Abstract
Primary Hyperoxaluria Type I (PH1) is a disorder of glyoxylate metabolism caused by mutations in the human AGXT gene encoding liver peroxisomal alanine:glyoxylate aminotransferase (AGT), a pyridoxal 5′-phosphate (PLP) dependent enzyme. Previous investigations highlighted that, although PH1 is characterized by a significant variability in terms of enzymatic phenotype, the majority of the pathogenic variants are believed to share both structural and functional defects, as mainly revealed by data on AGT activity and expression level in crude cellular extracts. However, the knowledge of the defects of the AGT variants at a protein level is still poor. We therefore performed a side-by-side comparison between normal AGT and nine purified recombinant pathogenic variants in terms of catalytic activity, coenzyme binding mode and affinity, spectroscopic features, oligomerization, and thermal stability of both the holo- and apo-forms. Notably, we chose four variants in which the mutated residues are located in the large domain of AGT either within the active site and interacting with the coenzyme or in its proximity, and five variants in which the mutated residues are distant from the active site either in the large or in the small domain. Overall, this integrated analysis of enzymatic activity, spectroscopic and stability information is used to (i) reassess previous data obtained with crude cellular extracts, (ii) establish which form(s) (i.e. holoenzyme and/or apoenzyme) and region(s) (i.e. active site microenvironment, large and/or small domain) of the protein are affected by each mutation, and (iii) suggest the possible therapeutic approach for patients bearing the examined mutations.
Abbreviations: PH1, primary hyperoxaluria type I; AGT, alanine:glyoxylate aminotransferase; PLP, pyridoxal 5′-phosphate; DLS, dynamic light scattering; CD, circular dichroism; KD(PLP), equilibrium dissociation constant for PLP; DSF, differential scanning fluorimetry; Tm, melting temperature
Keywords: Primary Hyperoxaluria Type I, Alanine:glyoxylate aminotransferase, Pyridoxal 5′-phosphate, Pathogenic mutations, Site-directed mutagenesis
Highlights
► We characterize the purified holo and apo recombinant forms of 9 PH1-causing variants. ► We analyze their enzyme activity, spectroscopic features and thermostability. ► We identify the structural and/or functional defect of each variant. ► We reassess previous data obtained with crude cellular extracts. ► We suggest possible therapeutic approaches for patients bearing the examined mutations.
1. Introduction
Primary Hyperoxaluria Type I (PH1) is a human metabolic disease whose principal hallmark is the formation of calcium oxalate stones at first in the kidneys and urinary tract and then, should renal failure occur, in the whole body [1]. Although the first clinical manifestations are related to renal dysfunction, the cause of the disease is the deficiency of an hepatic peroxisomal protein, namely alanine:glyoxylate aminotransferase (AGT) [2]. AGT is a pyridoxal 5′-phosphate (PLP)-dependent enzyme that converts l-alanine and glyoxylate into pyruvate and glycine. In the absence of AGT, the oxidation of glyoxylate by lactate dehydrogenase results in the synthesis of oxalate with the consequent formation and deposition of insoluble calcium oxalate crystals [1]. AGT is encoded by the AGXT gene [3], which is present in humans as two polymorphic variants, the “major allele” (AGT-Ma) and the less common “minor allele” (AGT-Mi). AGT-Mi differs from AGT-Ma by a 74-bp duplication in intron 1 and by two mutations leading to the P11L and I340M amino acid substitutions, respectively [4,5].
PH1 is characterized by a remarkable heterogeneity in terms of enzymatic phenotypes. Among the more than 150 pathogenic mutations in the AGXT gene identified so far [6], missense mutations are the most common type and lead to AGT deficiency by a variety of different mechanisms. Indeed, some mutations reduce AGT catalytic activity, others affect either protein folding, stability or localization inside the cell, while others, that represent the vast majority, influence at varying degrees both AGT catalytic activity and folding. Moreover, some mutations cosegregate and functionally interact with the minor allele polymorphism [7–9]. It should be pointed out that the effects of most pathogenic mutations have been identified by measuring the AGT transaminase activity (expressed as specific activity) and/or the protein expression level in crude cellular extracts or cell-free expression systems [9–16]. However, in cases of low specific activity and low expression level, this approach does not allow one to assess if a mutation exerts a structural and/or a functional impact. Since in diseases related to protein malfunction it is diagnostically and therapeutically essential to understand the multiple mechanisms that relate the specific mutants with the pathology, the knowledge of the structural and/or functional effect(s) that each amino acid substitution produces on AGT would be highly desirable. A relevant step in this direction has been the resolution of the crystal structure of AGT in complex with the inhibitor aminooxyacetic acid [17]. The structure revealed that AGT is dimeric and belongs to the aspartate aminotransferase family of PLP-dependent enzymes. Each subunit is comprised of an N-terminal extension (residues 1–21) that wraps over the surface of the other subunit, a large domain (residues 22–282) containing most of the active site and the dimerization interface, and a C-terminal domain (residues 283–392) containing the peroxisomal targeting information (Fig. 1A). As in all PLP-enzymes, the cofactor is covalently bound to the apoprotein by a Schiff base linkage with a lysyl residue which is at position 209 in AGT. The analysis of the crystal structure of AGT not only provided information on the active site topography and on the residues involved in binding of coenzyme and substrates but also allowed one to rationalize and interpret the impact of some disease-specific mutations in terms of their likely effects on AGT tertiary and quaternary conformation [17]. Indeed, in the last 5 years, biochemical and bioinformatic investigations on several pathogenic variants in the recombinant purified form have uncovered their molecular defects. In particular, it has been demonstrated that (i) the dramatic loss of catalytic activity of the G82E-Ma variant is related to its inability to undergo an efficient transaldimination [18] rather than to an impaired PLP binding, as previously suggested [9], and (ii) the decreased catalytic activity and immunoreactivity of Gly41 variants are not primarily due to the destabilization of the dimeric structure, as previously reported [9,17,19], but to the propensity of these variants in the dimeric form to undergo aggregation and degradation [20]. Additionally, evidence has been provided that the defect of G170R-Mi [21] and F152I-Mi [22] consists of a reduced dimer stability of their apo-forms. In this regard, it must be pointed out that, although the crystal structure of apoAGT is not available yet, near-UV CD and fluorescence spectra strongly suggest a different conformation between the apo- and holo-forms of AGT [20]. Importantly, the identification of the mechanism(s) leading to AGT loss of function in these variants has allowed to predict that patients carrying mutations at Gly82 or Gly41 will be unresponsive to pyridoxine treatment and to explain why patients bearing the G170R and F152I mutations have been found to be responsive to B6 therapy [23–25]. Following these considerations, it would be desirable to extend the analysis on the structural and functional properties to as many purified recombinant pathogenic variants as possible in both the holo- and apo-forms.
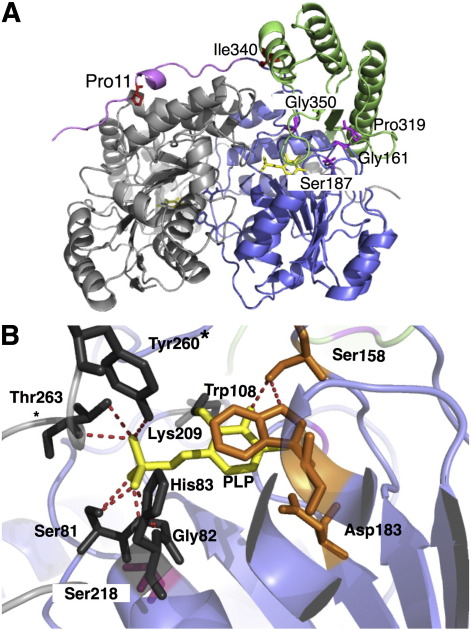
Fig. 1.
