RNA from untreated or treated cells using primers amplifying 18S ribosomal RNA as reference gene. Results are presented as fold increase of α-globin and γ-globin mRNAs with respect to untreated cells. (B,C) Effect of compounds 9, 11 and 10 on accumulation of α-globin mRNA (B) and γ-globin mRNA (C). The results of untreated cells were taken as 1. Results represent the average ± S.D. of three independent experiments.
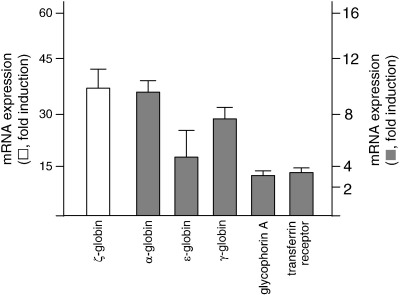
Further qRT-PCR data demonstrated that also the embryonic-type ζ- and ε-globin mRNAs are induced by compound 9 (Fig. 6). In addition, the effects of compound 9 on other genes involved in the expression of erythroid program. To this aim the mRNA coding glycophorin A (Ida et al., 2009; Kohmura et al., 2004; Watanabe et al., 1985) and transferrin receptor (Gambari et al., 1986; Harigae et al., 2006) were studied by qRT-PCR. The results obtained are shown in Fig. 6 and demonstrate that both relative amounts of glycophorin A and transferrin receptor mRNAs increase following treatment with compound 9, confirms the induction of erythroid pathway by this compound.
Fig. 6.
Accumulation (fold increase in respect to control uninduced cells) of the α-like ζ- and α-globin mRNAs, the β-like ε- and γ-globin mRNA, the glycophorin A and transferrin receptor mRNAs in K562 cells treated for 6 days with 30 μM compound 9. Results represent the average ± S.D. of three independent experiments.
3.6. The induction of erythroid differentiation of compound 9 is not associated with the activation of the apoptotic pathway
Since several inducers of erythroid differentiation are also strong inducers of apoptosis of K562 cells (for instance mithramycin and psoralens) (Bianchi et al., 2001; Lampronti et al., 2003a, b) the experiments described in Supplementary Figs. 3, 4 and 5 were performed. Flow cytometry analysis (Supplementary Fig. 3) demonstrates no major alteration of cell cycle parameters and no accumulation of sub-G1 cells (a hallmark of apoptosis). Apoptosis was not evident also when Annexin V–FITC staining was performed (Supplementary Fig. 4) and using the DeadEnd TUNEL assay (Supplementary Fig. 5).
4. Discussion
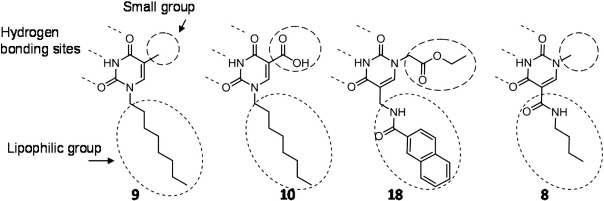
The biological activity of the derivatives tested in this study can lead to the following alternative effects: (a) a strong antiproliferative effect linked to a high level of erythroid differentiation activity and (b) an antiproliferative effect independent from the activation of the erythroid program (Table 2). The highest antiproliferative effect and erythroid induction ability were exhibited by compound 9, a thymine derivative bearing a n-octyl chain on nitrogen N(1), whereas thymine (compound 2) did not show any effect, suggesting the importance of the linear alkyl chain in position N(1). The insertion of the alkyl chain in both N(1) and N(3) positions of thymine (compound 12) brought a loss of erythroid induction activity with retention of antiproliferative effect, suggesting the fundamental role of hydrogen in position N(3) of thymine for the erythroid induction activity. Appreciably, even if in some cases much lower, erythroid differentiation activity was shown by derivatives 8, 10 and 18. Activity of compound 10 can be rationalized considering its structural similarities to compound 9 (methyl group substituted by COOH); compounds 8 and 18 show the same pattern, when properly oriented (Fig. 7); therefore, they can be considered as “C-nucleoside” equivalent (Lu et al., 2009) of compound 9 with exchange of the role of N(1) and C(5), which leads to N(3)-H in the same relative orientation with respect to the lipophilic group as in thymine derivatives.
Fig. 7.
Structural similarities of the compounds most effective as erythroid inducers; compounds 18 and 8 are oriented as C-nucleoside analogs.
In conclusion we found a simple thymine derivative, N(1)-octyl-thymine that exhibit strong antiproliferative activity, high ability to induce terminal erythroid differentiation without activation of the apoptotic pathway. To our knowledge this compound should be considered among the most efficient inducers of erythroid differentiation of K562 cells.
