Abstract
Background:
Although the majority of smooth muscle neoplasms found in the uterus are benign, uterine leiomyosarcoma is extremely malignant, with high rates of recurrence and metastasis. The development of gynecologic tumors is often correlated with secretion of female hormone; however, the development of human uterine leiomyosarcoma is not substantially correlated with hormonal conditions, and the risk factors are unclearly understood. Importantly, a diagnostic-biomarker, which distinguishes malignant human uterine leiomyosarcoma from benign tumor leiomyoma is yet to be established.
Aims:
It is necessary to analyze risk factors associated with human uterine leiomyosarcoma, in order to establish a diagnostic-biomarker and a clinical treatment method.
Patients and Methods:
Histology and Immunofluorescence Staining: Uteri obtained from LMP2–/– mice or its parental mice (C57BL/6 mice) were fixed in 10% buffered formalin, incubated in 4% paraformaldehyde for 8 hours, and embedded in paraffin. Tissue sections (5 μm) were prepared and stained with H&E for routine histological examination or were processed further for immunofluorescence staining with appropriate antidodies. Furthermore, a total of 101 patients between 32 and 83 years of age and diagnosed as having smooth muscle tumors of the uterus were selected from pathological files. Immunohistochemistry staining for LMP2 was performed on serial human uterine leiomyosarcoma, leiomyoma and myometrium sections.
Results:
Homozygous deficient mice for a proteasome β1i subunit, LMP2 spontaneously develop uterine leiomyosarcoma, with a disease prevalence of ~40% by 14 months of age. Defective LMP2 expression in human uterine leiomyosarcoma was demonstrated, but present in human leiomyoma and myometrium.
Conclusions:
Loss in LMP2 expression may be one of the risk factors for human uterine leiomyosarcoma. LMP2 may be a potential diagnostic-biomarker and targeted-molecule for a new therapeutic approach.
Keywords: LMP2, uterine leiomyosarcoma, uterine leiomyoma, diagnostic-biomarker
Introduction
The uterus is the female reproductive organ, located at the center of the pelvis between the left and right ovaries. The uterus, the organ in which the embryo grows, is composed of three layers, the uterine endometrium, myometrium and a serious membrane enveloping the uterus, the myometrium is composed of smooth muscle. In general, the term uterine tumor refers to an epithelial malignant tumor of the uterus, which is roughly classified as a tumor of the uterine cervix or the uterine body. Because of the prevalence of screening, uterine cervix cancer is decreasing in incidence, and usually detected at an early stage, including stage 0. In contrast, cancer of the uterine body is increasing in incidence, and rarely detected at the initial stages. While most tumors of the uterine body are adenocarcinoma derived from the subintimal gland, tumors of the uterine cervix are classified into squamous cancer and adenocarcinoma. Smooth muscle tumors (SMTs) which develop in the myometrium have been traditionally divided into benign leiomyoma (LMA) and malignant leiomyosarcoma (LMS) based on cytological atypia, mitotic activity and other criteria. Uterine LMS is a rare gynecologic malignancy in the female genital tract, having an estimated annual incidence of 0.64 per 100,000 women[1]. Uterine LMS accounts for 2% to 5% of tumors of the uterine body and develops more often in the muscle layer of the uterine body than in the uterine cervix[2,3]. Distinguishing uterine LMA from uterine LMS is very difficult, and a diagnosis generally requires surgery and cytoscopy.