3D representation of the AGT structure (PDB file 1H0C). (A) Overall structure of the AGT dimer. One monomer is colored gray, while in the opposite monomer the N-terminus, the large domain and the small domain are colored magenta, blue and green, respectively. PLP is represented as yellow sticks. Pro11 and Ile 340 are represented as red sticks, while Gly161, Ser187, Pro319 and Gly350 are represented as magenta sticks. (B) The AGT active site is shown. PLP is represented as yellow sticks. Residues directly interacting with PLP are represented as dark gray sticks or, if their mutation is analyzed in this study, as orange sticks. Ser218 is represented as magenta sticks. The dotted lines indicate possible hydrogen bond interactions. The figure was rendered using PyMol [26].
To this aim, we decided to elucidate the molecular basis underlying AGT loss of function in a subset of known pathogenic mutations spread over the structure of AGT. Specifically, we chose mutations affecting (i) residues at the active site directly interacting with the PLP coenzyme (Trp108, Ser158 and Asp183), (ii) a residue located in the large domain of AGT in the proximity of the active site (Ser218), and (iii) residues located far from the AGT active site either in the large (Gly161, Ser187) or in the small domain (Pro319 and Gly350) (Figs. 1A and B). Enzyme activity, PLP binding mode and affinity, oligomerization, and thermal unfolding were analyzed for each of these variants in its purified holo- and apo-forms. The integrative analysis of the kinetic, spectroscopic and thermostability data allows one to (i) establish that, in contrast with previous data obtained on crude lysates, not all the variants have both structural and functional defects, (ii) define if a mutation causes a structural defect limited to the active site or extended to the large and/or the small domain of AGT, and (iii) suggest the treatment that could be effective for patients carrying the examined mutations.
2. Materials and methods
2.1. Materials
PLP, SYPRO orange, l-alanine, sodium glyoxylate, pyruvate, rabbit muscle l-lactic dehydrogenase, and isopropyl β-d-thiogalactopyranoside were all purchased from Sigma. All other chemicals were of the highest purity available.
2.2. In silico analyses
In silico mutagenesis analyses were performed by means of the software PyMol [26] (DeLano Scientific) starting from the three-dimensional structure of AGT (pdb files 1H0C_A and 1H0C_B).
2.3. Site-directed mutagenesis
The polymorphic and pathogenic variants of AGT were constructed starting from the pAGT-His construct that contains the complete open reading frame of AGT cloned in a pTrcHis2A expression plasmid [9].The mutations were introduced by using the QuikChange site-directed mutagenesis kit (Stratagene) and the oligonucleotides used for mutagenesis are reported in Supplementary Table 1. All the mutations were confirmed by the entire DNA sequence analysis.
The polymorphic and pathogenic variants of AGT were constructed starting from the pAGT-His construct that contains the complete open reading frame of AGT cloned in a pTrcHis2A expression plasmid [9]. The mutations were introduced by using the QuikChange site-directed mutagenesis kit (Stratagene) and the oligonucleotides used for mutagenesis are reported in Supplementary Table 1. All the mutations were confirmed by the entire DNA sequence analysis.
2.4. Protein expression and purification
Mutant enzymes in their histidine-tagged form were expressed in E. coli and purified with the procedure already described [18]. The apo-form of each variant was prepared as previously described [18]. The protein concentration in the AGT samples was determined by absorbance spectroscopy using an extinction coefficient of 9.54 × 104 M− 1 cm− 1 at 280 nm [18]. The PLP content of the holoenzymes was determined by releasing the coenzyme in 0.1 M NaOH and by using an εM = 6600 M− 1 cm− 1at 388 nm.
2.5. Determination of the equilibrium dissociation constants of the variants for PLP
The equilibrium dissociation constant for PLP (KD(PLP)) of G161C-Mi, G161S-Mi, P319L-Ma and G350D-Mi variants was determined by measuring the quenching of the intrinsic fluorescence of the apoenzymes (0.1 μM) in the presence of PLP at a concentration range of 0.01–10 μM. In the case of W108R-Mi, S158L-Ma, D183N-Ma and S218L-Ma variants, the KD(PLP) was determined by measuring the CD signal at 430 nm of the apo forms at a concentration of 10 μM in the presence of PLP at concentrations ranging from 5 to 300 μM. All the experiments were carried out in 100 mM potassium phosphate buffer, pH 7.4. The KD(PLP) values for the mutant–coenzyme complexes were obtained by using the following equation:
where [E]t and [PLP]t represent the total concentrations of the mutant and PLP, respectively, Y refers to either the intrinsic fluorescence quenching or the 430 nm dichroic signal changes at a PLPt concentration, [PLP], and Ymax refers to the aforementioned changes when all enzyme molecules are complexed with coenzyme.
The KD(PLP) for the S187F-Ma variant has been estimated as the upper limit value by the following procedure: samples of holo- and apo-S187F-Ma at various protein concentrations (from 0.01 to 1 μM, being 0.01 μM the detection limit) were incubated overnight at 25 °C in 100 mM potassium phosphate buffer, pH 7.4. The intrinsic fluorescence emission at 336 nm of each sample was registered and the percentage of fluorescence quenching at each protein concentration was then calculated.
2.6. Enzyme activity measurements
The kinetic parameters for the overall transamination reaction for the pair alanine/glyoxylate of the AGT variants (0.1–10 μM) were determined in the presence of saturating PLP concentration by varying the substrate concentration at a fixed saturating co-substrate concentration. For the G161C-Mi, G161S-Mi, S218L-Ma, P319L-Ma and G350D-Mi variants pyruvate formation was measured by a spectrophotometric assay using the coupled lactate dehydrogenase system previously described [18], while for the W108R-Mi, S158L-Ma, D183N-Ma and S187F-Ma variants a more sensitive assay based on measuring the 2,4-dinitrophenylhydrazine derivative of pyruvate by HPLC analysis was used [27]. Data of initial velocity as a function of substrate concentration were fitted to the Michaelis–Menten equation.
2.7. Dynamic light scattering (DLS)
Stock solutions of all AGT variants, either in the holo- or the apo-form, were diluted to 10 μM concentration with 100 mM potassium phosphate buffer, pH 7.4. PLP at saturating concentrations was added to the holoenzyme solutions. Each sample was incubated for 10 min at 25 °C and DLS analysis was performed on a ZetasizerNano S device (Malvern Instruments) by using disposable 12.5 × 45-mm cells with stopper. The temperature was kept at 25 ± 0.1 °C during the measurements by a Peltier temperature controller.
2.8. Thermostability
The thermostability studies on purified AGT variants in the holo- (in the presence of saturating PLP) or the apo-form were performed by both CD-monitored thermal unfolding and differential scanning fluorimetry (DSF). In the first method, the CD signal at 222 nm of AGT variants at 10 μM concentration in 100 mM potassium phosphate buffer, pH 7.4, was registered with temperature increasing from 25 to 90 °C at a heating rate of 1.5 °C/min. For the holo-forms the PLP dissociation during thermal unfolding was also monitored by measuring the CD signal at 430 nm under the same experimental conditions reported above. Calculation of the melting temperatures (Tm) was carried out by fitting the CD signal either to a two or a three state unfolding model using the Origin Pro7 software according to the method of Pace [28]. The rates of PLP dissociation and loss of secondary structure at the melting temperature for both AGT-Ma and AGT-Mi were measured by monitoring the CD signal at 430 and 222 nm with time, and fitting the data to a single exponential curve.DSF experiments were performed with purified AGT variants at the concentration of 1 μM in 100 mM potassium phosphate buffer pH 7.4 containing 5× SYPRO orange, according to the method of Nielsen et al. [29]. Considering the high background fluorescence of the holo- and apo-forms of W108R-Mi at 25 °C, the SYPRO orange final concentration was 2.5×. Proteins were subjected to a ramp of 2.15° C/min in a Mastercicle EP Realplex 4 (Eppendorf) at a temperature gradient from 25° C–90°C. Graph generation and calculation of Tm were carried out by using the Realplex software.
