Abstract
Changes in natural killer (NK) cells according to their phenotype and expression of certain regulatory receptors were analyzed in a double-blind, controlled study of antiretroviral therapy (ART)-untreated HIV-seropositive patients, who had been vaccinated with monocyte-derived dendritic cells pulsed with inactivated HIV-1 autologous virus. This work extends other recently published studies of the same group of HIV-1+ vaccinated patients, which demonstrated that the viral load significantly decreases and correlates inversely with an increase in HIV-specific T-cell responses in vaccinated patients, but not in controls who received placebo. Our results indicate that this vaccine raises the level of the NK CD56neg cell subpopulation, while levels of the NK CD56dim and NK CD56bright cells expressing the inhibitory receptor CD85j/ILT-2 fell in vaccinated patients. Taken together, these results suggest that this vaccine might enhance innate immunity by amplifying the inflammatory and cytolytic capacity.
Introduction
The adoption of antiretroviral therapy (ART) in the treatment of HIV-1-seropositive patients was an important advance in the control of the progression of this infection (14,24,32). However, the clinical use of ART has several drawbacks, such as its high toxicity when treatment is continued for a prolonged period, and the occasional emergence of viral resistance in patients treated with ART (4,19).
These limitations justify the expenditure of extra effort to generate new modes of treatment for HIV-1+ patients (4). For this reason, several groups, including ours, are attempting to develop a vaccine based on the administration of autologous dendritic cells (DCs) pulsed with HIV-1 obtained from the same patient (25,15,36). The potential effects of these vaccines are based on the capacity of DCs to enhance HIV-1-specific immune responses due to their ability to act as “professional” antigen-presenting cells (5,6,31).
In this study, the patients selected were immunized using autologous monocyte-derived dendritic cells (MD-DCs), and their effects were analyzed by measuring viral parameters and certain components of the adaptative and innate immune response at different time points. The results related to the viral parameters and the adaptative response have recently been published (26), showing a decrease in viral load and an increase in HIV-specific T-cell responses in vaccinated patients, but not in placebo subjects.
This article presents the results of the analyses of the natural killer (NK) cells obtained from the same cohort of patients used in our previous publication. The relevance of this study is based on the demonstration that NK cells play an essential role in the surveillance of viral infections due to their secretory (mainly CD56bright) and cytolytic (mainly CD56dim) functions. These cells are modulated by their regulatory receptors (11), and by their effects on the innate and adaptative responses, resulting from cross-talk between NK and dendritic cells (22,16,42,41) and T cells (48,49). Although it has been shown that NK cell dysfunction contributes to the progression of HIV-1 viral infection (3,18,20,38,41), these cells have never previously been analyzed in trials of HIV-1 vaccines.
We studied the following NK subpopulations in patients treated with three MD-DC-HIV-1 vaccines: NK CD56dim, NK CD56bright, and NK CD56neg. We also studied the receptors CD85j/ILT-2, CD94, NKG2A, and NKG2C in the NK subpopulations, as these are known to be involved in regulating NK-cell functions (27,33,46).
Materials and Methods
Patients
We recruited 22 untreated chronic HIV-1 patients who had not received ART for at least 2 y before enrollment, from the Infectious Diseases Unit of the Hospital Clinic of Barcelona. Inclusion criteria were: baseline CD4+ T-lymphocyte count >450/mm3 (nadir CD4+ T-cell count above 350 cells/mL), and a plasma viral load (PVL) >10,000 copies/mL. The procedure followed in this double-blind study has been described in detail by Garcia et al. (25). The objective and other aspects of the study were explained to the patients in detail, and all gave their written informed consent prior to participation. The study was approved by our respective ethical review boards and by the Spanish regulatory authorities (clinical trial NCT0042142).
Study design
MD-DC treatment
The patients were blindly randomized to receive three immunizations, at weeks 0, 2, and 4, of at least 8×106 MD-DCs pulsed with heat-inactivated autologous virus (DC-HIV arm, 109 copies/dose), or three immunizations with non-pulsed autologous MD-DCs (DC-placebo arm), according to the procedure explained in detail in Garcia et al. (25). Blood samples were obtained at weeks −2, −1, 1, 3, 16, 24, and 48 for immunological determinations, including NK cells and their cytotoxic regulatory receptors. The results of weeks −2 and −1 were used as baselines. Inactivated autologous viruses were prepared as previously described (26).