A main factor in the development of tumors in the uterine body is the hormonal environment. Patients with uterine body tumors often are unmarried, have never been pregnant, and are taking a hormonal agent. High estrogen levels are considered to significantly influence the development of such tumors. The mechanisms by which uterine LMA and LMS develop are not yet understood, though tumor that have developed in the myometrium for some reason gradually become larger due to the influence of the female hormone, estrogen, and generate tumors. However, no correlation between the development of uterine LMS and hormonal conditions, and no obvious risk factors have been identified. The prognosis of uterine LMS is not good, and the five-year survival rate is approximately 35%, although the five-year survival rate depends on disease stage[2,3]. It is worth noting that, when adjusted for stage and mitotic count, LMS has a significantly worse prognosis than carcinosarcoma[4]. As uterine LMS is resistant to chemotherapy and radiotherapy, and thus surgical intervention is virtually the only means of treatment, developing an efficient adjuvant therapy is expected to improve the prognosis of the disease[5–7]. The identification of a risk factor associated with the development of uterine LMS would significantly contribute to the development of preventive and therapeutic treatments.
Materials and Methods
Mice: C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). LMP2–/– mice were a generous donation from Dr. Luc Van Kaer (Vanderbilt University School of Medicine, Nashville, TN).
Histology and Immunofluorescence Staining: Uteri obtained from LMP2–/– mice or its parental mice (C57BL/6 mice) were fixed in 10% buffered formalin, incubated in 4% paraformaldehyde for 8 h, and embedded in paraffin. Tissue sections (5 μm) were prepared and stained with H & E for routine histological examination or were processed further for immunofluorescence staining with appropriate antidodies. After removal of paraffin by 100% xylene, the sections were treated at 95°C for 5 min and incubated for 2 h at room temperature with antibodies to Ki-67 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA.). The sections were then washed in PBS, incubated with FITC-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA.), and examined with a fluorescence microscope.
Immunohistochemistry (IHC)
IHC staining for LMP2 and Ki-67 was performed on serial human uterine LMS or LMA sections. Antibody for Ki-67(MIB-1) were purchased from Immunotech (Marseille, France). The LMP2 antibody was produced by SIGMA-Aldrich Israel Ltd. (Rehovot, Israel). IHC was performed using the avidin-biotin complex method as described previously. Briefly, one representative 5-μm tissue section was cut from a paraffin-embedded sample of a radical hysterectomy specimen from each patient with uterine LMS. Sections obtained from patients were deparaffinized and rehydrated in graded concentrations of alcohol, incubated with normal mouse serum for 20 min, and then incubated at room temperature for 1 h with primary antibody. Afterwards, sections were incubated with a biotinylated secondary antibody (Dako, CA, USA.) and then exposed to a streptavidin complex (Dako). The completed reaction was revealed by 3, 3’-diaminobenzidine, and the slide was counterstained with hematoxylin. Normal myometrium portions in the specimens were used as positive controls. Negative controls consisted of tissue sections incubated with normal rabbit IgG instead of the primary antibody. These experiments were registered at Shinshu University in accordance with local guidelines (approval no. M192).
Biological Functions of the Proteasome
Cytoplasmic proteins are mostly degraded by a protease complex, which has many substrates consisting of twenty-eight 20 to 30-kDa subunits, referred to as the 20S proteasome[8]. The proteasomal degradation is essential for many cellular processes, including the cell cycle, the regulation of gene expression. The proteasomal degradation pathway is also essential for the production of peptide antigens presented by the major histocompatibility complex (MHC) class I, the proteasome plays a key role in the presentation of immunological self markers on the cell surface by MHC[8]. Interferon-γ (IFN-γ) induces the expression of large numbers of responsive genes, proteasome subunits, i.e., low-molecular mass polypeptide (LMP) 2, LMP7, and LMP10 are also markedly induced by IFN-γ signal[9–12]. The biological function of reconstructive proteasome by IFN-γ signal, called immuno-proteasome, plays a key role in MHC class I-mediated tumor rejection[11,13]. Further, a molecular approach to studying the correlation of IFN-γ with tumor cell growth has drawn attention. Here we identify LMP2, a single IFN-γ-responsive gene product, as obligatory for tumor surveillance[12] and demonstrate a tissue-specific role for LMP2 in protection from spontaneous neoplasms of the uterus.