However, this work should be considered as the starting point for the development of more effective and specific drugs for the induction of terminal erythroid differentiation, with the aim of leading new insights in the treatment of neoplastic diseases with molecules acting by inducing differentiation rather than simply by exerting cytotoxic and/or antiproliferative effects. Compound 9 might be of some interest since it strongly inhibits cell growth (the activity is similar to that displayed by 5-fluorouracil), but at the same time does induce erythroid differentiation. In this respect, the development of new compounds against chronic myelogenous leukemia cell lines might be of great interest, since it has been already demonstrated that synergistic effects of molecules inducing differentiation can be useful for the efficient treatment of chronic myelogenous leukemia (Jakubowska et al., 2008; Rabizadeh et al., 2007).
In addition, and more importantly, Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines can be treated with erythroid inducers, as recently demonstrated using the phytoalexin resveratrol (Puissant et al., 2008). It should be interesting to determine the activity of compound 9 in Imatinib-resistant cell lines, since the effects of resveratrol as erythroid-inducing agent are significantly different from those displayed by compound 9 in respect to induction of globin mRNAs (Bianchi et al., 2009) and activation of apoptosis and cell-cycle alterations (Puissant et al., 2008).
On the other hand, molecules similar to compound 9 might be of interest for the experimental treatment of β-thalassemic erythroid cells, for which the induction of γ-globin mRNA could be very beneficial (Fibach et al., 2003; Gambari and Fibach, 2007).
In this respect it has been demonstrated that inducers of K562 erythroid differentiation are often able to induce fetal hemoglobin production in erythroid cells isolated from β-thalassemia patients (Gambari and Fibach, 2007).
Acknowledgments
This work was partially supported by a grant by MIUR (PRIN2005 grant n. 2005038704). R.G. is granted by Associazione Italiana Ricerca sul Cancro (AIRC), Fondazione Cariparo (Cassa di Risparmio di Padova e Rovigo), Associazione Veneta per la Lotta alla Talassemia, Rovigo, Italy (AVLT), and Telethon (grant GGP10214). We thank the EU Project ITHANET (eInfrasctructure for Thalassemia Research Network) for support.
Appendix A. Supplementary data
Supplementary material.
References
- Accetta A., Corradini R., Sforza S., Tedeschi T., Brognara E., Borgatti M., Gambari R., Marchelli R. New uracil dimers showing erythroid differentiation inducing activities. J. Med. Chem. 2009;52:87–94. doi: 10.1021/jm800982q. [DOI] [PubMed] [Google Scholar]
- Accetta, A., 2010. Molecular Engineering of PNA Using Modified Uracil Derivatives and Porphyrins. PhD Thesis, University of Parma.
- Aoki S., Kong D., Matsui K., Kobayashi M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in K562 cells. Anticancer Drugs. 2004;15:363–369. doi: 10.1097/00001813-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Aoki S., Kong D., Matsui K., Kobayashi M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer. Res. 2004;24:2325–2330. [PubMed] [Google Scholar]
- Bianchi N., Chiarabelli C., Borgatti M., Mischiati C., Fibach E., Gambari R. Accumulation of gamma-globin mRNA and induction of erythroid differentiation after treatment of human leukaemic K562 cells with tallimustine. Br. J. Haematol. 2001;113:951–961. doi: 10.1046/j.1365-2141.2001.02843.x. [DOI] [PubMed] [Google Scholar]
- Bianchi N., Onagro F., Chiarabelli C., Gualandi L., Mischiati C., Bergamini P., Gambari R. Induction of erythroid differentiation of human K562 cells by cisplatin analogs. Biochem. Pharmacol. 2000;60:31–40. doi: 10.1016/s0006-2952(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Bianchi N., Osti F., Rutigliano C., Corradini F.G., Borsetti E., Tomassetti M., Mischiati C., Feriotto G., Gambari R. The DNA-binding drugs mithramycin and chromomycin are powerful inducers of erythroid differentiation of human K562 cells. Br. J. Haematol. 1999;104:258–265. doi: 10.1046/j.1365-2141.1999.01173.x. [DOI] [PubMed] [Google Scholar]