Cell staining and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll gradient centrifugation from 150-mL samples of EDTA-treated venous blood taken from 11 chronic HIV-1-infected patients and 11 immunized HIV-1-infected patients. Cells were frozen at −80°C for 1 wk and then cryopreserved in liquid nitrogen at −200°C for transport and processing. Cryopreserved PBMCs were thawed and washed with PBS supplemented with 1% bovine serum albumin and 2 mM EDTA (FACS buffer).
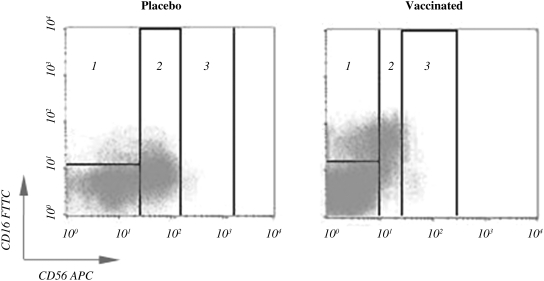
The cell subpopulations NK CD56bright, NK CD56dim, and NK CD56neg, defined as CD3−CD56bright, CD3−CD56dim, and CD3−CD16+CD56neg, respectively, were measured by flow cytometry in a four-color FACScalibur flow cytometer (Becton Dickinson, San Jose, CA), using the fluorochrome-labeled antibodies anti-CD3-PerCP (clone SK7), anti-CD16-FITC (clone 3G8), and anti-CD56-APC (clone B159), obtained from Becton Dickinson. A representative illustration of cytometry analysis of NK cell subpopulations is shown in Fig. 1.
FIG. 1.
Distribution of natural killer (NK) cell subsets in a representative cytometry study in placebo and vaccinated patients. Quadrants represent the individual NK cell subpopulations, NK CD56neg (1) in placebo (17.79%) and vaccinated patients (50.04%); NK CD56dim (2) in placebo (77.49%) and vaccinated patients (33.39%); and NK CD56bright (3) in placebo (4.72%) and vaccinated patients (16.55%).
In each one of the NK cell subpopulations, the NK cell regulatory receptor CD85j/ILT-2 was analyzed using the fluorochrome-labeled antibody anti-CD85j-PE (clone GHI/75) obtained from Becton Dickinson. In the NK CD56bright and NK CD56dim subpopulations, the regulatory receptors CD94-NKG2A and CD94-NKG2C were analyzed using the fluorochrome-labeled antibodies anti-CD94-FITC (clone HP-3D9) obtained from Becton Dickinson, and anti-NKG2A-PE (clone 131411) and anti-NKG2C-PE (clone 134591), obtained from R&D Systems, Inc. (Minneapolis, MN).
The percentage of antibody-positive cells was calculated by comparison with the appropriate isotype control antibodies. Cytometry data were analyzed using the CellQuest software package (Becton Dickinson). All the results of the analysis related to NK-cell subpopulations and their receptors are expressed as percentages of the total lymphocyte population.
Statistical analyses were performed using IBM SPSS software (version 18.0.0). A p value <0.05 was considered to be statistically significant.
Results
Changes in the percentages of the NK subpopulations NK CD56dim, NK CD56bright, and NK CD56neg, and NK subsets expressing the receptors CD85j/ILT-2, CD94, NKG2A, and NKG2C, in immunized patients are shown in Tables 1 and 2.
Table 1.
Percent Changes in Levels of Natural Killer (NK) Cell Subsets
| |
NK CD56dim |
NK CD56bright |
NK CD56neg |
||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
Placebo |
Immunized |
|
Placebo |
Immunized |
|
Placebo |
Immunized |
| Time | p Value | Median | Median | p Value | Median | Median | p Value | Median | Median |
| W1 | 0.83 | 1.71 | −4.10 | 0.13 | −0.08 | 0.09 | 0.35 | −8.95 | 4.34 |
| W3 | 0.14 | −7.87 | 0.08 | 0.96 | −0.07 | 0.02 | 0.66 | 2.44 | −0.99 |
| W16 | 0.56 | 3.06 | −1.83 | 0.67 | 0.06 | −0.27 | 0.89 | 0.54 | 2.22 |
| W24 | 0.04 | 8.49 | −18.64 | 0.87 | 0.05 | −0.84 | 0.03 | −8.75 | 10.62 |
| W48 | 0.49 | 2.84 | −13.72 | 0.82 | −1.11 | −0.04 | 0.69 | −6.87 | 10.80 |
Table 2.
