Abstract
Background
Previous studies have shown that thyroxine (T4) is stored as T4 glucuronide (T4G) in the kidney, and that 24 hours after administration of [125I]T4 to mice, 17% of the radioactivity was present in the kidneys, whereas only 4% was found in the liver. The present study was carried out to determine the relative amounts of conjugated and unconjugated T4 and 3,5,3′-triiodothyronine (T3) in the kidney and liver, and whether the conjugated hormones are extracted from tissues using our established extraction protocols, and detected in our radioimmunoassays (RIAs) for T4 and T3.
Methods
Mice were injected with 10 μCi [125I]T4 or [125I]T3 and 24 hours later, the labeled compounds present in serum, kidney, liver, and urine were extracted and analyzed by paper chromatography before and after treatment with β-glucuronidase. In addition, the amounts of endogenous T4 and T3 in extracts of mouse kidney and liver were measured by RIA before and after treatment with β-glucuronidase.
Results
After [125I]T4, more than 95% of the total kidney and liver radioactivity was extracted, and in the kidney, almost all of it was present in a conjugated form, mostly as T4G. The liver also contained T4G, but none was present in serum or urine. T3 glucuronide (T3G) was also found in the kidney and liver after the administration of [125I]T3. Analysis by RIA of the endogenous T4 content in extracts of kidney before and after hydrolysis by β-glucuronidase revealed that a substantial fraction of the T4 in both tissues was present as T4G, and the T4G was not detected in the RIA. Furthermore, the combined T4+T4G content in the kidney expressed per gram of tissue was significantly higher than that in the liver or serum. In contrast, the kidney content of T3+T3G was very low compared with that of T4+T4G.
Conclusions
In summary, we have shown that the kidney stores a significant amount of T4 as T4G. Since T4G deconjugation can occur rapidly in the kidney, it is possible that this tissue participates in maintaining extrathyroidal serum T4 homeostasis.
Introduction
The ability of tissues, especially the liver, to catalyze the conjugation of thyroxine (T4) and 3,5,3′-triiodothyronine (T3) with glucuronic acid has been recognized since 1952, when Taurog et al. reported that the administration of [131I]T4 to rats was followed by the appearance in bile of a compound that they identified as T4 glucuronide (T4G) (1). They subsequently showed that the T4G in bile underwent deconjugation in the intestine, and only unconjugated T4 was excreted in the feces (2).
Evidence that the kidney also forms significant amounts of T4G was provided in 1959 by Galton and Pitt-Rivers, who showed that 72 hours after the injection of 10 μCi [131I]iodide to mice, the major labeled radioactive compound in the kidney was T4G (3). At that time, although it was not technically possible to determine what proportion of the total extrathyroidal T4 was present in the kidney as T4G, on the basis of a comparison of the level of radioactivity present in kidney with that in other tissues, including serum, it appeared to be substantial. The authors, therefore, suggested that the conjugating system is part of a mechanism whereby the kidney participates in the regulation of the serum T4 concentration.
As part of our ongoing studies of thyroid hormone (TH) economy under different conditions, we recently examined the distribution of radioactivity in the entire body and excreta of mice 24 hours after the injection of [125I]T4 or [125I]T3. In the case of T4, it was noted that more than 17% of the injected radioactivity was located in the kidneys, whereas only 4% was found in the liver. In contrast, after the administration of [125I]T3, only 1% and 2% of the radioactivity was found in the kidney and liver, respectively (Galton, unpublished data).
These findings have raised several important questions. First: Is a substantial fraction of the radioactivity found in the kidney after the administration of [125I]T4 present in the form of T4G? Second: What are the relative amounts of T4 and T4G in the mouse kidney? Third: Is T4G extracted along with the TH from tissues using our established TH extraction protocols (4)? Fourth: Does T4G bind to the T4 antibody in our T4 radioimmunoassay (RIA) (4)?
In the present study, we have investigated these issues. We show that not only is most of the T4 in the mouse kidney present as T4G, but also that the kidneys contain considerably more T4 (T4+T4G) per unit weight than is found in either serum or liver. Furthermore, although T4G is extracted from tissue homogenates using our standard extraction protocols, it is not detected in our RIA. Thus, it is likely that in previous studies in which the kidney T4 content had been determined by RIA, the fraction of T4 present as T4G was not included (5–8).
