Abstract
Chronic hepatitis B is characterized by an impaired immune response to hepatitis B virus (HBV). Telbivudine treatment has significantly improved the clinical outcome of chronic HBV infection. However, the underlying mechanism behind the antiviral response of patients treated with nucleoside analogs remains unclear. To gather more evidence about the mechanism responsible for the weak immune response, in this study we analyzed the effects on HBV viral load of treatment with the nucleoside analogue telbivudine and the percentage of Tregs, programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) expression, and related cytokine production. Peripheral blood mononuclear cells (PBMCs) and serum of 28 patients with chronic hepatitis B were collected at baseline, and 3 mo and 6 mo after therapy was begun. In parallel with the decline in viral load and serum ALT normalization, we found a decline in circulating CD4+CD25high Tregs, PD-L1 on CD4+ T cells, and IL-9 production. The expression of PD-1 on CD4+ T cells and the production of IFN-γ did not increase during therapy. Our findings suggest that the antiviral effect of the nucleoside analogs may be attributable not only to their direct effect on virus suppression, but also to their immunoregulatory capabilities.
Introduction
Hepatitis B virus (HBV) infection is still a global public health problem, with an estimated 400 million chronic HBV carriers all over the world (14). Chronic hepatitis B (CHB) always results in HBV-related liver failure, cirrhosis, and hepatocellular carcinoma, leading to about 1 million deaths annually. In China, the hepatitis B surface antigen (HBsAg) carrier rate was 7.18% based on a seroepidemiological survey done in 2006. There were approximately 93 million HBV carriers, and among them 30 million were patients with CHB (15,29).
In patients with acute self-limited HBV infection, multispecific CD4+ T helper (Th) cell and CD8+ cytotoxic T lymphocyte (CTL) responses with interferon-γ (IFN-γ) production are important for control of the infection. In contrast, cellular immune responses are weak or undetectable in CHB patients, which leads to a state of relative collapse of HBV-specific adaptive immunity (2,9). However, the precise mechanism responsible for T-cell hyporesponsiveness or tolerance in CHB is still not completely understood. In recent years, negative immunoregulatory cells and molecules have raised interest in immunological research of HBV infection, especially CD4+CD25high regulatory T cells (Tregs) and the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway. Patients with chronic HBV infection have increased percentages of Tregs in their peripheral blood (25,32) and liver (31,32). HBV specificity of CD4+CD25+ Tregs and CD4+CD25+ depletion reveal enhanced proliferation of the remaining peripheral blood mononuclear cells (PBMCs) in response to HBV, supporting the role of Tregs in HBV persistence (8,25,35). PD-1/PD-L1 also represents a mechanism of T-cell dysfunction in HBV persistence. PD-1 is upregulated in virus-specific T cells in peripheral blood (21) and liver (4), leading to the impairment of T cells. Anti-HBV peripheral and intrahepatic T-cell responses can be restored by blockade of PD-1/PD-L1 interactions, which may increase CD8+ T-cell proliferation and production of interferon-γ (IFN-γ) and interleukin (IL)-2 (7). Moreover, upregulation of circulating PD-1/PD-L1 is associated with a poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma (33). Our previous work also demonstrated the increased frequency of CD4+CD25high Tregs and PD-1 expression on CD4+ T lymphocytes, which may inhibit the cellular immune response and affect viral clearance, leading to chronic HBV infection (18,35).
The high viral load present in peripheral blood of HBV patients could provide a stimulus to sustain the function of Tregs and the PD-1/PD-L1 pathway. To gain further understanding of the role of Tregs and PD-1/PD-L1 in chronic HBV infection, we longitudinally investigated the relationship between Tregs, PD-1/PD-L1 expression, and viral load in patients with chronic hepatitis B undergoing treatment with telbivudine (β-L-2′-deoxythymidine), which is an L-nucleoside with potent and specific antiviral activity against HBV.