- Bianchi N., Zuccato C., Lampronti I., Borgatti M., Gambari R. Fetal hemoglobin inducers from the natural world: a novel approach for identification of drugs for the treatment of β-thalassemia and sickle-cell anemia. Evid.-Based Complement. Altern. Med. (eCAM) 2009 doi: 10.1093/ecam/nem139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarabelli C., Bianchi N., Borgatti M., Prus E., Fibach E., Gambari R. Induction of gamma-globin gene expression by tallimustine analogs in human erythroid cells. Haematologica. 2003;88:826–827. [PubMed] [Google Scholar]
- Coutouli-Argyropoulou E., Zachariadou C. Synthesis of 5-substituted uracils and 2,4-dimethoxypyrimidines by Wittig olefination. J. Heterocycl. Chem. 2005;42:1135–1142. [Google Scholar]
- Fibach E., Bianchi N., Borgatti M., Prus E., Gambari R. Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood. 2003;102:1276–1281. doi: 10.1182/blood-2002-10-3096. [DOI] [PubMed] [Google Scholar]
- Fibach E., Bianchi N., Borgatti M., Zuccato C., Finotti A., Lampronti I., Prus E., Mischiati C., Gambari R. Effects of rapamycin on accumulation of alpha-, beta- and gamma-globin mRNAs in erythroid precursor cells from beta-thalassaemia patients. Eur. J. Haematol. 2006;77:437–441. doi: 10.1111/j.1600-0609.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- Frieden M., Giraud M., Reese C.B., Song Q. Synthesis of 1-[cis-3-(hydroxymethyl)cyclobutyl]-uracil, -thymine and –cytosine. J. Chem. Soc. Perkin Trans. 1. 1998:2827–2832. [Google Scholar]
- Gambari R., Barbieri R., Buzzoni D., Bernardi F., Marchetti G., Amelotti F., Piva R., Viola L., del Senno L. Human leukemic K562 cells: suppression of hemoglobin accumulation by a monoclonal antibody to human transferrin receptor. Biochim. Biophys. Acta. 1986;886:203–213. doi: 10.1016/0167-4889(86)90138-2. [DOI] [PubMed] [Google Scholar]
- Gambari R., Fibach E. Medicinal chemistry of fetal hemoglobin inducers for treatment of beta-thalassemia. Curr. Med. Chem. 2007;14:199–212. doi: 10.2174/092986707779313318. [DOI] [PubMed] [Google Scholar]
- Guerrini A., Lampronti I., Bianchi N., Zuccato C., Breveglieri G., Salvatori F., Mancini I., Rossi D., Potenza R., Chiavilli F., Sacchetti G., Gambari R., Borgatti M. Bergamot (Citrus bergamia Risso) fruit extracts as gamma-globin gene expression inducers: phytochemical and functional perspectives. J. Agric. Food Chem. 2009;57:4103–4111. doi: 10.1021/jf803489p. [DOI] [PubMed] [Google Scholar]
- Kohmura K., Miyakawa Y., Kawai Y., Ikeda Y., Kizaki M. Different roles of p38 MAPK and ERK in STI571-induced multi-lineage differentiation of K562 cells. J. Cell. Physiol. 2004;198:370–376. doi: 10.1002/jcp.10426. [DOI] [PubMed] [Google Scholar]
- Harigae H., Okitsu Y., Yokoyama H., Fujiwara T., Inomata M., Takahashi S., Minegishi N., Kaku M., Sasaki T. Induction of erythroid-specific genes by overexpression of GATA-2 in K562 cells. Int. J. Hematol. 2006;84:38–42. doi: 10.1532/IJH97.06020. [DOI] [PubMed] [Google Scholar]
- Ida C., Ogata S., Okumura K., Taguchi H. Induction of differentiation in k562 cell line by nicotinic acid-related compounds. Biosci. Biotechnol. Biochem. 2009;73:79–84. doi: 10.1271/bbb.80483. [DOI] [PubMed] [Google Scholar]
- Jakubowska J., Wasowska-Lukawska M., Czyz M. STI571 and morpholine derivative of doxorubicin collaborate in inhibition of K562 cell proliferation by inducing differentiation and mitochondrial pathway of apoptosis. Eur. J. Pharmacol. 2008;596:41–49. doi: 10.1016/j.ejphar.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Lagoja I.M. Pyrimidine as costituent of natural biologically active compounds. Chem. Biodivers. 2005;2:1–50. doi: 10.1002/cbdv.200490173. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Bianchi N., Zuccato C., Medici A., Bergamini P., Gambari R. Effects on erythroid differentiation of platinum(II) complexes of synthetic bile acid derivatives. Bioorg. Med. Chem. 2006;14:5204–5210. doi: 10.1016/j.bmc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Bianchi N., Borgatti M., Fibach E., Prus E., Gambari R. Accumulation of gamma-globin mRNA in human erythroid cells treated with angelicin. Eur. J. Haematol. 2003;71:189–195. doi: 10.1034/j.1600-0609.