2.9. Spectroscopic measurements
Absorption measurements were made with a Jasco V-550 spectrophotometer with a 1 cm path length quartz cuvette at a protein concentration of 1–10 μM. Visible and far-UV CD spectra were recorded on a Jasco J-710 spectropolarimeter equipped with a thermostatically controlled compartment at 25 °C, by using 1 cm path-length quartz cuvettes. The enzyme concentration was 1–10 μM and the spectra of the holoenzymes were recorded in the presence of saturating PLP concentrations. Routinely, three spectra were recorded at a scan speed of 50 nm/min− 1 with a bandwidth of 2 nm and averaged automatically. Intrinsic fluorescence emission spectra were recorded on a Jasco FP-750 spectrofluorometer equipped with a thermostatically controlled cell holder.
3. Results
3.1. In silico analyses
To investigate the possible local effect of each of the studied amino acid substitutions on AGT we mapped them onto the available crystal structure of the enzyme in its major allelic form [17] and performed in silico mutagenesis by the software PyMol [26]. The location of the residues whose mutation has been analyzed in this work is shown in Figs. 1A and B. Trp108, Ser158 and Asp183 are active site residues that directly interact with the coenzyme. The side chain of Ser158 is hydrogen-bonded to the 3′ hydroxyl group of PLP, the indolic ring of Trp108 forms a base-stacking interaction with the pyridine ring of the coenzyme, and the carboxylic group of Asp183 forms a salt-bridge with the positive pyridine nitrogen of PLP. Thus, it can be predicted that the W108R, S158L and D183N mutations could have a functional effect on AGT, by possibly altering the binding of the coenzyme and/or the catalytic properties of the enzyme. Ser218, a residue located near the active site, does not interact with the coenzyme, and is located at the end of the strand 6 of the β-barrel. Since its hydroxyl group could interact with the peptydic oxygens of Ser81, Gly80 and Ile78, this residue might be involved in the proper positioning of the helix 4 that also comprises Gly82, a residue hydrogen-bonded to the PLP phosphate. The S218L mutation is predicted to have a local structural effect on the large domain, possibly resulting in the distortion of the coenzyme binding cleft. Gly161 and Ser187 are two large domain residues located far from the active site. Gly161 is located at the interface between the large and the small domain and the in silico analysis predicts that both G161C and G161S mutations would alter the inter-domain contacts of the AGT subunit. Ser187 is located in a random coil region on the re face of the coenzyme and its substitution with a phenylalanine residue would possibly change the positioning of the loop 154–169 comprising Gly161, thus indirectly affecting the large domain/small domain contacts. Finally, Pro319 and Gly350 belong to two random coil regions of the small domain. It can be predicted that the P319L mutation could have a structural effect on the small domain by leading to a mispositioning of the helix 11 located on the surface of the protein. Gly350 faces the active site on the si side of the coenzyme and the Gly350-to-Asp substitution could induce steric clashes of the small domain, in particular of the loop 343–357.
3.2. E. coli expression and purification
E. coli expression vectors encoding for the selected pathogenic variants were constructed by site-directed mutagenesis. Each mutation was inserted on the background of the major or the minor allele according to the genotype of the PH1 patients in whom the mutation was identified. Then, by a previously developed protocol [18], the nine AGT variants were expressed and purified to homogeneity, as indicated by a single band on SDS–PAGE at an apparent molecular weight of about 45 KDa (data not shown). The majority of the mutant proteins was present in good amounts in the soluble extract as compared to AGT-Ma or AGT-Mi, except Gly161 variants whose yield is ~ 10% that of AGT-Mi. The far-UV CD spectrum of each variant in the apo-form was registered and found to be identical to that of apoAGT-Ma or apoAGT-Mi, thus indicating that the mutations do not introduce major changes on the secondary structure of the protein. Moreover, the molecular dimensions of the variants in both the holo- and apo-forms have been determined by DLS. For each protein at 10 μM concentration in 100 mM potassium phosphate buffer pH 7.4, a peak with a size ranging from 9.2 to 10.8 nm, corresponding to that of a dimer, could be seen. This indicates that the dimer is the most abundant species in solution, given that the intensity of the signal on DLS varies with the sixth power of a particle diameter. However, the presence of low amounts of small aggregates (100–400 nm) could be noticed for W108R-Mi, S218L-Ma, S187F-Ma, G161C-Mi, G161S-Mi and P319L-Ma in both the holo- and apo-forms as well as for G350D-Mi in the apo-form (data not shown).
3.3. PLP binding mode and affinity
The equilibrium dissociation constants (KD(PLP)) for the complexes between PLP and the variants were determined by titrating the apo-form of each enzymatic species with increasing concentrations of PLP and by fitting the data to the appropriate equation. The obtained KD(PLP) values are reported in Table 1 along with the previously determined KD(PLP) values for AGT-Ma and AGT-Mi [18,22]. Basing on these data, it can be observed that: (i) W108R-Mi, S158L-Ma, D183N-Ma and S218L-Ma, even if at different extents, show a significantly reduced affinity for the coenzyme while (ii) G161C-Mi, G161S-Mi, P319L-Ma and G350D-Mi display an unaltered or even increased affinity for PLP. In the case of S187F-Ma, the enzyme concentration required for fluorescence measurements far exceeds the KD(PLP), which precludes the determination of a precise value for KD(PLP). Thus, only an upper limit of 0.01 μM has been estimated for the KD(PLP) of the S187F-Ma variant.
Table 1.
Equilibrium dissociation constants for PLP (KD(PLP)) and optical activity of AGT-Ma, AGT-Mi and pathogenic variants.
| Enzymatic species | KD(PLP) (μM) | Optical activity |
|---|---|---|
| AGT-Ma | 0.27 ± 0.03a | 97 mdeg/Abs 420 nma |
| AGT-Mi | 0.26 ± 0.02b | 97 mdeg/Abs 420 nmb |
| W108R-Mi | 96 ± 19 | 4.73 mdeg/Abs 434 nm |
| S158L-Ma | 272 ± 47 | 45 mdeg/Abs 430 nm |
| G161S-Mi | 0.46 ± 0.07 | 66 medeg/Abs 430 nm |
| G161C-Mi | 0.56 ± 0.09 | 57 mdeg/Abs 414 nm |
| D183N-Ma | 2.1 ± 0.1 | 84 mdeg/Abs 430 nm |
| S187F-Ma | < 0.01 | 103 mdeg/Abs 414 nm |
| S218L-Ma | 24.2 ± 0.1 | 31 mdeg/Abs 428 nm |
| P319L-Ma | 0.19 ± 0.04 | 88 mdeg/Abs 420 nm |
| G350D-Mi | 0.031 ± 0.005 | 88 mdeg/Abs 420 nm |
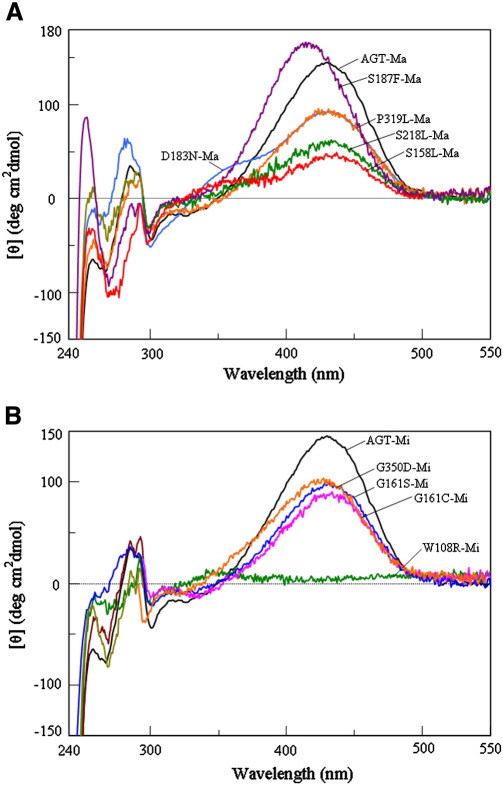
Coenzyme binding to AGT gives rise to typical absorbance bands at 430 and 340 nm associated with positive and negative dichroic signals, respectively [18]. In order to define if the analyzed PH1-causing mutations could alter the AGT coenzyme binding mode, the UV–visible absorbance and CD spectrum of each enzymatic species at 10 μM concentration has been registered in the presence of saturating PLP concentrations (Fig. 2), and the corresponding optical activity (expressed as millidegrees/absorbance at 410–430 nm) has been calculated (Table 1). The results indicate that, at varying degrees, only the W108R,G161C, G161S, S158L and S218L mutations decrease the optical activity with respect to that of AGT-Ma or AGT-Mi, thus indicating that these mutations cause a change in the microenvironment of the internal aldimine. It is worth noting that, although the S187F-Ma variant does not show an altered value of the optical activity, its internal aldimine is characterized by about 6 nm-blue shifted absorbance and dichroic maxima (Fig. 2A). This finding, along with the increase of at least about 27-fold in the KD(PLP) value, suggests that the mutation should induce some local structural changes at the AGT active site.