Materials and Methods
Patients
Twenty-eight CHB patients (20 males and 8 females; average age 29.01±1.77 y; HBV DNA 7.686±0.285 log10 copies/mL; alanine aminotransferase [ALT] 117.92±10.61 U/L), including 23 who were HBV envelope antigen (HBeAg)-positive and 5 were HBeAg-negative, were enrolled in the study. All patients were followed-up in the Center for Infectious Diseases, Tangdu Hospital. CHB diagnoses were made according to the diagnostic standards of the Chinese National Program for Prevention and Treatment of Viral Hepatitis. Patients co-infected with human immunodeficiency virus (HIV) or other hepatitis viruses were excluded from the study. In addition, patients who were pregnant or had received antiviral, immunosuppressive, or immunomodulatory treatment during the last year were also excluded. The study was approved by the ethics committee of Tangdu Hospital, and all participants gave informed consent before sampling. All patients took telbivudine 600-mg tablets (Novartis, Beijing, China) orally once daily in the morning. There was no restriction regarding food intake. The investigations were focused on three time points, with EDTA anticoagulant blood used for the separation of PBMCs obtained serially from all patients at baseline and 3 and 6 mo after antiviral treatment was begun.
Virological assessment
HBsAg, HBeAg, and anti-HBe antibody levels of the patients were determined using commercial enzyme immunoassay kits (Kehua Biotech, Shanghai, China). Serum HBV DNA was quantified by a commercial real-time PCR kit (PG Biotech, Shenzhen, China) according to the manufacturer's instructions. The HBV DNA detection threshold was 291 copies/mL.
Isolation of PBMCs
PBMCs were isolated from 20 mL of fresh EDTA anticoagulant blood by Ficoll-Hypaque density gradient centrifugation (Sigma-Aldrich, St. Louis, MO). After isolation, the PBMCs were immediately cryopreserved in medium containing 90% heat-inactivated fetal bovine serum (FBS) and 10% dimethylsulfoxide (DMSO).
Flow cytometry analysis
The PBMCs were thawed using a step-by-step gradual dilution method, and cultured (106/well) in 24-well plates in two different wells at 37°C in a 5% CO2 environment overnight. Cells were harvested by centrifugation at 300×g for 10 min at 4°C and washed with 2 mL of phosphate-buffered saline (PBS) with 1% FBS. The following antibodies were used for staining: anti-CD4-FITC (eBioscience, San Diego, CA), anti-CD25-PE-Cy5 (eBioscience), anti-PD-1-APC (eBioscience), anti-PD-L1-PE (eBioscience); anti-CD14-FITC (eBioscience), anti-TLR2-APC (eBioscience), and anti-TLR4-PE (eBioscience). The PBMCs were incubated and stained at 4°C in the dark for 30 min, and then were analyzed with a four-color FACSCalibur analyzer (BD Biosciences Immunocytometry Systems). Acquisitions were performed with CellQuest Pro software (BD Biosciences Immunocytometry Systems, San Jose, CA), and analyses were performed with FlowJo version 5.7.2 for Windows (Tree Star Inc., Ashland, OR). Isotype control antibodies were used to separate positive and negative cells in the FITC, PE-Cy5, PE, and APC fluorescence channels.
Cytokine measurements
Serum concentrations of IFN-γ and IL-9 were measured with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Genway Biotech, San Diego, CA) according to the manufacturer's instructions.
Statistical analysis
All data were analyzed using SPSS version 13.0 for Windows. The Wilcoxon matched pairs test was used to perform the comparison between groups. Pearson correlation tests were performed for correlation analysis. All tests were two-tailed, and p values <0.05 were considered significant.