Percent Changes in Levels of Receptor Expression in Natural Killer (NK) Cell Subsets
| |
|
NK CD56dim |
NK CD56bright |
NK CD56neg |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Placebo |
Immunized |
Placebo |
Immunized |
Placebo |
Immunized |
|||
| Time | p Value | Median | Median | p Value | Median | Median | p Value | Median | Median | |
| CD85j/ILT2 | W1 | 0.04 | −1.98* | −9.63* | 0.04 | −0.74* | −6.19* | 0.70 | −1.84 | −4.68 |
| W3 | 0.19 | −4.57 | −8.47 | 0.01 | −1.07* | −6.25* | 0.49 | −6.03 | −7.55 | |
| W16 | 0.01 | 1.03* | −11.16* | <0.01 | 0.50* | −5.45* | 0.21 | 2.27 | −12.45 | |
| W24 | 0.10 | −3.28 | −13.30 | 0.06 | −1.99 | −5.10* | 0.96 | −9.07 | −16.14 | |
| W48 | 0.11 | −6.04 | −15.54 | 0.05 | −0.82* | −5.97* | 0.91 | −10.35 | −8.29 | |
| CD94-NKG2A | W1 | 0.04 | 10.98* | −0.27* | 0.47 | 1.97 | 1.90 | |||
| W3 | 0.16 | 2.73 | −0.34 | 0.46 | 2.47 | −4.06 | ||||
| W16 | 0.43 | −4.42 | −0.80 | 0.43 | −13.17 | −2.12 | ||||
| W24 | 0.34 | −3.42 | 2.03 | 0.06 | −3.73 | 8.38 | ||||
| W48 | 0.43 | −6.40 | −1.81 | 0.87 | −5.55 | −14.69 | ||||
| CD94-NKG2C | W1 | 0.22 | 3.75 | −1.06 | 0.19 | 3.68 | −4.22 | |||
| W3 | 1.00 | −1.79 | 1.82 | 0.65 | 0.57 | 0.94 | ||||
| W16 | 0.70 | 2.53 | −2.91 | 0.73 | 1.77 | 3.13 | ||||
| W24 | 0.26 | −1.35 | 3.18 | 0.74 | −2.35 | −1.49 | ||||
| W48 | 0.85 | 1.29 | −4.04 | 0.85 | 3.69 | 0.88 | ||||
Differences statistically significant.
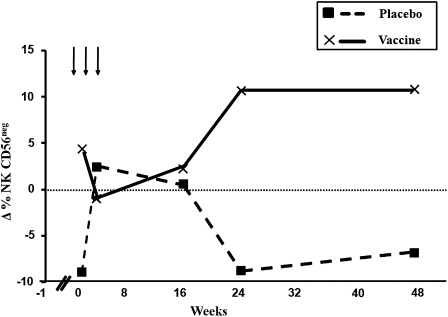
The levels of the three types of NK subpopulations analyzed in HIV-1-immunized and placebo patients, as a percentage of the total lymphocyte counts, indicate that there were no significant differences between placebo and immunized patients in the NK CD56bright cell subpopulation. However, a tendency for NK CD56dim cell subpopulations to fall in immunized patients could be observed at week 24 (Table 1). Interestingly, the levels of the NK CD56neg cell subpopulation rose in immunized patients from week 24 until the end of follow-up, although this difference became statistically significant only at the 24th week (p=0.013; Fig. 2).
FIG. 2.
Distribution of natural killer (NK) CD56neg cells in HIV-1-seropositive vaccinated and placebo patients. A rise in NK CD56neg cells can be observed from the 24th week to the end of the study period in HIV-1-immunized patients. The values were statistically significant at the 24th week of treatment (p=0.013).