Correlation Between LMP2-Deficient and Leiomyosarcome Tumorigenesis
Malignant tumors originate from a single cancerous cell and develop as a result of unlimited cell proliferation. Malignant tumor cells have properties that are biologically different from those of normal cells, thus, the host immune-system should be able to distinguish malignant tumor cells from corresponding normal cells. That is, malignant tumor cells present intrinsic antigens as tumor-antigens (TA) on the tumor cell surface with the aid of MHC. In many cases, however, almost no reaction by the immune-system is observed. Also, the incidence of major tumors is not very different between immunodeficient (i.e., lymphocyte-deficient) mice and control mice having normal immune-systems. Specifically, tumor cells can avoid the immune monitoring system via several means[14,15]. Naturally-occurring tumor cells seem to have lost the expression of peptide antigens, TA, or cell-adhesion factors intrinsic to tumors, tumor cells therefore may avoid the host immune reaction.
The homozygous deficient mice for LMP2 show tissue- and substrate-dependent abnormalities in the biological functions of the proteasome, and impaired functioning of the proteasome in the splenocytes or hepatic cells[16]. We found that uterine LMS occurred in female LMP2–/– mice at age 6 months or older, and the incidence at 14 months of age was about 40%[17] (Figure 1). The curve indicating the incidence in mice is similar to that indicating the incidence of human uterine LMS, which occurs after menopause. Histological studies of LMP2-lacking uterine tumors revealed characteristic abnormalities of uterine LMS[17]. The tumors lacked lymphoid infiltrates, a sign of immune recognition, and consisted of uniform elongated myometrium cells arranged into bundles. The nuclei of the tumor cells varied in size and shape; furthermore, mitosis was frequent, in contrast, the myometrium cells of C57BL/6 mice were normal in appearance[17]. Whereas relatively few Ki-67-positive cells, the proliferating cells of solid tumors, were observed in the basal cell layer of the normal myometrium, most of the basal cells vividly expressed Ki-67 in LMP2–/– mice[17]. This immunological staining indicates abnormal proliferation of the LMP2-lacking cells in the basal layer[17]. LMP2–/– mice that have developed uterine LMS undergo considerable weight loss, and then die by 14 months of age[17] (Figure 1). The LMP2–/– mice also exhibit skeletal muscle metastasis from uterine LMS. Therefore it is like that LMP2–/– mice with uterine LMS have died of mass effect and metastasis. In general, it is not easy to distinguish uterine LMA from LMS, however, in mice, because of such characteristic pathological findings, significant weight loss, and exhibition of skeletal muscle metastasis, a tumor that develops in the uterus of an LMP2–/– mouse can be considered malignant, i.e., a uterine LMS. Furthermore, IHC revealed a serious loss in the ability to induce LMP2 expression in human uterine LMS tissue in comparison with LMA or normal myometrium located in the same section[18]. Of the 54 cases we examined with uterine LMS, 46 were negative for LMP2 expression, 4 were focally positive, and 2 were partially positive. Two LMS cases were stained for LMP2. LMP2 levels were also evaluated in skeletal muscle and rectum metastases from individual uterine LMS patients. Pathological examination of surgical samples showed the presence of a mass measuring 3 cm at its largest diameter in the lumbar quadrate muscle without a fibrous capsule. All lymph nodes were negative for LMS metastases, and IHC analyses showed positivity for Ki-67 and negativity for LMP2. Histological findings were consistent with metastatic LMS for the skeletal muscle and rectum lesions.
Fig. 1.