Results
HBV DNA decreased and ALT levels normalized during telbivudine treatment
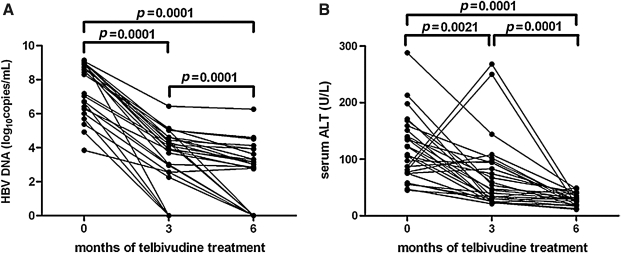
Serum HBV DNA and ALT levels were determined at baseline, as well as after 3 and 6 mo of telbivudine treatment. The reduction in serum HBV DNA from baseline to 6 mo was 5.1 log10 (baseline, 7.69±0.29 log10 copies/mL; 3 months, 3.71±0.25 log10 copies/mL; 6 months, 2.54±0.31 log10 copies/mL; Fig. 1A). After 3 mo of therapy, 4 patients achieved HBV DNA levels below the limit of detection. After 6 mo of treatment, HBV DNA was undetectable in 10 (35.71%) patients, and surprisingly, ALT levels were normalized in all 28 (100%) patients (baseline, 117.90±10.61 U/L; 3 mo, 69.61±11.87 U/L; 6 mo, 33.07±3.562 U/L; Fig. 1B). Two patients had seroconversion from HBeAg to anti-HBe during treatment. No patients exhibited loss of serum HBsAg.
FIG. 1.
HBV DNA decreased and ALT levels normalized during telbivudine treatment. (A) The serum HBV DNA levels of the 28 patients at baseline, after 3 mo, and after 6 mo of telbivudine therapy. (B) The serum ALT levels of the 28 patients at baseline, after 3 mo, and after 6 mo of telbivudine therapy. HBV DNA and ALT levels were significantly lower at 3 and 6 mo compared to baseline (p<0.05).
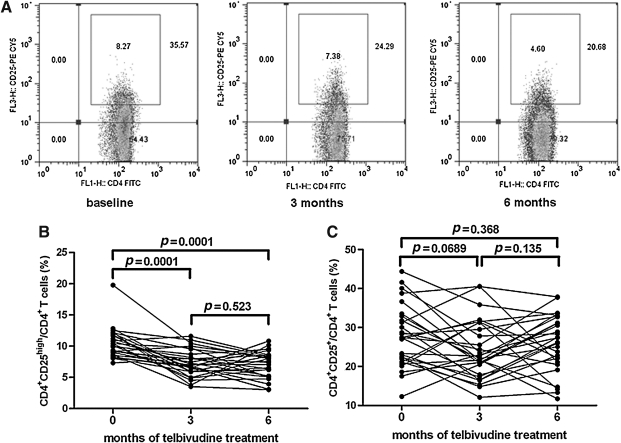
The percentage of peripheral CD4+CD25high Tregs decreased during telbivudine-induced viral load reduction
To assess the effect of treatment with telbivudine on the immune response, we first determined the percentage of Tregs from cells that stained positive for CD4. Tregs were defined as the percentage of cells positive for CD4 and CD25high divided by the proportion of CD4-positive cells (Fig. 2A). There was a rapid reduction in CD4+CD25high Tregs after 3 mo of treatment. Also, we found a notable decrease in the percentage of Tregs after 3 and 6 mo of therapy compared to baseline (p<0.0001), but there was no significant difference in Treg proportions between 3 and 6 mo (baseline, 10.43±0.45%; 3 mo, 7.31±0.40%; 6 mo, 7.09±0.40%; Fig. 2B). Moreover, the frequency of CD4+CD25+ Tregs in CD4+ T cells was 27.95±1.55% at baseline, 24.03±1.50% at 3 mo, and 25.69±1.43% at 6 mo. No changes were seen in the CD4+CD25+ Treg population in response to antiviral therapy (Fig. 2C). Furthermore, no correlation was observed between the decrease in HBV DNA and the proportion of CD4+CD25high Tregs (data not shown).