Fig. 2.
CD spectra of AGT-Ma, AGT-Mi and pathogenic variants. (A) CD spectra of AGT-Ma (black), S158L-Ma (red), D183N-Ma (blue), S187F-Ma (violet), S218L-Ma (green) and P319L-Ma (orange).(B) CD spectra of AGT-Mi (black), W108R-Mi (green), G161C-Mi (fucsia), G161S-Mi (blue) and G350D-Mi (orange). All CD spectra are registered in the presence of saturating PLP concentrations in 100 mM potassium phosphate buffer, pH 7.4, at an enzyme concentration of 9 μM.
3.4. Enzymatic activity
The steady-state kinetic parameters of the overall transamination of the alanine-glyoxylate pair were measured on the variants and compared with those of either AGT-Ma or AGT-Mi. A summary of the resulting steady-state parameters is listed in Table 2. The kcat/Km value of D183N-Ma vs AGT-Ma and W108R-Mi vs AGT-Mi is decreased by ~ 23,000- and 30,000-fold, respectively. The reduction in the catalytic efficiency is mainly driven by the decrease in the kcat value, being about 0.02% and 0.04% that of AGT-Ma and AGT-Mi, respectively. Both S158L and S218L mutations do no significantly change the Km values, but reduce the kcat values of AGT-Ma by ~ 100-fold and ~ 16-fold, respectively, thus causing a reduction in the catalytic efficiency. As for S187F-Ma, the Km and the kcat values decrease by ~ 2- and 360-fold, respectively, as compared to AGT-Ma. Taken together, these results agree with those previously obtained on both purified proteins [13] and crude bacterial extracts [12]. On the other hand, a moderate, if any, reduction of the kcat value (≥ 50% residual activity) has been observed for the P319L-Ma variant (vs AGT-Ma) and for the G161C-Mi, G161S-Mi, and G350D-Mi variants (vs AGT-Mi). This is in contrast with previous reports showing that the specific activity of Gly161 variants and G350D-Mi in crude cellular extracts was less than 1% [12] and 2.9% [30], respectively, that of AGT-Ma. However, the subsaturating substrate concentration used in these studies (150 mM l-alanine, by comparison see the Km values for l-alanine listed in Table 2) coupled with the low expression level of these variants might explain this discrepancy.
Table 2.
Steady-state kinetic parameters of AGT-Ma, AGT-Mi and pathogenic variants for the pair alanine-glyoxylate.
| Enzymatic species | Substrate | Cosubstrate | kcat (s− 1) | Km l-alanine(mM) | Km Glyoxylate (mM) | kcat/Km (s− 1/mM− 1) |
|---|---|---|---|---|---|---|
| AGT-Maa | l-Alanine | Glyoxylate | 45 ± 2a | 31 ± 4a | 1.4 ± 0.2a | |
| Glyoxylate | l-Alanine | 45 ± 2a | 0.23 ± 0.05a | 196 ± 4a | ||
| AGT-Mib | l-Alanine | Glyoxylate | 33 ± 5b | 28 ± 2b | 1.2 ± 0.2b | |
| Glyoxylate | l-Alanine | 37 ± 1b | 0.22 ± 0.01b | 168 ± 8b | ||
| W108R-Mi | l-Alanine | Glyoxylate | 0.0013 ± 0.0003 | 36 ± 3 | 0.00004 ± 0.00001 | |
| Glyoxylate | l-Alanine | n.d. | n.d. | n.d. | ||
| S158L-Ma | l-Alanine | Glyoxylate | 0.41 ± 0.03 | 25 ± 6 | 0.016 ± 0.001 | |
| Glyoxylate | l-Alanine | 0.41 ± 0.04 | 0.35 ± 0.09 | 1.2 ± 0.3 | ||
| G161S-Mi | l-Alanine | Glyoxylate | 55 ± 2 | 70 ± 9 | 0.8 ± 0.1 | |
| Glyoxylate | l-Alanine | 58 ± 2 | 0.63 ± 0.07 | 92 ± 2 | ||
| G161C-Mi | l-Alanine | Glyoxylate | 36.1 ± 0.7 | 103 ± 6 | 0.35 ± 0.02 | |
| Glyoxylate | l-Alanine | 36.6 ± 0.7 | 0.21 ± 0.02 | 174 ± 1 | ||
| D183N-Ma | l-Alanine | Glyoxylate | 0.008 ± 0.003 | 140 ± 17 | 0.00006 ± 0.00001 | |
| Glyoxylate | l-Alanine | n.d. | n.d. | n.d. | ||
| S187F-Ma | l-Alanine | Glyoxylate | 0.13 ± 0.01 | 12 ± 2 | 0.0108 ± 0.0002 | |
| Glyoxylate | l-Alanine | 0.12 ± 0.01 | 0.13 ± 0.04 | 0.92 ± 0.09 | ||
| S218L-Ma | l-Alanine | Glyoxylate | 2.7 ± 0.1 | 29 ± 6 | 0.09 ± 0.02 | |
| Glyoxylate | l-Alanine | 2.95 ± 0.08 | 0.19 ± 0.02 | 15 ± 2 | ||
| P319L-Ma | l-Alanine | Glyoxylate | 27.5 ± 0.8 | 44 ± 5 | 0.63 ± 0.01 | |
| Glyoxylate | l-Alanine | 26.2 ± 0.7 | 0.11 ± 0.02 | 238 ± 44 | ||
| G350D-Mi | l-Alanine | Glyoxylate | 15.0 ± 0.7 | 328 ± 51 | 0.046 ± 0.007 | |
| Glyoxylate | l-Alanine | 15 ± 1 | 0.29 ± 0.04 | 52 ± 7 |
3.5. Thermal stability studies
To investigate whether the analyzed PH1-associated mutations could affect the stability of AGT, which could give information about the steady-state amount of active enzyme, we compared the thermal unfolding profiles of the purified variants with those of AGT-Ma or AGT-Mi.