When the changes in the percentages of NK CD56+ subpopulations expressing CD94-NKG2A and CD94-NKG2C heterodimers were analyzed, no significant differences were observed (Table 2). However, the percentages of NK subpopulations expressing CD85j/ILT-2 in NK CD56dim and NK CD56bright cells was lower in immunized patients than in placebo individuals.
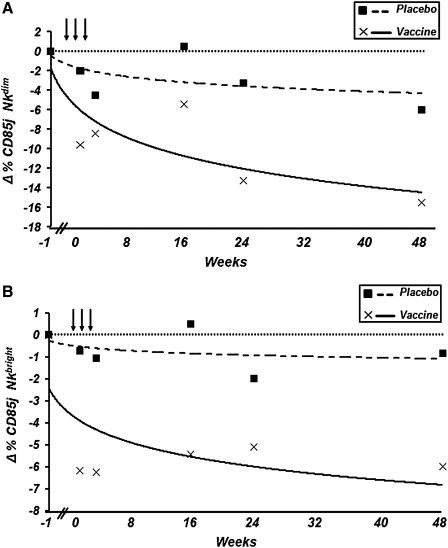
In the case of the NK CD56dim cell subpopulation, the decrease in the percentage of expression of CD85j/ILT-2 in immunized patients compared with the placebo group was statistically significant at the first (p=0.04) and 16th (p=0.017) weeks of follow-up (Fig. 3A and Table 1).
FIG. 3.
Trend lines reflecting changes in the percentages of (A) natural killer (NK) CD56dim-and (B) NK CD56bright-expressing CD85j/ILT-2 cells in HIV-1-seropositive vaccinated and placebo individuals. (A) Trend line [equation: y=−3.912ln(x) – 1.7675], which shows that changes in the percentage of NK CD56dim cells expressing CD85j/ILT-2 were reduced in vaccinated patients relative to the placebo group. (B) Trend line [equation: y=−1.341ln(x) – 2.4406], which shows that changes in the percentage of NK CD56bright cells expressing CD85j/ILT-2 were reduced in vaccinated patients relative to the placebo group.
With regard to the NK CD56bright cell subpopulation, the decrease in the percentage of expression of CD85j/ILT-2 in immunized patients compared to the placebo group was statistically significant at the first, third, 16th, and 48th weeks of follow-up (p=0.004, p=0.01, p=0.009, and p=0.05, respectively; Fig. 3B and Table 2). No changes in the CD56neg NK cell subpopulation expressing CD85j/ILT-2 were observed (Table 2).
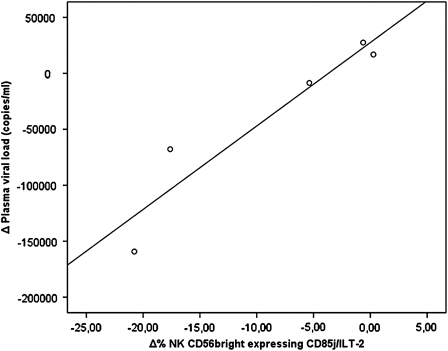
Finally, when we compared the values of NK cells expressing the CD85j/ILT2 receptor with plasma viral loads, no significant relationship was found between these parameters during the follow-up period, although a positive correlation was seen in the case of NK CD56bright cells, which was statistically significant at the 48th week (p=0.015, R=0.89; Fig. 4).
FIG. 4.
Relationship between natural killer (NK) CD56bright cells expressing the CD85j/ILT2 receptor and the plasma viral load found in vaccinated patients at the 48th week after the vaccinations were initiated (p=0.015, R=0.89).
It is noteworthy that the three vaccinated patients who best controlled their viral load as a consequence of the vaccine (data not shown), displayed greater decreases in the expression of CD85j/ILT-2 in NK CD56bright cells than other patients.
Discussion
NK cell subpopulations
Recent evidence suggests that abnormalities not only in the number of the NK cells, but also in their secretory and cytolytic capacity, are relevant to the progression of HIV-1 infections (37). Specifically, in HIV-1 infection a larger subpopulation of dysfunctional NK CD56neg cells (39), and an augmented expression of certain inhibitory receptors (38,30), have been observed. Defective cytolytic (29) and ADCC activity (1,12,45), and dysfunction in cytokine production have also been detected (34,38).