FIG. 2.
The frequency of peripheral CD4+CD25high regulatory T cells (Tregs) during telbivudine-induced viral load reduction. (A) CD4+ T cells were separated into CD25high, CD25low, and CD25− T-cell subsets, as defined by the fluorescence intensity of CD25 obtained using isotypic control antibody. Typical PBMC samples from one of the patients were analyzed with flow cytometry at baseline, and after 3 mo and 6 mo of telbivudine treatment. The percentages of CD4+CD25high Tregs (B) and CD4+CD25+ Tregs (C) in the 28 CHB patients are shown.
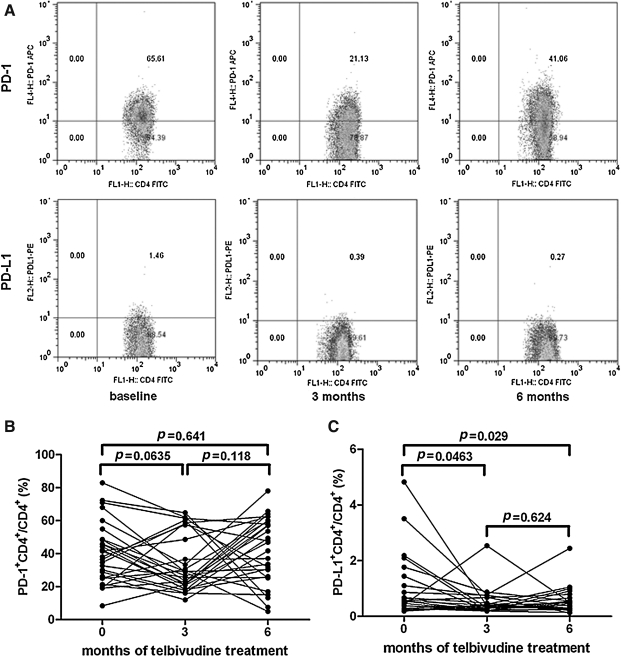
Downregulation of PD-L1, but not PD-1, expression on CD4+ T cells during telbivudine-induced viral load reduction
CD4+PD-1+ and CD4+PD-L1+ cells were determined at baseline, and after 3 and 6 mo of therapy. A representative PBMC sample analyzed by flow cytometry is shown in Fig. 3A. There were significant differences in the percentages of PD-1 in CD4+ T cells at baseline (42.25±4.39%), and after 3 (33.67±3.35%), and 6 mo (43.50±11.93%) of telbivudine treatment (Fig. 3B). Surprisingly, there was a consistent trend toward downregulated PD-L1 levels after therapy (baseline, 0.93±0.22%; 3 mo, 0.53±0.09%; 6 mo, 0.48±0.09%), and these differences achieved statistical significance compared with baseline (p=0.0463, p=0.029; Fig. 3C).
FIG. 3.
PD-1 and PD-L1 expression in circulating CD4+ T cells in patients with chronic hepatitis B during telbivudine therapy. (A) Surface expression of PD-1 and PD-L1 on CD4+ T cells in a representative chronic hepatitis B patient at baseline, and after 3 and 6 mo of telbivudine treatment. (B) Percentage of CD4+PD-1+ T cells within CD4+ T cells in patients during telbivudine therapy. (C) Percentage of CD4+PD-L1+ T cells in CD4+ T cells in CHB patients during telbivudine therapy.