Thermal protein denaturation was studied either by monitoring the decrease of the dichroic signal at 222 nm and 430 nm, which are indicative of the loss of the protein secondary structure and of the PLP-bound content, respectively, or by differential scanning fluorimetry (DSF), that allows one to monitor unfolding events by measuring the exposure of hydrophobic groups of the denaturing protein [29]. This analysis was performed both in the holo- and apo-forms of each enzymatic species and the obtained results are listed in Tables 3 and 4, respectively. The far-UV (222 nm) (Fig. 3) and the visible (430 nm) CD-monitored heating scans as well as the DSF experiments reveal that the holo-forms of AGT-Ma and AGT-Mi are denatured and release PLP in a single-step process with melting temperatures at about 78 °C and 74 °C, respectively. This is in agreement with previous results obtained by differential scanning calorimetry [20] and thermal inactivation experiments [22]. In order to assess whether the loss of secondary structure and the PLP release are concerted events in these enzymatic species, the kinetics of changes of the 430 nm and 222 nm have been measured at their Tm values. We found that while the rate constant of the loss of the 430 nm dichroic signal is 0.0042 ± 0.0001 s− 1 and 0.0057 ± 0.003 s− 1 for AGT-Ma and AGT-Mi, respectively, that of the loss of the 222 nm dichroic band is 0.0024 ± 0.0001 s− 1 and 0.0021 ± 0.001 s− 1. These data provide evidence for a higher local stability of the PLP binding site in AGT-Ma than in AGT-Mi, in line with the higher sensitivity to urea stress of the PLP-bound state of the latter form [21]. They also indicate that both the allelic forms of AGT release the coenzyme before losing their secondary structure, as corroborated by the finding that, when the coenzyme is covalently bound to the protein by NaBH4-reduction, the mid-point transition of AGT-Ma and AGT-Mi is at 85.6 °C and 79.1 °C, respectively.
Table 3.
Tmholo and TmPLP values of AGT-Ma, AGT-Mi and pathogenic variants.
| Enzymatic species | Tmholoa(°C) CD222 nm |
TmPLPa(°C) CD420 nm |
Tmholoa(°C) DSF |
|---|---|---|---|
| AGT-Ma | 77.5 | 78 | 78.6 |
| AGT-Mi | 73.6 | 73.6 | 74.1 |
| W108R-Mi | 54.1 | 53.4 | 53.9 |
| S158L-Ma | 67 | 64 | 64.7 |
| G161S-Mi | 62.5 | 62.8 | 61.8 |
| G161C-Mi | 64 | 64.2 | 63.8 |
| D183N-Ma | 80.8 | 79.6 | 80.1 |
| S187F-Ma | 76.7 | 74.4 | 73.6 |
| S218L-Ma | 57.8 | 57.9 | 56 |
| P319L-Ma | 77.9 | 76.3 | 77.5 |
| G350D-Mi | 73.5 | 73.7 | 72.6 |
They represent apparent Tm values and are the mean of two independent experiments. The error is within ± 0.3 °C.
Table 4.
Tmapo values of AGT-Ma, AGT-Mi and pathogenic variants.
| Enzymatic species | Tm1apoa(°C) CD 222 nm |
Tm2apoa (°C) CD 222 nm |
Tm1apoa (°C) DSF |
Tm2apoa (°C) DSF |
|---|---|---|---|---|
| AGT-Ma | 61.1 | 66.4 | 61.9 | 67.3 |
| AGT-Mi | 53.1 | 66 | 54.2 | 66.5 |
| W108R-Mi | 48.9 | 54 | 53.3 | |
| S158L-Ma | 65.9 | 64.3 | ||
| G161S-Mi | 46.8 | 46.5 | ||
| G161C-Mi | 51.1 | 51.3 | ||
| D183N-Ma | 70.2 | 70.3 | ||
| S187F-Ma | 48.2 | 45.3 | ||
| S218L-Ma | 50.9 | 57.8 | 47.2 | 55.7 |
| P319L-Ma | 61.6 | 60.1 | ||
| G350D-Mi | 53.6 | 52.5 |
They represent Tm values and are the mean of two independent experiments. The error is within ± 0.3 °C.
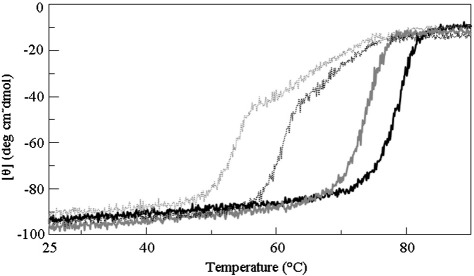
Fig. 3.
Far-UV CD-monitored heating scans of AGT-Ma and AGT-Mi. Far-UV CD changes of holoAGT-Ma (black curve, straight line) and holoAGT-Mi (gray curve, straight line) in the presence of 10 μM exogenous PLP, and of apoAGT-Ma (black curve, dotted line) and apoAGT-Mi (gray curve, dotted line). The enzyme concentration was 10 μM, and the buffer was 100 mM potassium phosphate, pH 7.4.
Like holoAGT-Ma and holoAGT-Mi, the nine pathogenic variants in the holo-form display a single thermal transition with the Tmholo values, derived from the 222 nm CD and DSF measurements, comparable to the corresponding resulting from the 430 nm CD measurements (TmPLP). The D183N mutation appears to exert a stabilizing effect, while the S187F, G350D and P319L mutations do not exert any effect on protein stability. For all the remaining variants the transition from the native state to the unfolded state occurs at temperatures lower than the corresponding wild-type, i.e. AGT-Ma or AGT-Mi. The variants W108R-Mi and S218L-Ma show the most pronounced alteration with Tmholo of 54° and 57 °C, respectively. However, it should be noted that W108R-Mi displays a considerably (~ 10-fold) elevated ground state fluorescence signal at 25 °C in comparison with AGT-Mi, thus suggesting an increased hydrophobicity due to partial protein unfolding even without the application of the thermal stress.
Interestingly, the apo-forms of AGT-Ma and AGT-Mi display a first unfolding phase with a mid-denaturation at 61 °C and 53 °C for AGT-Ma and AGT-Mi, respectively, and then a second unfolding transition with a mid-denaturation at 66 °C for both AGT-Ma and AGT-Mi (Fig. 3). In order to define the impact of the nine pathogenic mutations on the intrinsic stability of AGT, the thermal unfolding of the apo-form of each variant has been measured by far-UV CD (Supplementary Fig. 1) and DSF experiments. Apo-S158L-Ma unfolds in a single-step process with a Tmapo value identical to that of the high-temperature transition (Tm2apo) of apoAGT-Ma, thus suggesting that this large domain mutation does not significantly affect the Tm2apo value. On the other hand, the small domain mutations G350D and P319L, associated with the major and the minor alleles, respectively, cause the complete loss of the high-temperature transition (Tm2apo) without affecting the low-temperature transition (Tm1apo). All together, these findings indicate that the mutations of residues located in the large or in the small domain only affect the Tm1apo or the Tm2apo values, respectively.
Interestingly, the apo-forms of AGT-Ma and AGT-Mi display a first unfolding phase with a mid-denaturation at 61 °C and 53 °C for AGT-Ma and AGT-Mi, respectively, and then a second unfolding transition with a mid-denaturation at 66 °C for both AGT-Ma and AGT-Mi (Fig. 3). In order to define the impact of the nine pathogenic mutations on the intrinsic stability of AGT, the thermal unfolding of the apo-form of each variant has been measured by far-UV CD (Supplementary Fig. 1) and DSF experiments. Apo-S158L-Ma unfolds in a single-step process with a Tmapo value identical to that of the high-temperature transition (Tm2apo) of apoAGT-Ma, thus suggesting that this large domain mutation does not significantly affect the Tm2apo value. On the other hand, the small domain mutations G350D and P319L, associated with the major and the minor alleles, respectively, cause the complete loss of the high-temperature transition (Tm2apo) without affecting the low-temperature transition (Tm1apo). All together, these findings indicate that the mutations of residues located in the large or in the small domain only affect the Tm1apo or the Tm2apo values, respectively.
Distinct alterations of thermal unfolding parameters were observed for the variants S187F-Ma, G161C-Mi and G161S-Mi in the apo-form. These residue replacements in the large domain give rise to a single thermal transition with a mid-point at temperatures lower than those of the Tm1apo of AGT-Ma or AGT-Mi. On the other hand, a two step process was observed for apo-S218L-Ma and apo-W108R-Mi with both transition left-shifted, and Tmapo values lower than those of apoAGT-Ma and apoAGT-Mi, respectively. These data suggest that these large domain mutations not only exert destabilizing effects of the domain in which they are located but also influence the structural stability of the region responsible for the high-temperature transition.