A further objective of this work was to determine whether the balance of NK cell subsets in HIV-1-infected patients is altered when they are vaccinated with autologous HIV-1-pulsed dendritic cells. This would tell us if the vaccine has the ability to modify NK cell distributions, as normally expressed in HIV-1-positive individuals (2,28,39), and whether any such changes are actually a consequence of the specific vaccine used.
Our results identified no large differences between the changes in percentages of NK CD56bright and NK CD56dim cell subpopulations in immunized patients and placebo individuals, which suggests that the vaccine probably has no effect on these subpopulations. However, we found that the level of the NK CD56neg cell subpopulation rose in immunized patients from week 24 until the end of the follow-up period (Fig. 2). The rise seen in NK CD56neg cells could be a consequence of the improvement in the adaptative immune response observed in vaccinated patients, as reported in the preliminary article including these same patients (26), although it does not exclude the possibility that it may be due to the effect of the vaccine on T-regulatory lymphocytes (47,35).
We do not know the reason for the rise in the NK CD56neg cell subpopulation observed in the immunized patients, although this may be due to the loss of NK CD56+ cells, as originally described by Brunetta et al. (13) under other conditions.
The observed increase in CD56neg cells after the administration of the vaccine may be of significance as a mechanism that blocks the progression of HIV-1 infection in vaccinated individuals. Obviously, these important immunological effects of the vaccine could be based on the biological characteristics of these cells, which may act via their cytotoxic capacity, and via the production of cytokines and IFN-γ (9). However, most importantly, they could interfere with the progression of the infection, because CD56neg NK cells are particularly relevant due to their capacity to produce and secrete chemokines such as CCL3, CCL4, and CCL5 (10), which are not only capable of acting as macrophage inflammatory proteins, facilitating chronic inflammation (9), but can also interfere with the entry of HIV-1 into host cells (8), and recruitment of NK cells, T cells, and macrophages to block HIV infection and reinforce the cytotoxic function of these cells.
The ability of these three chemokines to bind to CC-chemokine receptor 5 (CCR5) and to interfere with the receptor that acts as a co-ligand of HIV, has also been frequently described. NK CD56neg cells are thus able to inhibit HIV-1 entry into CD4+ and other HIV-1 target cells (17,) and subsequently to suppress HIV-1 replication (21,43). However, this potential beneficial effect of the vaccine caused by NK CD56neg cell activity needs further study.
NK cell receptors
As the expression of CD94, NKG2A, and NKG2C, measured in NK CD56+bright and CD56+dim cell subpopulations displayed no statistically significant differences between placebo and immunized patients, we can conclude that the vaccine has no effect on these receptors. However, when the expression of the inhibitory receptor CD85j/ILT-2 was measured, we found significant changes between both groups. Our data show a statistically significant decrease in the expression of CD85j/ILT-2 in both NK cell subpopulations, NK CD56bright and NK CD56dim, in immunized patients, while no changes were observed in the placebo group.
Given that CD85j/ILT-2 is expressed in NK cells and in the majority of myelomonocytic cells (11), and that it binds to different forms of HLA-I, including the regulatory molecule HLA-G (44), our results should be of both biological and clinical significance. Our finding of diminished expression of this receptor, which has an inhibitory functional effect on NK cells (11), and also inhibits the adhesion of NK cells to their target cells (23), is of importance in that its low expression in NK CD56dim and NK CD56bright cell subsets in immunized patients may facilitate enhanced NK cell function. Specifically, it may be that an increase in the secretory activity of NK CD56bright NK cells and the cytolytic function of NK CD56dim cells are consequences of the vaccine. This may have been due to a rise in the cytotoxicity and secretory capability of NK cells obtained from MD-DC-immunized patients (7). Furthermore, this effect could even be beneficial, as CD85j/ILT-2 has a tolerogenic effect on the production of IL-15 by dendritic cells, and it also inhibits the adhesion of NK cells to target cells (40).