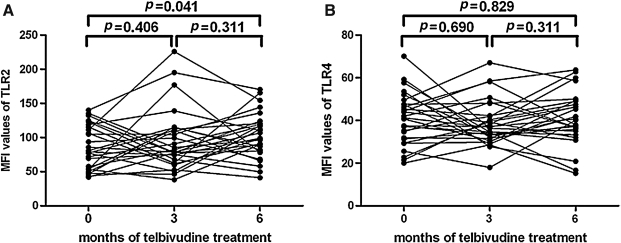
Longitudinal analysis of TLR2/4 on monocytes in response to telbivudine therapy
We gated CD14-positive populations as monocytes, and the levels of TLR2/4 expression were measured in these cells as we did previously (35). The values of mean fluorescence intensity (MFI) of TLR2/4 on monocytes of CHB patients were tested before and after treatment. There was slightly increased TLR2 expression after 6 mo of therapy (102.93±6.63) compared to baseline (89.07±6.09; p=0.041), but not after 3 mo of treatment (96.12±8.80, p<0.05; Fig. 4A). However, the MFI values corresponding to TLR4 were not affected (baseline, 41.17±2.39; 3 mo, 38.20±2.12; 6 mo 40.48±2.37, p>0.05; Fig. 4B).
FIG. 4.
TLR2 and TLR4 expression on monocytes in response to telbivudine therapy. (A) The expression of TLR2 in the 28 patients at baseline, and after 3 and 6 mo of telbivudine therapy. (B) The expression of TLR4 in the 28 patients at baseline, and after 3 and 6 mo of telbivudine therapy.
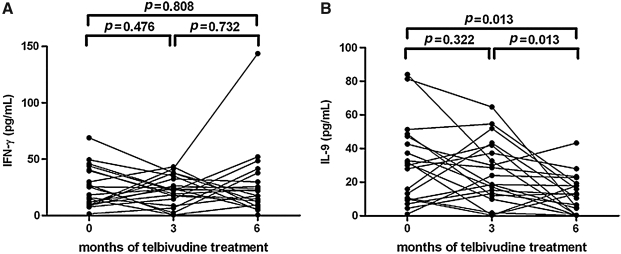
The serum concentrations of IFN-γ and IL-9 in response to telbivudine therapy
The IFN-γ and IL-9 levels in serum were tested at baseline, as well as after 3 and 6 mo of treatment. The concentrations of IFN-γ were 24.49±3.86 pg/mL at baseline, 21.86±2.87 pg/mL at 3 mo, and 27.28±6.58 pg/mL at 6 mo, and there was no notable difference among the three groups (Fig. 5A). In addition, IL-9 concentrations were significantly reduced after 6 mo of treatment (12.76±2.52 pg/mL), compared with both baseline (30.89±5.28 pg/mL; p=0.013) and 3 mo post-therapy (25.80±4.16 pg/mL, p=0.013; Fig. 5B).
FIG. 5.
IFN-γ and IL-9 concentrations in the serum during telbivudine treatment. (A) Serum IFN-γ concentrations in the 28 patients at baseline, and after 3 and 6 mo of telbivudine therapy. (B) Serum IL-9 concentrations in the 28 patients at baseline, and after 3 and 6 mo of telbivudine therapy.
Discussion
Standard antiviral treatment with nucleoside analogs has remarkably ameliorated the clinical management of chronic hepatitis B, which is one of the major health problems in China. Clinical trials in China and globally in both HBeAg-positive and HBeAg-negative chronic hepatitis B patients have demonstrated that telbivudine therapy, which provides greater antiviral and clinical efficacy with less viral resistance, is superior to lamivudine treatment (10,13). In this study, we examined telbivudine treatment of chronic HBV patients in China and showed that after 6 mo of therapy, 32.41% (9/28) of patients demonstrated seroclearance of HBV DNA, and 100% (28/28) had normalization of serum ALT levels. These results are consistent with earlier reports (10,13), and further prove that telbivudine is a safe and effective therapeutic agent against chronic hepatitis B.