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Bianchi N., Zuccato C., Dall'acqua F., Vedaldi D., Viola G., Potenza R., Chiavilli F., Breveglieri G., Borgatti M., Finotti A., Feriotto G., Salvatori F., Gambari R. Increase in gamma-globin mRNA content in human erythroid cells treated with angelicin analogs. Int. J. Hematol. 2009;90:318–327. doi: 10.1007/s12185-009-0422-2. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Martello D., Bianchi N., Borgatti M., Lambertini E., Piva R., Jabbar S., Choudhuri M.S., Khan M.T., Gambari R. In vitro antiproliferative effects on human tumor cell lines of extracts from the Bangladeshi medicinal plant Aegle marmelos Correa. Phytomedicine. 2003;10:1300–1308. doi: 10.1078/094471103322004794. [DOI] [PubMed] [Google Scholar]
- Lozzio C.B., Lozzio B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- Lu J., Li N.S., Koo S.C., Piccirilli J.A. Synthesis of pyridine, pyrimidine and pyridinone C-nucleoside phosphoramidites for probing cytosine function in RNA. J. Org. Chem. 2009;74:8021–8030. doi: 10.1021/jo9016919. [DOI] [PubMed] [Google Scholar]
- Mini E., Coronnello M., Carotti S., Gerli A., Pesciullesi A., Moroson B.A., Mazzei T., Periti P., Bertino J.R. Biochemical modulation of fluoropyrimidines by antifolates and folates in an in vitro model of human leukemia. J. Chemother. 1990;(Suppl. 1):17–27. doi: 10.1080/1120009x.1990.11739000. [DOI] [PubMed] [Google Scholar]
- Olivieri N.F. Reactivation of fetal hemoglobin in patients with beta-thalassemia. Semin. Hematol. 1996;33:24–42. [PubMed] [Google Scholar]
- Osti F., Corradini F.G., Hanau S., Matteuzzi M., Gambari R. Human leukemia K562 cells: induction to erythroid differentiation by guanine, guanosine and guanine nucleotides. Haematologica. 1997;82:395–401. [PubMed] [Google Scholar]
- Puissant A., Grosso S., Jacquel A., Belhacene N., Colosetti P., Cassuto J.P., Auberger P. Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines exhibit high sensitivity to the phytoalexin resveratrol. FASEB J. 2008;22:1894–1904. doi: 10.1096/fj.07-101394. [DOI] [PubMed] [Google Scholar]
- Rabizadeh E., Merkin V., Belyaeva I., Shaklai M., Zimra Y. Pivanex, a histone deacetylase inhibitor, induces changes in BCR-ABL expression and when combined with STI571, acts synergistically in a chronic myelocytic leukemia cell line. Leuk. Res. 2007;31:1115–1123. doi: 10.1016/j.leukres.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Reddick J.J., Saha S., Lee J., Melnick J.S., Perkins J., Begley T.P. The mechanism of action of bacimethrin, a naturally occurring thiamin antimetabolite. Bioorg. Med. Chem. Lett. 2001;11:2245–2248. doi: 10.1016/s0960-894x(01)00373-0. [DOI] [PubMed] [Google Scholar]
- Salvatori F., Cantale V., Breveglieri G., Zuccato C., Finotti A., Bianchi N., Borgatti M., Feriotto G., Destro F., Canella A., Breda L., Rivella S., Gambari R. Development of K562 cell clones expressing beta-globin mRNA carrying the beta039 thalassaemia mutation for the screening of correctors of stop-codon mutations. Biotechnol. Appl. Biochem. 2009;9:41–52. doi: 10.1042/BA20080266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilsky R.L. Methotrexate: an effective agent for treating cancer and building careers. The polyglutamate era. Stem Cells. 1996;14:29–32. doi: 10.1002/stem.140029. [DOI] [PubMed] [Google Scholar]
- Szulawska A., Arkusinska J., Czyz M. Accumulation of gamma-globin mRNA and induction of irreversible erythroid differentiation after treatment of CML cell line K562 with new doxorubicin derivatives. Biochem. Pharmacol. 2007;73:175–184. doi: 10.1016/j.bcp.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Mitchell T., Sariban E., Sabbath K., Griffin J., Kufe D. Effects of 1-beta-d-arabinofuranosylcytosine and phorbol ester on differentiation of human K562 erythroleukemia cells. Mol. Pharmacol. 1985;27:683–688. [PubMed] [Google Scholar]
- Zuccato C., Bianchi N., Borgatti M., Lampronti I., Massei F., Favre C., Gambari R. Everolimus is a potent inducer of erythroid differentiation and gamma-globin gene expression in human erythroid cells. Acta Haematol. 2007;117:168–176. doi: 10.1159/000097465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.