Although an increase in NK cells expressing the CD85j/ILT-2 receptor when ART is interrupted has been reported (37), this study demonstrates the opposite effect in immunized patients, since even though our patients were off ART treatment, the level of expression of this receptor was reduced. Further studies are needed to extend our knowledge of the real effects of downregulating CD85j/ILT-2 expression, and its influence on the inhibition of the activity of NK cells and other immunoreactive cells as consequences of employing the DC vaccine in HIV-1-infected individuals.
The positive correlation seen between the levels of NK CD56bright-expressing CD85j/ILT-2 cells and the viral load in the 48th week of follow-up (Fig. 4) could be of biological interest, although we do not know why this relationship did not appear at any other time point when both NK CD56dim and NK CD56neg cell subpopulations expressing the CD85j/ILT-2 receptor were analyzed.
These results regarding NK cells and their receptors could be of clinical relevance, since to our knowledge no data concerning the role of NK cells in previous therapeutic vaccines are available. It may also be of interest in view of the cross-talk described between NK cells and dendritic cells, which were used as part of the vaccine in this work (16,22,42). In any case, further functional studies to clarify the hypotheses suggested here are required to better understand the changes seen in NK cells and their receptors in vaccinated patients.
Acknowledgments
This study was partially supported by grants from the Health Research Fund (FIS-PS09/00424, 01297 and 04272, FIS-PI07/0291 and PI10/02984) of the Spanish Ministry of Health, the Ministry of Science (SAF2005-06984), the Andalusian Department of Health (PI0293/2007), the Spanish Foundation for Research and Prevention of AIDS (FIPSE 36536/05; FIPSE is a non-profit foundation whose members include the Ministry of Health, Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, and Roche), Red Temática Cooperativa de Grupos de Investigación en Sida del Fondo de Investigación Sanitaria (FIS), and Objectif Recherche Vaccin Sida (ORVACS). Dr. Felipe García was the recipient of a research grant from Institut D'Investigacions Biomédiques August Pi i Sunyer (IDIBAPS), Barcelona. Dr. Montserrat Plana is a researcher at the August Pi I Sunyer Institute of Biomedical Investigation, and is supported by the Carlos III Health Institute (ISCIII), and the Department of Health of the Catalan Government (Generalitat de Catalunya).
Centers and principal investigators of the Dc2-Manon07 vaccine research group: Carlos III Hospital (Madrid), J.M. Benito; Hivacat-Hospital Clinic (Barcelona), J.M. Gatell; Germans Trias and Pujol Hivacat-Hospital (Badalona), B. Clotet; Gregorio Marañón Hospital (Madrid), M.A. Muñoz; Reina Sofía University Hospital (Córdoba), J. Peña, A. Rivero; and Carlos III Institute of Health (Madrid), J. Alcamí.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahmad A. Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996;10:258–266. doi: 10.1096/fasebj.10.2.8641559. [DOI] [PubMed] [Google Scholar]
- 2.Alter G. Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2009;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altfeld M. Fadda L. Frleta D. Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autran B. Debre P. Walker B. Katlama C. Therapeutic vaccines against HIV need international partnerships. Nat Rev Immunol. 2003;3:503–508. doi: 10.1038/nri1107. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J. Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J. Briere F. Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Bellon T. Kitzig F. Sayos J. Lopez-Botet M. Mutational analysis of immunoreceptor tyrosine-based inhibition motifs of the Ig-like transcript 2 (CD85j) leukocyte receptor. J Immunol. 2002;168:3351–3359. doi: 10.4049/jimmunol.168.7.3351. [DOI] [PubMed] [Google Scholar]
- 8.Berger EA. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl A):S3–S16. [PubMed] [Google Scholar]