Tregs are of the utmost importance in maintaining immune homeostasis and a strong immunological inhibitory effect (17). Ours and many other groups have demonstrated that Tregs are elevated in CHB patients, and that they contribute to the dysfunction of HBV-specific CTL, causing immunotolerance, viral persistence, and chronic infection (1,8,11,12,18,20,25,31,32,35). Herein our findings show that telbivudine-induced suppression of HBV DNA replication resulted in a decrease in the frequency of circulating Tregs within 3 mo, and consistently inhibited them after 6 mo of therapy. Although we failed to observe any correlation between the decrease in viral loads and Tregs, the results are still consistent with previously published data indicating that anti-HBV treatment with nucleoside analogs or pegylated IFN-α can cause a decrease in Tregs (24,26,34). Due to the mechanism of action of telbivudine, which suppresses the activity of HBV DNA polymerase and directly inhibits viral replication, it seems likely that treatment results in a notable decline in HBV DNA levels, which in turn leads to a change in the cytokine and chemokine environment, which may result in a decrease in Tregs.
Recent studies have demonstrated that there is overexpression of PD-1 and PD-L1 in both HBV-specific T cells and PBMCs (3,18), and that blocking the PD-1/PD-L1 pathway can increase HBV-specific T-cell cytokine secretion and cell proliferation (3). Similarly, in an HBV transgenic mice model, in vivo blocking of the PD-1/PD-L1 interaction resulted in an increase in the absolute number of IFN-γ-producing CTLs in the liver (16). In the current study, we found that there was a trend toward PD-L1 suppression, but not PD-1 suppression, in CD4+ T cells in response to telbivudine therapy. This may indicate that inhibition of viral replication may block the PD-1/PD-L1 inhibitory pathway. Previous studies have demonstrated that blockade of the PD-1/PD-L1 interaction may improve the function of circulating HBV-specific CD8+ T cells, which may partially reverse T-cell depletion and cause a restoration of T-cell function (3,7,16). It is well known that persistent exposure to high antigen concentrations promotes HBV-specific T-cell dysfunction. Telbivudine directly inhibits viral replication and concomitantly downregulates the levels of HBsAg and HBeAg, and thus may activate antiviral T-cell functioning. Suppression of the PD-1/PD-L1 pathway may further improve T-cell function. However, in natural infection, peripheral cells may only partially reflect their intrahepatic counterparts. Others also found that upregulation of intrahepatic PD-1/PD-L1 was significantly associated with hepatic inflammation and ALT elevations in patients with chronic HBV infection (5,30). Moreover, the function of liver-infiltrating HBV-specific T cells can be ameliorated by PD-1/PD-L1 inhibition (7). Taken together, these findings indicate that T-cell exhaustion by high antigen concentrations promotes HBV-specific T-cell dysfunction by affecting both the phenotype and function of peripheral and intrahepatic T cells.
Inhibition of Tregs and/or the PD-1/PD-L1 pathway may also lead to changes in the expression profile of cytokines and chemokines. Depletion of Tregs led to an increase in IFN-γ production by HBV-Ag-stimulated PBMCs (31). In addition, PD-1/PD-L1 blockade increased CD8+ cell proliferation and IFN-γ and IL-2 production by intrahepatic lymphocytes, and induced variable levels of functional T-cell restoration both in the liver and in peripheral blood, with functional improvement of intrahepatic T cells (7,16). Our findings of the slightly increased expression of IFN-γ 6 mo after therapy, with no statistically significant difference compared with baseline, may indicate that the impaired immune responses of CHB patients are not restored by telbivudine therapy. Surprisingly, the levels of IL-9, which plays an important role in inflammatory diseases (19,27), were decreased in serum by telbivudine therapy. As we learn more about the role of FoxP3+ Tregs in apoptosis-induced liver inflammation (23), it is likely that the changes seen in Tregs and PD-L1 expression in peripheral blood after antiviral treatment with telbivudine reflect improvements in liver inflammation.