It should be noted that the analysis of apo-W108R-Mi performed using DSF, unlike that performed using CD, reveals a single Tmapo value similar to the Tm2apo measured by CD. This could be explained by the fact that this apovariant has a ground state fluorescence 100-fold higher than that of apoAGT-Mi, reflecting an increased hydrophobicity due to partial protein unfolding of the native apoprotein.
4. Discussion
To date, about 150 point mutations leading to PH1 have been identified that span throughout all the 11 exons of the AGXT gene and about half of them are missense mutations [6]. While prediction of the effect of splice-site mutations, premature stop-codon insertion and major deletions is straightforward, missense point mutations or small insertions/deletions are more dubious and suitable instruments should be available to evaluate their consequences on the AGT structural and/or functional properties. Until now, the effect of many PH1-causing mutations has been evaluated by either the analysis of patient liver samples or by eukaryotic and/or prokaryotic cell expression experiments [9,11–13,15,16,19,31–35]. These studies led to classify all the known pathogenic mutations according to their effect on the AGT catalytic activity and/or immunoreactivity [7,9,36]. During the last years, however, a more detailed biochemical and bioinformatic characterization of several purified recombinant pathogenic variants in both the holo- and apo-forms allowed identification of their defect(s) at a molecular level [18,20–22]. A more complex scenario of the pathogenic mechanisms responsible for AGT deficiency has emerged, thus highlighting that the heterogeneity of the PH1-causing enzymatic phenotypes could be even wider than previously thought and substantiating the idea that a definition of the defect of the AGT variants at molecular level would be highly desirable.
On these bases, we have explored the structural and functional properties of nine pathogenic variants by using in silico predictions based on the available crystal structure of the protein [17] and by defining some biochemical properties of the variants in their holo- and apo- recombinant purified forms. Each mutation was inserted on the background of either the major or the minor allele on the basis of the genotype of PH1 patients. We report the consequences of each amino acid substitution on: (i) the coenzyme binding mode and affinity and the catalytic activity, which are indicative of the effect of a mutation on the AGT functional properties, and (ii) the quaternary structure and the thermal stability of the protein in the holo- and apo-forms, which together are indicative of the structural effects of a particular mutation and of the possible role of the coenzyme on protein stability.
The first observation that comes from our data concerns the thermostability of AGT-Ma and AGT-Mi in the holo form measured either by the loss of secondary/tertiary structure or by the PLP release. Interestingly, in both of these species (i) the Tmholo and TmPLP values are identical, (ii) the rate of PLP release is higher than that of the global unfolding, and (iii) the Tmholo is lower than the mid-point transition of the NaBH4-reduced form. Thus, since a conversion from the holo- to the apo-form occurs during the thermal transition, the Tmholo values are affected by the sensitivity of the active site to the thermal stress. Nevertheless, our results clearly show that, in agreement with previous studies [21,37], AGT-Ma and AGT-Mi in the apo-form are significantly less stable than the corresponding holo-forms. In addition, while a single transition occurs during the thermal unfolding of the holo-proteins, two transitions with melting temperatures (Tm1apo and Tm2apo) lower than the corresponding Tmholo characterize the thermal unfolding of the apoproteins. Taken together, these data indicate that (i) PLP located at the interface between the two subunits is responsible for the higher stability of holoAGT-Ma and holoAGT-Mi with respect to the corresponding apo-forms, and (ii) the large and small domains unfold in a concerted and cooperative way in the holo-forms, while appear to be differentially stabilized in the apoenzymes. Considering that PLP makes several contacts with the large domain only [17], it can be speculated that in the apoenzymes, but not in the holoenzymes, the large domain could be less stable than the small one. Accordingly, the three state model of thermal denaturation of apoAGT can be described as a process in which a low-temperature transition (Tm1apo), that represents the unfolding of the large domain, is followed by a high-temperature transition (Tm2apo), that represents the unfolding of the small domain, and by irreversible protein denaturation. This interpretation might explain the reason why the two polymorphic mutations typical of the minor allele induce a ~ 8 °C reduction only in the Tm1apo. Previous evidences have indicated that the reduced stability of AGT-Mi might depend on a distortion of the N-terminal arm of the protein caused by the P11L substitution that, in turn, likely destabilizes the dimeric structure of AGT [21]. Since the N-terminal arm wraps over the surface of the opposite subunit that interacts with the large domain, it follows that the distortion of the N-terminus could specifically destabilize the large domain of AGT.
In Table 5 the major defect(s) of the nine pathogenic variants analyzed on crude lysates and on recombinant purified protein are listed. On the basis of the functional and structural features of the pathogenic variants in the holo-form, it can be inferred that (i) D183N-Ma, G350D-Mi and S187F-Ma display exclusively a functional defect. It is reasonable that Asp183, in analogy with what observed upon replacement of Asp222 with asparagine in E. coli aspartate aminotransferase [38], plays a role in stabilizing the positive charge at N(1) of PLP, thus enhancing the electron withdrawing capacity of the coenzyme. Moreover, the functional defect of G350D-Mi, that could be ascribed to as one possible cause of its pathogenicity, might be its high Km l-alanine value. According to the in silico analysis, the substitution of Gly350 with an aspartate residue changes the conformation of the loop 343–357 at the entrance of the AGT active site, thus possibly affecting substrate binding. On the other hand, considering that Ser187 is a residue far from the active site, the features of S187F-Ma in terms of specific activity, PLP binding affinity and mode are unexpected and difficult to explain. (ii) W108R-Mi, S158L-Ma and S218L-Ma are characterized by a functional defect (decreased catalytic activity and PLP binding affinity as well as altered coenzyme binding mode) associated with an increased sensitivity to thermal stress limited to the active site microenvironment. This is not surprising, taking into account that Ser158, Ser218, and Trp108, residues belonging to the large domain, interact directly or indirectly with functional groups of PLP. (iii) HoloGly161 variants show exclusively a structural defect consisting in a decreased thermostability of their active site, and (iv) P319L-Ma in the holo form appears to display neither a functional nor a structural defect. Notably, by looking at the thermal unfolding profiles of these variants in the apo-form, a more detailed and enlightening picture of their molecular defects comes out. Unlike AGT-Ma and AGT-Mi, all the apo forms of the examined variants, except S218L-Ma and W108R-Mi, exhibit a single transition. Based on the Tmapo values reported in Table 4, and considering the thermal unfolding model proposed for apoAGT-Ma and apoAGT-Mi, it can be deduced that the large domain D183N and S158L mutations do not decrease AGT stability, being the Tmapo values of S158L-Ma and D183N-Ma similar to or higher than the Tm2apo of AGT-Ma. On the other hand, the large domain G161S, G161C, S187F, W108R, and S218L mutations cause a destabilizing effect not limited to the large domain but extended to the small domain. In fact, the Tmapo values of S187F-Ma and Gly161 variants are lower than the Tm1apo of AGT-Ma and AGT-Mi, respectively, and both the Tm1apo and the Tm2apo of S218L-Ma and W108R-Mi are lower than the corresponding of AGT-Ma and AGT-Mi, respectively. While this finding is not easy to explain for W108R-Mi and S218L-Ma, it is consistent with the in silico analysis in the case of Gly161 variants and S187F-Ma. In fact, Gly161 is just located at the interface between the large and small domains, and the bulky hindrance of the side chain of phenylalanine at position 187 could cause a mispositioning of the loop 154–168 involved in interdomain interactions. Finally, the small domain P319L and G350D mutations have essentially a structural effect limited to the small domain, being the Tmapo values of the P319L-Ma and G350D-Mi similar to the Tm1apo of AGT-Ma and AGT-Mi, respectively.
Table 5.