Methods
Animal and treatments
Experiments were performed on 10–12 week old wildtype (WT) mice and mice deficient in the types 1 and 2 5′-deiodinases (D1/D2KO mice). Both genotypes were in the C57Bl/6 background. The mice were bred and housed in the barrier section of the Dartmouth Medical School animal research facility, under conditions of controlled temperature and lighting (12 hours of light and 12 hours of darkness). Animal protocols were approved by the Institutional animal care and use committee.
Two types of experiments were performed. In the first, WT and D1/D2KO mice were injected ip with either [125I]T4 or [125I]T3, ∼10 μCi in 0.1 mL phosphate-buffered saline containing 0.01% bovine serum albumin (BSA). They were then placed in individual cages designed to allow the separate collection of urine and feces, and offered food and water ad libitum. After 24 hours, the mice were euthanized with CO2, and exsanguinated via the inferior vena cava. The serum, kidneys, and liver were harvested and stored at −80°C for subsequent extraction of TH. An aliquot of urine was also stored frozen.
In the second experiment, untreated WT mice were euthanized, and the serum, kidneys, and liver were harvested and stored as just indicated.
Extraction of TH from tissues
Pieces of kidney and liver were weighed and homogenized 1:10 wt/volume in ice-cold 95% methanol containing 10−4 M propylthiouracil (MeOH/PTU). Two 0.5 mL aliquots of each homogenate were transferred to 2.0 mL microfuge tubes, 0.5 mL MeOH/PTU was added, and the mixture was agitated for 10 minutes. After centrifuging at 12,000 g for 10 minutes, the supernatants were transferred to a fresh tube, and the residue was re-extracted with 0.5 mL MeOH/PTU. The pooled supernatants were evaporated to dryness in a rotovac apparatus.
Hydrolysis with β-glucuronidase
The kidney and liver extracts were resuspended in 1 mL of 75 mM sodium phosphate buffer, pH 6.5. For each pair of extracts, β-glucuronidase (Sigma, type 1X-A from Escherichia coli, sulfatase free) was added to one extract (100 units in 10 μL of phosphate buffer), while the second extract received 10 μL buffer. After incubation at 37°C for 1 hour, the mixture was evaporated to dryness in the rotovac. The residue was extracted twice with 0.5 mL aliquots of MeOH/PTU, and the pooled supernatants evaporated to dryness.
Chromatographic analysis of TH
Extracts of tissues prepared from mice treated with [125I]T4 or [125I]T3 were resuspended in ∼50 μL methanol/ammonia 3:1, and the labeled compounds present were separated by paper chromatography using tertiary amyl alcohol/2 N NH4OH as the solvent system (3). The locations of the compounds on the strips were determined by exposing the strips to X-ray film. Urine and serum samples were chromatographed without extraction.
RIA of TH
The contents of T4 and T3 in the liver and kidney extracts prepared from untreated WT mice were determined using our highly sensitive nonequilibrium RIA procedures previously described (9). The T4 and T3 antibodies were obtained from a commercial source (Fitzgerald Industries International, Inc.; T3, catalog no. 20-TR45, cross-reactivity with T4, 0.38%; T4, catalog no. 20-TR40, cross-reactivity with T3, 7.5%). Briefly, the RIA buffer consisted of 0.2 M glycine, 0.13 M sodium acetate (pH 8.6), containing 0.02% BSA. The dried tissue extracts were dissolved in 2 mL of RIA buffer, and incubation was carried out for 5 days at 4°C. Preliminary tests indicated that up to 20 μL of sample could be assayed before linearity with the standard curve was lost. Ten microliters of the samples were used in the assay. A combined polyethylene glycol/second antibody separation step was employed. Assay sensitivity was ∼2 pg/tube for T3 and 4 pg/tube for T4. Total T4 and T3 concentrations in serum were determined using the Coat-A-Count® RIA total T4 and total T3 kits (Diagnostic Systems Laboratories, Inc.).
Statistical analysis
Data are expressed as mean±SE, and values between two groups of mice were compared using Student's t-test. Statistical significance was defined as p<0.05.