- 9.Bjorkstrom NK. Ljunggren HG. Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol. 2010;31:401–406. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Bluman EM. Bartynski KJ. Avalos BR. Caligiuri MA. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J Clin Invest. 1996;97:2722–2727. doi: 10.1172/JCI118726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrego F. Kabat J. Kim DK, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–660. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 12.Brenner BG. Gryllis C. Wainberg MA. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. J Leukoc Biol. 1991;50:628–640. doi: 10.1002/jlb.50.6.628. [DOI] [PubMed] [Google Scholar]
- 13.Brunetta E. Hudspeth KL. Mavilio D. Pathologic natural killer cell subset redistribution in HIV-1 infection: new insights in pathophysiology and clinical outcomes. J Leukoc Biol. 2010;88:1119–1130. doi: 10.1189/jlb.0410225. [DOI] [PubMed] [Google Scholar]
- 14.Carcelain G. Debre P. Autran B. Reconstitution of CD4+ T lymphocytes in HIV-infected individuals following antiretroviral therapy. Curr Opin Immunol. 2001;13:483–488. doi: 10.1016/s0952-7915(00)00245-4. [DOI] [PubMed] [Google Scholar]
- 15.Connolly NC. Whiteside TL. Wilson C. Kondragunta V. Rinaldo CR. Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) peptide-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper MA. Fehniger TA. Fuchs A. Colonna M. Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Choe H. Farzan M. Sun Y, et al. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 18.Chung AW. Navis M. Isitman G, et al. Activation of NK cells by ADCC responses during early HIV infection. Viral Immunol. 2011;24:171–175. doi: 10.1089/vim.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorrell L. Therapeutic immunization for the control of HIV-1: where are we now? Int J STD AIDS. 2006;17:436–441. doi: 10.1258/095646206777689035. quiz 442. [DOI] [PubMed] [Google Scholar]
- 20.Fauci AS. Mavilio D. Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 21.Fehniger TA. Herbein G. Yu H. Para MI. Bernstein ZP. O'Brien WA. Caligiuri MA. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol. 1998;161:6433–6438. [PubMed] [Google Scholar]
- 22.Fernandez NC. Flament C. Crepineau F. Angevin E. Vivier E. Zitvogel L. Dendritic cells (DC) promote natural killer (NK) cell functions: dynamics of the human DC/NK cell cross talk. Eur Cytokine Netw. 2002;13:17–27. [PubMed] [Google Scholar]
- 23.Forte P. Pazmany L. Matter-Reissmann UB. Stussi G. Schneider MK. Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167:6002–6008. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 24.Garcia F. Plana M. Vidal C, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS. 1999;13:F79–F86. doi: 10.1097/00002030-199907300-00002. [DOI] [PubMed] [Google Scholar]
- 25.Garcia F. Lejeune M. Climent N, et al. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J Infect Dis. 2005;191:1680–1685. doi: 10.1086/429340. [DOI] [PubMed] [Google Scholar]
- 26.Garcia F. Climent N. Assoumou L, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis. 2011;203:473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez VD. Falconer K. Michaelsson J. Moll M. Reichard O. Alaeus A. Sandberg JK. Expansion of CD56- NK cells in chronic HCV/HIV-1 co-infection: reversion by antiviral treatment with pegylated IFNalpha and ribavirin. Clin Immunol. 2008;128:46–56. doi: 10.1016/j.clim.2008.03.521. [DOI] [PubMed] [Google Scholar]
- 28.Hong HS. Eberhard JM. Keudel P, et al. Phenotypically and functionally distinct subsets contribute to the expansion of CD56-/CD16+ natural killer cells in HIV infection. AIDS. 2010;24:1823–1834. doi: 10.1097/QAD.0b013e32833b556f. [DOI] [PubMed] [Google Scholar]
- 29.Kottilil S. Chun TW. Moir S, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187:1038–1045. doi: 10.1086/368222. [DOI] [PubMed] [Google Scholar]
- 30.Kottilil S. Shin K. Planta M, et al. Expression of chemokine and inhibitory receptors on natural killer cells: effect of immune activation and HIV viremia. J Infect Dis. 2004;189:1193–1198. doi: 10.1086/382090. [DOI] [PubMed] [Google Scholar]