Major defect(s) of AGT pathogenic variants analyzed on crude lysates and on recombinant purified proteins.
| Analysis on crude lysates |
Analysis on purified proteins |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AGT variants | Specific activity | Expression level | PLP binding affinity | PLP binding mode | Specific activity | Tmholo and TmPLP | Tmapo | Functional (F) and/or strucural (S) defect | Suggested therapy |
| W108R-Mi | Decreaseda | Decreaseda | Decreased | Altered | Decreased | Decreased | Decreased | F + S | ? |
| S158L-Ma | Decreasedb | Decreasedb | Decreased | Altered | Decreased | Decreased | Slightly increased | F + S | ? |
| G161S-Mi | Decreasedb | Decreasedb | Slightly decreased | Altered | Sligthy decreased | Decreased | Decreased | S | Pyridoxine + chemical chaperones |
| G161C-Mi | Decreasedb | Decreasedb | Slightly decreased | Altered | Slightly decreased | Decreased | Decreased | S | Pyridoxine + chimica chaperones |
| D183N-Ma | Decreaseda | Decreaseda | Decreased | Slightly altered | Decreased | Slightly increased | Slightly increased | F | ? |
| S187F-Ma | Decreaseda | Decreaseda | Increased | Unaltered | Decreased | Unaltered | Decreased | F + S | ? |
| S218L-Ma | Decreased | Decreased | Decreased | Altered | Decreased | Decreased | Decreased | F + S | Chemical chaperones |
| P319L-Ma | ? | ? | Slightly increased | Slightly altered | Slightly decreased | Unaltered | Slightly decreased | S | Pyridoxine |
| G350D-Mi | Decreasedc | ? | Increased | Slightly altered | Decreasedf | Unaltered | Decreased | F + S | l-Alanine + pyridoxine |
Overall, with respect to the analysis performed on crude lysates [9–13,15,16,33,34], the data obtained on purified recombinant variants make clear the diversity of the effect(s) caused by each mutation and strength the importance of looking at both holo and apo forms of pathogenic variants. Finally, the new insights into the molecular basis of the defects of the examined AGT pathogenic variants could be instrumental in suggesting possible therapeutic approaches (Table 5). B6 supplementation is expected to be beneficial for those patients carrying the P319L mutation on the major allele. Since the major impact of this mutation is on the thermostability of the apo-form of AGT, the coenzyme is expected to shift the equilibrium toward the holo-form. Patients with G161 mutations on the minor allele could benefit by pyridoxine treatment in association with chemical chaperones because these mutations reduce the thermostability of both the holo and apo-forms of G161 variants. In the case of patients bearing the G350D mutation on the minor allele, a combination of l-alanine and pyridoxine could be suggested to overcome the reduced affinity for the substrate and the reduced thermal stability of the apo form. Again, the administration of molecules acting as chemical chaperones could be effective for patients harboring the S218L mutation on the major allele, considering that the S218L-Ma variant has a reduced thermal stability in both the holo and apo forms and retains a rather significant specific activity. On the other hand, no suggestions can be advanced for a pharmacological treatment of patients carrying the W108R mutation on the minor allele or the S158L, D183N and S187F mutations on the major allele.
5. Conclusions
In conclusion, our characterization of the AGT variants in their recombinant purified form provided evidence for structural and/or functional defects of each enzymatic species. The in vitro approach presented in this study permits to: (i) reassess previous data obtained in crude cellular lysates, (ii) define whether the impact of each mutation is limited to the active site microenvironment or extended to the large and/or the small domain, thus posing itself as a valid instrument to shed light on the molecular defects of other PH1-causing variants and (iii) suggest possible treatments for patients bearing the examined mutations.
The following are the supplementary materials related to this article
Far-UV CD-monitored heating scans of AGT pathogenic variants. Far-UV CD changes of the labeled pathogenic AGT variants in the holo-form (panels A and B) in the presence of saturating PLP concentrations, and in the apo-form (panels C and D). In each case, the enzyme concentration was 10 μM, and the buffer was 100 mM potassium phosphate, pH 7.4.
Oligonucleotide primers used in site-directed mutagenesis.
Supplementary materials related to this article can be found online at doi:10.1016/j.ymgme.2011.09.033
Footnotes
This work was supported by Telethon Foundation (grant GGP10092 to C.B.V.).
References
- 1.Danpure C.J., Rumsby G. Molecular aetiology of primary hyperoxaluria and its implications for clinical management. Expert Rev. Mol. Med. 2004;6:1–16. doi: 10.1017/S1462399404007203. [DOI] [PubMed] [Google Scholar]
- 2.Danpure C.J., Jennings P.R. Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett. 1986;201:20–24. doi: 10.1016/0014-5793(86)80563-4. [DOI] [PubMed] [Google Scholar]
- 3.Purdue P.E., Lumb M.J., Fox M., Griffo G., Hamon-Benais C., Povey S., Danpure C.J. Characterization and chromosomal mapping of a genomic clone encoding human alanine:glyoxylate aminotransferase. Genomics. 1991;10:34–42. doi: 10.1016/0888-7543(91)90481-s. [DOI] [PubMed] [Google Scholar]
- 4.Purdue P.E., Lumb M.J., Allsop J., Danpure C.J. An intronic duplication in the alanine: glyoxylate aminotransferase gene facilitates identification of mutations in compound heterozygote patients with primary hyperoxaluria type 1. Hum. Genet. 1991;87:394–396. doi: 10.1007/BF00197154. [DOI] [PubMed] [Google Scholar]
- 5.Purdue P.E., Takada Y., Danpure C.J. Identification of mutations associated with peroxisome-to-mitochondrion mistargeting of alanine/glyoxylate aminotransferase in primary hyperoxaluria type 1. J. Cell Biol. 1990;111:2341–2351. doi: 10.1083/jcb.111.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams E.L., Acquaviva C., Amoroso A., Chevalier F., Coulter-Mackie M., Monico C.G., Giachino D., Owen T., Robbiano A., Salido E., Waterham H., Rumsby G. Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum. Mutat. 2009;30:910–917. doi: 10.1002/humu.21021. [DOI] [PubMed] [Google Scholar]
- 7.Danpure C.J., Cooper P.J., Wise P.J., Jennings P.R. An enzyme trafficking defect in two patients with primary hyperoxaluria type 1: peroxisomal alanine/glyoxylate aminotransferase rerouted to mitochondria. J. Cell Biol. 1989;108:1345–1352. doi: 10.1083/jcb.108.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danpure C.J., Jennings P.R., Fryer P., Purdue P.E., Allsop J. Primary hyperoxaluria type 1: genotypic and phenotypic heterogeneity. J. Inherit. Metab. Dis. 1994;17:487–499. doi: 10.1007/BF00711363. [DOI] [PubMed] [Google Scholar]
- 9.Lumb M.J., Danpure C.J. Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations. J. Biol. Chem. 2000;275:36415–36422. doi: 10.1074/jbc.M006693200. [DOI] [PubMed] [Google Scholar]
- 10.Coulter-Mackie M.B., Lian Q., Applegarth D., Toone J. The major allele of the alanine:glyoxylate aminotransferase gene: nine novel mutations and polymorphisms associated with primary hyperoxaluria type 1. Mol. Genet. Metab. 2005;86:172–178. doi: 10.1016/j.ymgme.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Purdue P.E., Lumb M.J., Allsop J., Minatogawa Y., Danpure C.J. A glycine-to-glutamate substitution abolishes alanine:glyoxylate aminotransferase catalytic activity in a subset of patients with primary hyperoxaluria type 1. Genomics. 1992;13:215–218. doi: 10.1016/0888-7543(92)90225-h. [DOI] [PubMed] [Google Scholar]