Results
Identification of [125I]T4G in kidney
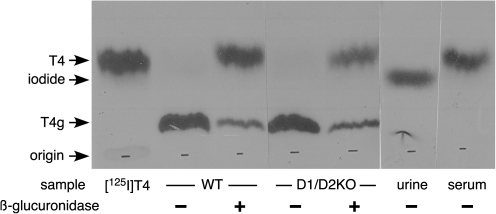
When pieces of kidney and liver obtained from WT and D1/D2KO mice 24 hours after the injection of [125I]T4 were subjected to the TH extraction procedure, it was found that at least 95% of the total radioactivity in both tissues was present in the dried extracts. The labeled compounds present in the extract before and after hydrolysis by β-glucuronidase as determined by paper chromatography are shown in Figure 1. In both genotypes, almost all the radioactivity in the unhydrolyzed extract was located in a single band with a relatively low mobility in the solvent system employed. This is where T4G would be expected (3). After hydrolysis with β-glucuronidase, the level of radioactivity in this band was greatly reduced, and the majority of it now migrated in parallel with the T4 marker, indicating that the vast majority of the T4 in the kidney was in fact in the form of T4G. The kidney is known to contain a high level of D1 activity (10). However, the similarity between the results obtained in the WT and D1/D2KO kidney suggests that the presence of this enzyme has little, if any, influence on the extent of T4G formation.
FIG. 1.
Identification of thyroxine glucuronide (T4G) in kidney extracts prepared from wild type and D1/D2KO mice. Extracts prepared from mice treated for 24 hours with [125I]T4 were incubated with and without β-glucuronidase, and the radioactive compounds present were separated by paper chromatography using tertiary amyl alcohol/2 N NH4OH as the solvent system. The X-ray film of these strips and also two additional strips prepared with urine and serum from these mice is shown, and the locations for T4 and iodide are indicated.
No T4G could be detected in either urine or serum of WT mice; iodide was the only detectable radioactive compound in urine, and essentially all of the radioactivity in serum was T4 (Fig. 1).
Since the liver extracts contained relatively low levels of radioactivity, satisfactory analysis of the radioactive compounds present was limited. However, after several weeks of exposure to X-ray film, very faint bands were just visible in the locations of both T4G and T4 in the liver preparation.
Similar studies with [125I]T3 indicated that at least 95% of the total radioactivity in the liver and kidney was extracted by our protocol. However, chromatographic analysis was not carried out, because the levels of radioactivity present in the extracts were too low for accurate determination of the labeled components present.
Identification of endogenous T4G in kidney and liver
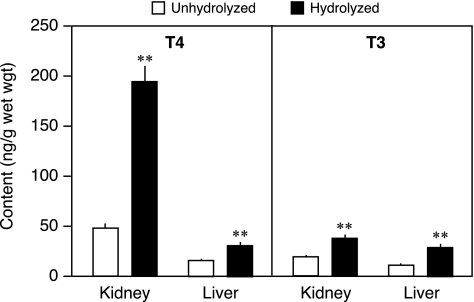
The TH in kidney and liver of WT mice was extracted, and the T4 and T3 present in the extracts before and after hydrolysis by β-glucuronidase were determined by RIA. As shown in Figure 2, a substantial fraction of the T4 in both the kidney and liver was present in the form of T4G, most, if not all, of which was not detected in the RIA. It is also notable that the total T4 content in the kidney (T4+T4G) was almost 200 ng/g wet wt. This was approximately fivefold the content in the liver. These two findings have been confirmed in several subsequent experiments.
FIG. 2.
The T4 and triiodothyronine (T3) contents in liver and kidney from adult WT mice before and after exposure to β-glucuronidase. Bars indicate the mean±SE of 9–12 mice/group. **p<0.001.
Similar studies were performed to determine the level of T3G present in kidney and liver. In both tissues, some T3G was detected, but whereas in the liver, the T3 and T3G contents were comparable to the T4 and T4G contents; it is notable that in the kidney the content of T3+T3G was very low compared with that of T4+T4G (Fig. 2).
Serum T4 and T3 levels in the mice used in this study were 27±2.0 and 1.12±0.044 ng/mL respectively.
Discussion
The results of this study indicate that the majority of the T4 in the mouse kidney is present in the conjugated form, primarily as T4G. However, in addition to conjugation with glucuronic acid, iodothyronines can also be conjugated with sulfate in several tissues, including kidney and liver. The sulfate conjugates of T4 and T3 are excellent substrates for the inner-ring deiodinase activity of the type 1 deiodinase, and, thus, sulfation of the TH is thought to be an important step in their inactivation (11,12). However, for several reasons, it is unlikely that a significant fraction of the T4 conjugate identified herein as T4G is T4 sulfate. First, the β-glucuronidase employed in this study is claimed to be essentially free of sulfatases. Second, T4 sulfate appears to be a very poor substrate for hydrolysis by known sulfatases (13,14). Third, T4 sulfate is readily hydrolyzed by heating in 1 N HCl at 80°C for 1 hour (13), and we have previously shown that heating the extracted kidney T4 conjugate in 2 N HCl at 100°C for 18 hours did not release the T4 (3).