- 31.Larsson M. Fonteneau JF. Bhardwaj N. Cross-presentation of cell-associated antigens by dendritic cells. Curr Top Microbiol Immunol. 2003;276:261–275. doi: 10.1007/978-3-662-06508-2_12. [DOI] [PubMed] [Google Scholar]
- 32.Letvin NL. Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- 33.Lima M. Almeida J. Montero AG, et al. Clinicobiological, immunophenotypic, and molecular characteristics of monoclonal CD56-/+dim chronic natural killer cell large granular lymphocytosis. Am J Pathol. 2004;165:1117–1127. doi: 10.1016/s0002-9440(10)63373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin SJ. Roberts RL. Ank BJ. Nguyen QH. Thomas EK. Stiehm ER. Effect of interleukin (IL)-12 and IL-15 on activated natural killer (ANK) and antibody-dependent cellular cytotoxicity (ADCC) in HIV infection. J Clin Immunol. 1998;18:335–345. doi: 10.1023/a:1023290932154. [DOI] [PubMed] [Google Scholar]
- 35.Lozano J M. Gonzalez R. Luque J. Frias M. Rivero A. Pena J. CD8(+)HLA-G(+) regulatory T cells are expanded in HIV-1-infected patients. Viral Immunol. 2009;22:463–465. doi: 10.1089/vim.2009.0041. [DOI] [PubMed] [Google Scholar]
- 36.Lu W. Arraes LC. Ferreira WT. Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 37.Luque J. Lozano JM. Garcia-Jurado G. Soriano-Sarabia N, et al. NK-associated regulatory receptors in a structured HAART interruption of HIV-1-positive individuals. AIDS Res Hum Retroviruses. 2008;24:1037–1042. doi: 10.1089/aid.2007.0285. [DOI] [PubMed] [Google Scholar]
- 38.Mavilio D. Benjamin J. Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci USA. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavilio D. Lombardo G. Benjamin J, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monsivais-Urenda A. Nino-Moreno P. Abud-Mendoza C. Baranda L. Layseca-Espinosa E. Lopez-Botet M. Gonzalez-Amaro R. Analysis of expression and function of the inhibitory receptor ILT2 (CD85j/LILRB1/LIR-1) in peripheral blood mononuclear cells from patients with systemic lupus erythematosus (SLE) J Autoimmun. 2007;29:97–105. doi: 10.1016/j.jaut.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Montaner LJ. Crowe SM. Aquaro S. Perno CF. Stevenson M. Collman RG. Advances in macrophage and dendritic cell biology in HIV-1 infection stress key understudied areas in infection, pathogenesis, and analysis of viral reservoirs. J Leukoc Biol. 2006;80:961–964. doi: 10.1189/jlb.0806488. [DOI] [PubMed] [Google Scholar]
- 42.Moretta A. Marcenaro E. Sivori S. Della Chiesa M. Vitale M. Moretta L. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Oliva A. Kinter AL. Vaccarezza M, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest. 1998;102:223–231. doi: 10.1172/JCI2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saverino D. Ghiotto F. Merlo A, et al. Specific recognition of the viral protein UL18 by CD85j/LIR-1/ILT2 on CD8+ T cells mediates the non-MHC-restricted lysis of human cytomegalovirus-infected cells. J Immunol. 2004;172:5629–5637. doi: 10.4049/jimmunol.172.9.5629. [DOI] [PubMed] [Google Scholar]
- 45.Sirianni MC. Mezzaroma I. Aiuti F. Moretta A. Analysis of the cytolytic activity mediated by natural killer cells from acquired immunodeficiency syndrome patients in response to phytohemagglutinin or anti-CD16 monoclonal antibody. Eur J Immunol. 1994;24:1874–1878. doi: 10.1002/eji.1830240824. [DOI] [PubMed] [Google Scholar]
- 46.Tarazona R. DelaRosa O. Casado JG. Torre-Cisneros J. Villanueva JL. Galiani MD. Pena J. Solana R. NK-associated receptors on CD8 T cells from treatment-naive HIV-infected individuals: defective expression of CD56. AIDS. 2002;16:197–200. doi: 10.1097/00002030-200201250-00008. [DOI] [PubMed] [Google Scholar]
- 47.Terzieva V. Regulatory T cells and HIV-1 infection. Viral Immunol. 2008;21:285–291. doi: 10.1089/vim.2008.0006. [DOI] [PubMed] [Google Scholar]
- 48.Zingoni A. Sornasse T. Cocks BG. Tanaka Y. Santoni A. Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 49.Zingoni A. Sornasse T. Cocks BG. Tanaka Y. Santoni A. Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol. 2005;42:451–454. doi: 10.1016/j.molimm.2004.07.025. [DOI] [PubMed] [Google Scholar]