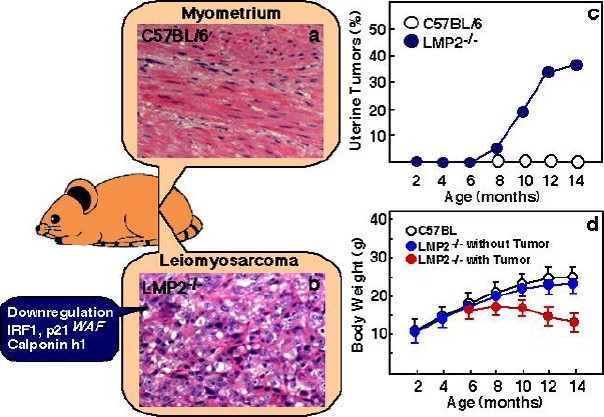
Histological findings of myometrium (a) and uterine LMS in LMP2-deficient mice (b). Among the histological findings of uterine LMS in LMP2-deficient mice, a cytoskeleton, which is characteristic of uterine LMS, is observed. (a and b magnification x200) Panel c, in LMP2-deficient females, uterine LMS is observed at 6 months of age. The incidence at age 14 months is as high as 40%. The curve indicating the incidence of mouse uterine LMS is very similar of that indicating the incidence of human uterine LMS, which is observed after menopause. In mice with tumors of the uterus, significant weight loss is observed (d). Thus, a tumor that develops in the uterus is diagnosed as malignant, i.e., uterine LMS. In LMP2-deficient cells, levels of the anti-oncogenic factor IRF-1, p21WAF are significantly reduced. Reduced expression of the calponin h1 transcript, which contributes to cell proliferation and tumorigenesis in myometrium cells, is detected in uterine LMS tissues. The inactivation of such anti-oncogenic factors is considered to transform LMP2-deficient cells into leiomyosarcoma cells.
Biological functions of Anti-oncogenic Factors on uterine Leiomyosarcoma Tumorigenesis
Uterine LMS was demonstrated to spontaneously develop in 6-month-old LMP2–/– mice at high frequency. The expression of cell-cycle regulators that are regulated by the IFN-γ signal or immuno-proteasome activity was examined under the LMP2-deficient condition. Signal transducer and activator of transcription (STAT) 1, having been activated by IFN-γ, significantly induced expression of tumor suppressors such as interferon regulatory factor 1 (IRF1), which markedly regulates LMP2 gene expression as a transcriptional regulator[18,19]. It was examined whether the IFN-γ signal induces the expression of each subunit of the immuno-proteasome, IFR1 and IRF2 in LMP2–/– mice and the its parental strain, C57BL/6. IFN-γ-induced phosphorylation of STAT1 would not be influenced by a lack of LMP2, however, the IRF1 expression was markedly reduced in splenocytes derived from LMP2–/– mice in comparison with wild-type mice. Recent reports suggest that function of proteasome subunit contributes to mRNA transcriptional activation[20,21]. Accordingly, the transcription of IRF1 mRNA may depend on the function of proteasome subunits.
Primary cultured tumor cells (LMP2-ULMS) were established from the uterine LMS of LMP2–/– mice, the IRF1 expression was also markedly reduced in the LMP2-ULMS. The IRF1 reportedly has the inhibiting effects on tumor cell proliferation[19,22]. Thus, a lowered level of IRF1 resulting from a deficiency in LMP2 might seem to be a risk factor for uterine LMS in mice. The effects of IRF1 on tumor cell proliferation are achieved through the expression of p21WAF, which induce G1 arrest[23]. Whether or not p21WAF expression or activation is affected in LMP2–/– mice should be examined further.
If the TP53 gene is damaged, tumor suppression is severely reduced. People who inherit only one functional copy of the TP53 gene will most likely develop tumors in early adulthood, more than 50 percent of human tumors contain a mutation or deletion of the TP53 gene[24]. To increase tumor incidence and better assess the role of systemic expression of TP53 in responses to initiation of uterine LMS tumorigenesis, LMP2–/– mice were bred with TP53-deficient mice to create Lmp2-/- TP53-/- double knockout mice. Uterine LMS incidence and death rates were similar in LMP2-/- TP53-/- mice and closely matched control LMP2-/-TP53+/+ mice. The correlation of defective TP53 function with uterine LMS tumorigenesis is unclearly understood. The tumor suppressor, retinoblastoma (Rb) is phosphorylated by a complex of Cyclin E/Cyclin dependent kinase 2 (CDK2) and then inactivated, the activity of CDK2 is also negatively regulated via degradation of Cyclin E by the proteasome[25–27]. A level of phosphorylated-Rb is clearly observed in mouse embryonic fibroblast (MEF)-lacking LMP2, and the activity of CDK2 for phosphorylation is determined to be stronger than that in normal MEF. However research over all, including experiments with gene-deficient mouse models and clinical studies, suggests that defective Rb expression does not take part in the onset of uterine LMS[28–30].