The liver is widely believed to be the primary site of TH conjugation, although the process is known to occur also in other tissues, including the kidney (3,12). Thus, the present finding that the concentration of T4G in the kidney is very much higher than that in the liver was unexpected. The difference in T4G content in the kidney and liver may be due at least in part to the fact that the T4G formed in the liver is secreted into the bile and then into the intestine (1). Indeed, after the injection of [125I]T4, the most abundant labeled compound in bile was T4G (15,16). In contrast, the available data suggest that little if any of the T4G produced in the kidney is released into either the serum or the urine in this form. Thus, in the earlier study in which endogenous TH was labeled with [131I]iodide, no [131I]T4G was detected in either the urine or serum (3). Similarly, in the present study, no [125I]T4G was detected in the urine or serum of mice after the administration of [125I]T4.
The fact that the content of T4G in the kidney in much higher than in the liver does not necessarily mean that the rate of T4G formation is higher in the kidney than in the liver. To determine this, a detailed study of the dynamics of T4 conjugation and deconjugation in the two tissues would be required. However, the finding suggests that the purpose of T4 conjugation in the two tissues is somewhat different. In the liver, T4 conjugation is believed to be a process that precedes and perhaps facilitates the elimination of the T4 in the bile and feces (12). In the kidney, however, although the conjugation process per se is possibly an inactivating process, the T4G is not eliminated in this form, and enough of it is stored in the kidney such that the total amount of T4 (T4+T4G) is several-fold higher than that in the liver, and presumably in other tissues too. Furthermore, since the mean serum T4 concentration in the WT mice was only 27 ng/mL, it appears that the kidney T4+T4G concentration, expressed per gram of wet tissue weight, is actually considerably higher than that in the serum.
What is the role of the T4G stored in the kidney? In the 1959 report, it was suggested that the conjugating system in the kidney is a part of a mechanism whereby the kidney assists in the regulation of the serum T4 level (3). At that time, aside from the knowledge that T4G could be deconjugated in the intestinal lumen, the way in which T4G is metabolized was unknown. However, Hayes has recently reported that within 15 minutes of an intravenous injection of synthetic [125I]T4G, significant deconjugation is evident in the kidney and to a much lesser extent in the liver; in the kidneys, almost 80% of the total [125I]T4 ([125I]T4+[125I]T4G) present was in the form of T4, whereas in the liver, all but 10% remained in the form of T4G (17). These findings strongly suggest that the kidney is also a major site of deconjugation of T4G, and lend credence to the original suggestion that the T4G sequestered in the kidney provides a readily available source of T4 that can assist in maintaining the serum T4 level.
Hayes also reported that little if any T4G was formed in the short 15 minutes interval after the injection of [131I]T4 (17), a finding which suggests that the rate of deconjugation is greater than that of conjugation. This conclusion is incompatible with our finding that most of the endogenous T4 in the kidney is in the form of T4G, or that 24 hours after the injection of [125I]T4, the major labeled compound in the kidney is T4G. However, it should be noted that the deconjugation data were obtained after the injection of [125I]T4G, and the kidney glucuronidase may be more readily accessible to T4G obtained directly from the serum, than to the T4G formed locally from T4 in the kidney. In the latter case, it may be stored such that it cannot be inappropriately deconjugated, perhaps in a cell that either does not contain or has a relatively low level of the deconjugating enzyme.
In summary, we have shown that the majority of the T4 in the mouse kidney is present as T4G, a form that escapes measurement in an RIA. Furthermore, the T4+T4G content per unit weight in the kidney is much higher than in other tissues including the serum and constitutes a significant fraction of the total extrathyroidal T4. These factors have not been noted in previous studies of kidney T4 content as determined by RIA, with the result that the substantial amount of T4G present in the kidney appears to have remained undetected (5–8). This high kidney T4+T4G content, together with the knowledge that T4G deconjugation can occur rapidly in the kidney, raises the possibility that the kidney participates in maintaining extrathyroidal serum T4 homeostasis.