We also investigated the changes in TLR2/4, which are essential pathogen-associated molecular elements of the innate immune response, during telbivudine treatment. Increasing evidence suggests that TLR2/4 may play a key role in the recognition and initiation of HBV infection (6,28,35). However, controversy remains about the numbers and functions of TLR2/4 in the immune response to HBV infection. We found that TLR2 expression on CD14+ monocytes was slightly increased during telbivudine therapy. Due to the high efficacy of anti-HBV function and e antigen seroconversion caused by telbivudine, we presume that the upregulation of TLR2 may be correlated with the reductions seen in HBeAg during treatment. Further studies are needed to clarify the mechanisms underlying the immune functions of these molecules.
Ribeiro et al. (22) demonstrated that the clearance of HBV DNA and infected hepatocytes was significantly faster in HBeAg-negative than in HBeAg-positive patients. Also, in HBeAg-negative patients, the baseline levels of HBV DNA showed a negative correlation with the half-life of infected cells. This advances the notion that HBeAg-positive and HBeAg-negative chronic HBV infections are very different in terms of immune status, hepatocyte turnover, and viral kinetics under antiviral treatment. Thus our findings of the reductions in HBV DNA and ALT normalization, as well as downregulation of Tregs and PD-L1, may also be different in these two populations. Thus we analyzed the changes in HBV DNA, ALT, IL-9, Tregs, PD-1, and PD-L1 in 23 HBeAg-positive patients after telbivudine treatment. The results were similar for the mixed population of HBeAg-positive and HBeAg-negative patients (data not shown), and revealed that these negative immunomodulatory factors may have identical functions in both HBeAg-positive and HBeAg-negative infections. However, as the sample size in our study was small, further studies are needed to confirm these results in larger study samples.
Conclusion
In summary, the HBV viral load reduction and serum ALT normalization induced by treatment with telbivudine results in a partial restoration of the impaired immune response, as shown by significant reductions in the percentages of CD4+CD25high Tregs, PD-L1 expression on CD4+ T cells, and IL-9 production. Our findings suggest that the antiviral effects of the nucleoside analogs may be attributable not only to their direct effect on viral suppression, but also to their immunoregulatory capabilities.
Author Disclosure Statement
No competing financial interests exist.
Acknowledgments
We thank the volunteers who generously participated in this study. This work was supported by grants from the National Key Technologies Research and Development Program of China (2008ZX10002-004, 2008ZX10002-006, 2008ZX10002-008, 2009ZX10002-027, and 2012ZX10002-007-001-006).
References
- 1.Barboza L. Salmen S. Goncalves L, et al. Antigen-induced regulatory T cells in HBV chronically infected patients. Virology. 2007;368:41–49. doi: 10.1016/j.virol.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Bertoletti A. Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr Opin Microbiol. 2000;3:387–392. doi: 10.1016/s1369-5274(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 3.Boni C. Fisicaro P. Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J. Wang XM. Wu XJ. Wang Y. Zhao H. Shen B. Wang GQ. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm Res. 2010;60:47–53. doi: 10.1007/s00011-010-0233-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen J. Wang XM. Wu XJ. Wang Y. Zhao H. Shen B. Wang GQ. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm Res. 2011;60:47–53. doi: 10.1007/s00011-010-0233-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z. Cheng Y. Xu Y, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Fisicaro P. Valdatta C. Massari M, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693. doi: 10.1053/j.gastro.2009.09.052. 693 e1–e4. [DOI] [PubMed] [Google Scholar]
- 8.Franzese O. Kennedy PT. Gehring AJ. Gotto J. Williams R. Maini MK. Bertoletti A. Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J Virol. 2005;79:3322–3328. doi: 10.1128/JVI.79.6.3322-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoofnagle JH. Doo E. Liang TJ. Fleischer R. Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 10.Hou J. Yin YK. Xu D, et al. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology. 2008;47:447–454. doi: 10.1002/hep.22075. [DOI] [PubMed] [Google Scholar]