- 12.Williams E., Rumsby G. Selected exonic sequencing of the AGXT gene provides a genetic diagnosis in 50% of patients with primary hyperoxaluria type 1. Clin. Chem. 2007;53:1216–1221. doi: 10.1373/clinchem.2006.084434. [DOI] [PubMed] [Google Scholar]
- 13.Coulter-Mackie M.B., Lian Q. Partial trypsin digestion as an indicator of mis-folding of mutant alanine:glyoxylate aminotransferase and chaperone effects of specific ligands. Study of a spectrum of missense mutants. Mol. Genet. Metab. 2008;94:368–374. doi: 10.1016/j.ymgme.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Basmaison O., Rolland M.O., Cochat P., Bozon D. Identification of 5 novel mutations in the AGXT gene. Hum. Mutat. 2000;15:577. doi: 10.1002/1098-1004(200006)15:6<577::AID-HUMU9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Minatogawa Y., Tone S., Allsop J., Purdue P.E., Takada Y., Danpur C.J., Kido R. A serine-to-phenylalanine substitution leads to loss of alanine:glyoxylate aminotransferase catalytic activity and immunoreactivity in a patient with primary hyperoxaluria type 1. Hum. Mol. Genet. 1992;1:643–644. doi: 10.1093/hmg/1.8.643. [DOI] [PubMed] [Google Scholar]
- 16.Danpure C.J., Purdue P.E., Fryer P., Griffiths S., Allsop J., Lumb M.J., Guttridge K.M., Jennings P.R., Scheinman J.I., Mauer S.M. Enzymological and mutational analysis of a complex primary hyperoxaluria type 1 phenotype involving alanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting and intraperoxisomal aggregation. Am. J. Hum. Genet. 1993;53:417–432. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Roe S.M., Hou Y., Bartlam M., Rao Z., Pearl L.H., Danpure C.J. Crystal structure of alanine:glyoxylate aminotransferase and the relationship between genotype and enzymatic phenotype in primary hyperoxaluria type 1. J. Mol. Biol. 2003;331:643–652. doi: 10.1016/s0022-2836(03)00791-5. [DOI] [PubMed] [Google Scholar]
- 18.Cellini B., Bertoldi M., Montioli R., Paiardini A., Borri Voltattorni C. Human wild-type alanine:glyoxylate aminotransferase and its naturally occurring G82E variant: functional properties and physiological implications. Biochem. J. 2007;408:39–50. doi: 10.1042/BJ20070637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulter-Mackie M.B., Lian Q. Consequences of missense mutations for dimerization and turnover of alanine:glyoxylate aminotransferase: study of a spectrum of mutations. Mol. Genet. Metab. 2006;89:349–359. doi: 10.1016/j.ymgme.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Cellini B., Montioli R., Paiardini A., Lorenzetto A., Maset F., Bellini T., Oppici E., Voltattorni C.B. Molecular defects of the glycine 41 variants of alanine glyoxylate aminotransferase associated with primary hyperoxaluria type I. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2896–2901. doi: 10.1073/pnas.0908565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellini B., Lorenzetto A., Montioli R., Oppici E., Voltattorni C.B. Human liver peroxisomal alanine:glyoxylate aminotransferase: different stability under chemical stress of the major allele, the minor allele, and its pathogenic G170R variant. Biochimie. 2010;92:1801–1811. doi: 10.1016/j.biochi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Cellini B., Montioli R., Paiardini A., Lorenzetto A., Voltattorni C.B. Molecular insight into the synergism between the minor allele of human liver peroxisomal alanine:glyoxylate aminotransferase and the F152I mutation. J. Biol. Chem. 2009;284:8349–8358. doi: 10.1074/jbc.M808965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monico C.G., Olson J.B., Milliner D.S. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am. J. Nephrol. 2005;25:183–188. doi: 10.1159/000085411. [DOI] [PubMed] [Google Scholar]
- 24.Monico C.G., Rossetti S., Olson J.B., Milliner D.S. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 2005;67:1704–1709. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 25.van Woerden C.S., Groothoff J.W., Wijburg F.A., Annink C., Wanders R.J., Waterham H.R. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 2004;66:746–752. doi: 10.1111/j.1523-1755.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 26.De Lano W. DeLano Scientifics; San Carlos, CA: 2002. The pyMol Molecular Graphics System. [Google Scholar]
- 27.Cellini B., Bertoldi M., Borri Voltattorni C. Treponema denticola cystalysin catalyzes beta-desulfination of l-cysteine sulfinic acid and beta-decarboxylation of l-aspartate and oxalacetate. FEBS Lett. 2003;554:306–310. doi: 10.1016/s0014-5793(03)01178-5. [DOI] [PubMed] [Google Scholar]
- 28.Pace C.N., Shirley B.A., Thompson J.T. Measuring the Conformational Stability of a Protein. In: Creighton T.E., editor. Protein Structure, a Pratical Approach. IRL Press; IRL Press, Oxford, England: 1989. pp. 311–330. [Google Scholar]
- 29.Niesen F.H., Berglund H., Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat. Protoc. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- 30.von Schnakenburg C., Rumsby G. Identification of new mutations in primary hyperoxaluria type 1 (PH1) J. Nephrol. 1998;11(Suppl 1):15–17. [PubMed] [Google Scholar]
- 31.Danpure C.J. Molecular etiology of primary hyperoxaluria type 1: new directions for treatment. Am. J. Nephrol. 2005;25:303–310. doi: 10.1159/000086362. [DOI] [PubMed] [Google Scholar]
- 32.Danpure C.J. Primary hyperoxaluria type 1: AGT mistargeting highlights the fundamental differences between the peroxisomal and mitochondrial protein import pathways. Biochim. Biophys. Acta. 2006;1763:1776–1784. doi: 10.1016/j.bbamcr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Coulter-Mackie M.B., Applegarth D., Toone J.R., Henderson H. The major allele of the alanine:glyoxylate aminotransferase gene: seven novel mutations causing primary hyperoxaluria type 1. Mol. Genet. Metab. 2004;82:64–68. doi: 10.1016/j.ymgme.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Coulter-Mackie M.B., Lian Q., Wong S.G. Overexpression of human alanine:glyoxylate aminotransferase in Escherichia coli: renaturation from guanidine-HCl and affinity for pyridoxal phosphate co-factor. Protein Expr. Purif. 2005;41:18–26. doi: 10.1016/j.pep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Salido E.C., Li X.M., Lu Y., Wang X., Santana A., Roy-Chowdhury N., Torres A., Shapiro L.J., Roy-Chowdhury J. Alanine–glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18249–18254. doi: 10.1073/pnas.0607218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danpure C.J., Fryer P., Griffiths S., Guttridge K.M., Jennings P.R., Allsop J., Moser A.B., Naidu S., Moser H.W., MacCollin M. Cytosolic compartmentalization of hepatic alanine:glyoxylate aminotransferase in patients with aberrant peroxisomal biogenesis and its effect on oxalate metabolism. J. Inherit. Metab. Dis. 1994;17:27–40. doi: 10.1007/BF00735393. [DOI] [PubMed] [Google Scholar]
- 37.Hopper E.D., Pittman A.M., Fitzgerald M.C., Tucker C.L. In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J. Biol. Chem. 2008;283:30493–30502. doi: 10.1074/jbc.M803525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano T., Kuramitsu S., Tanase S., Morino Y., Kagamiyama H. Role of Asp222 in the catalytic mechanism of Escherichia coli aspartate aminotransferase: the amino acid residue which enhances the function of the enzyme-bound coenzyme pyridoxal 5′-phosphate. Biochemistry. 1992;31:5878–5887. doi: 10.1021/bi00140a025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Far-UV CD-monitored heating scans of AGT pathogenic variants. Far-UV CD changes of the labeled pathogenic AGT variants in the holo-form (panels A and B) in the presence of saturating PLP concentrations, and in the apo-form (panels C and D). In each case, the enzyme concentration was 10 μM, and the buffer was 100 mM potassium phosphate, pH 7.4.
Oligonucleotide primers used in site-directed mutagenesis.