Acknowledgment
This work was supported by USPHS Grant # DK 079946.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Taurog A. Briggs FN. Chaikoff IL. I131-labeled L-Thyroxine. II. nature of the excretion product in bile. J Biol Chem. 1951;194:655–682. [PubMed] [Google Scholar]
- 2.Taurog A. Conjugation and excretion of the hormone. Brookhaven Symp Biol. 1955;7:111–136. [PubMed] [Google Scholar]
- 3.Galton VA. Pitt-Rivers R. Thyroid hormone metabolism in the kidney. Biochem J. 1959;72:314–318. doi: 10.1042/bj0720314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galton VA. Wood ET. St. Germain EA. Withrow C-A. Aldrich G. St. Germain GM. Clark AS. St. Germain DL. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient brain during development. Endocrinology. 2007;148:3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- 5.Escobar-Morreale H. Obregón MJ. Escobar del Rey F. Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palha JA. Hays MT. Morreale de Escobar G. Episkopou V. Gottesman ME. Saraiva MJ. Transthyretin is not essential for thyroxine to reach the brain and other tissues in transthyretin-null mice. Am J Phsiol. 1997;272:E485–E493. doi: 10.1152/ajpendo.1997.272.3.E485. [DOI] [PubMed] [Google Scholar]
- 7.Pedraza PE. Obregon MJ. Escobar-Morreale HF. Escobar del Rey F. Morreale de Escobar G. Mechanisms of adaptation to iodine deficiency in rats: thyroid status is tissue specific. Its relevance for man. Endocrinology. 2006;147:2098–2108. doi: 10.1210/en.2005-1325. [DOI] [PubMed] [Google Scholar]
- 8.Trajkovic-Arsic M. Visser TJ. Darras VM. Friesema ECH. Schlott B. Mittag J. Bauer K. Heuer H. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology. 2010;151:802–809. doi: 10.1210/en.2009-1053. [DOI] [PubMed] [Google Scholar]
- 9.St. Germain DL. Galton VA. Comparative study of pituitary-thyroid hormone economy in fasting and hypothyroid rats. J Clin Invest. 1985;75:679–688. doi: 10.1172/JCI111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonard JL. Biochemical basis of thyroid hormone deiodination. In: Wu S, editor. Thyroid Hormone Metabolism. Regulation and Clinical Implications. Blackwell Scientific Publications; Boston: 1991. pp. 1–28. [Google Scholar]
- 11.Visser TJ. Role of sulfation in thyroid hormone metabolism. Chem Biol Interact. 1994;92:293–303. doi: 10.1016/0009-2797(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 12.Wu S-Y. Green WL. Huang W-S. Hays MT. Chopra IJ. Alternate pathways of thyroid hormone metabolism. Thyroid. 2005;15:943–958. doi: 10.1089/thy.2005.15.943. [DOI] [PubMed] [Google Scholar]
- 13.Mol JA. Visser TJ. Synthesis and some properties of sulfate esters and sulfamates of iodothyronines. Endocrinology. 1985;117:1–7. doi: 10.1210/endo-117-1-1. [DOI] [PubMed] [Google Scholar]
- 14.Kester MHA. Kapstein E. van Dijk CH. Roest TJ. Tibboel D. Coughtrie MWH. Visser TJ. Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology. 2002;143:814–819. doi: 10.1210/endo.143.3.8686. [DOI] [PubMed] [Google Scholar]
- 15.Bastomsky CH. Papapetrou PD. The effect of methylcholanthrene on biliary thyroxine excretion in normal and gunn rats. J Endocrinol. 1973;56:267–273. doi: 10.1677/joe.0.0560267. [DOI] [PubMed] [Google Scholar]
- 16.Rutgers M. Pigmans IG. Bonthuis F. Docter R. Visser TJ. Effects of propylthiouracil on the biliary clearance of thyroxine (T4) in rats: decreased excretion of 3,5,3′-triiodothyronine glucuronide and increased excretion of 3,3′,5′-triiodothyronine glucuronide and T4 sulfate. Endocrinology. 1989;125:2175–2186. doi: 10.1210/endo-125-4-2175. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MT. Deconjugation of thyroxine glucuronide by the rat kidney. Thyroid. 2009;19:1001–1004. doi: 10.1089/thy.2008.0375. [DOI] [PubMed] [Google Scholar]