- 11.Kondo Y. Kobayashi K. Ueno Y, et al. Mechanism of T cell hyporesponsiveness to HBcAg is associated with regulatory T cells in chronic hepatitis B. World J Gastroenterol. 2006;12:4310–4317. doi: 10.3748/wjg.v12.i27.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian JQ. Wang XQ. Zhang Y. Huang CX. Bai XF. Correlation of circulating TLR2/4 expression with CD3+/4+/8+ T cells and Treg cells in HBV-related liver cirrhosis. Viral Immunol. 2009;22:301–308. doi: 10.1089/vim.2009.0039. [DOI] [PubMed] [Google Scholar]
- 13.Liaw YF. Gane E. Leung N, et al. 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Lok AS. McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 15.Lu FM. Zhuang H. Management of hepatitis B in China. Chin Med J (Engl) 2009;122:3–4. [PubMed] [Google Scholar]
- 16.Maier H. Isogawa M. Freeman GJ. Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 17.Miyara M. Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Nan XP. Zhang Y. Yu HT. Li Y. Sun RL. Wang JP. Bai XF. Circulating CD4+CD25high regulatory T cells and expression of PD-1 and BTLA on CD4+ T cells in patients with chronic hepatitis B virus infection. Viral Immunol. 2010;23:63–70. doi: 10.1089/vim.2009.0061. [DOI] [PubMed] [Google Scholar]
- 19.Nowak EC. Weaver CT. Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng G. Li S. Wu W. Sun Z. Chen Y. Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123:57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng G. Li S. Wu W. Tan X. Chen Y. Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro RM. Germanidis G. Powers KA. Pellegrin B. Nikolaidis P. Perelson AS. Pawlotsky JM. Hepatitis B virus kinetics under antiviral therapy sheds light on differences in hepatitis B e antigen positive and negative infections. J Infect Dis. 2010;202:1309–1318. doi: 10.1086/656528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speletas M. Argentou N. Germanidis G, et al. Foxp3 expression in liver correlates with the degree but not the cause of inflammation. Mediators Inflamm. 2011;827:565. doi: 10.1155/2011/827565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprengers D. Stoop JN. Binda RS, et al. Induction of regulatory T-cells and interleukin-10-producing cells in non-responders to pegylated interferon-alpha therapy for chronic hepatitis B. Antiviral Ther. 2007;12:1087–1096. [PubMed] [Google Scholar]
- 25.Stoop JN. van der Molen RG. Baan CC. van der Laan LJ. Kuipers EJ. Kusters JG. Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–778. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 26.Stoop JN. van der Molen RG. Kuipers EJ. Kusters JG. Janssen HL. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology. 2007;361:141–148. doi: 10.1016/j.virol.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Temann UA. Laouar Y. Eynon EE. Homer R. Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 28.Visvanathan K. Skinner NA. Thompson AJ, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y. Jia J. Control of hepatitis B in China: prevention and treatment. Expert Rev Anti Infect Ther. 2011;9:21–25. doi: 10.1586/eri.10.143. [DOI] [PubMed] [Google Scholar]
- 30.Xie Z. Chen Y. Zhao S, et al. Intrahepatic PD-1/PD-L1 up-regulation closely correlates with inflammation and virus replication in patients with chronic HBV infection. Immunol Invest. 2009;38:624–638. doi: 10.1080/08820130903062210. [DOI] [PubMed] [Google Scholar]
- 31.Xu D. Fu J. Jin L, et al. Circulating and liver resident CD4+ CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 32.Yang G. Liu A. Xie Q. Guo TB. Wan B. Zhou B. Zhang JZ. Association of CD4+ CD25+ Foxp3+ regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Z. Shi F. Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011;6:e23621. doi: 10.1371/journal.pone.0023621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JY. Song CH. Shi F. Zhang Z. Fu JL. Wang FS. Decreased ratio of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One. 2011;5:e13869. doi: 10.1371/journal.pone.0013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y. Lian JQ. Huang CX, et al. Overexpression of toll-like receptor 2/4 on monocytes modulates the activities of CD4(+)CD25(+) regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]