Conclusion
Uterine LMS mainly develops in the myometrium or endometrial stroma, and menstrual anomalies, such as hypermenorrhea and prolonged menstruation, and symptoms such as abnormal hemorrhage, hypogastric pain, lumbar pain, and abdominal strains, are observed[4]. As in the case of uterine LMA, however, a correlation between the development of uterine LMS, the female hormone and hormone receptors has yet to be elucidated[31,32]. A recent report showed the LMP2 expression in luminal and glandular epitheliua, placenta villi, trophoblastic shells, and arterial endothelial cells[33]. These results implicate LMP2 in the invasion of placental villi, degradation of the extracellular matrix, immune tolerance, glandular secretion, and angiogenesis[33]. The present study should help to elucidate the regulatory role of the proteasome pathway in the implantation of embryos. Uterine LMS often seems to develop in individuals exposed to radiation in the pelvis. Risk factors for its development however, have not been identified because of the absence of a suitable animal model. The LMP2–/– mouse was the first animal model of spontaneous uterine LMS to be established[17]. To demonstrate whether LMP2 is a potential biomarker for distinguishing LMS from LMA, we are investigating the reliability and characteristics of LMP2 as a diagnostic indicator with several clinical research facilities. The clinical research is yet to be concluded, and large-scale clinical studies need to be performed. Definitive histological studies must be performed, including the gene-expression profiling of several known pro-oncogenic factors as well as factors such as brain-specific polypeptide PEP-19 and a transmembrane tyrosine kinase receptor, c-kit[34–36]. The reduced expression of calponin h1 transcripts was reported to be associated with uterine LMS, and calponon h1 might function as a tumor suppressor in uterine LMS[37,38].
Since no spontaneous development of uterine LMS is observed in IRF1-, calponin h1-deficient mice or heterozygous Rb mice, the lack of LMP2 may be largely associated with the expression of other known or unknown cell-cycle regulatory factors or oncogenic factors (Figure 1). Further research is required to demonstrate the correlative functions of LMP2 and other anti-oncogenic factors with calponin h1 in the tumorigenesis of uterine LMS. Clarification of the correlation between these factors and the development of uterine LMS and the identification of specific risk factors may lead to the development of new treatments for the disease. Uterine LMS is refractory to chemotherapy and has a poor prognosis. The molecular biological and cytological information obtained from LMP2-deficient mice will contribute remarkably to the development of preventive methods, a potential diagnostic- biomarker, and new therapeutic approaches against uterine LMS.
Acknowledgments
We sincerely thank Professor Luc Van Kaer (Vanderbilt University Medical Center). This study was supported in part by grants from the Ministry of Education, Culture, Science and Technology, and The Foundation of Osaka Cancer Research, The Ichiro Kanehara Foundation for the Promotion of Medical Science and Medical Care, The foundation for the Promotion of Cancer Research, The Kanzawa Medical Research Foundation, The Shinshu Medical Foundation, and The Takeda Foundation for Medical Science.
References
- 1.Zaloudek C, Hendrickson MR. Mesenchymal tumors of the uterus. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. (ed 5) New York, Springer-Verlag: 2002. pp. 561–578. [Google Scholar]
- 2.Lin JF, Slomovitz BM. Uterine sarcoma Curr Oncol Rep. 2008;10:512–518. doi: 10.1007/s11912-008-0077-9. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006;48:97–105. doi: 10.1111/j.1365-2559.2005.02292.x. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Dusenbery KE. Limitations of adjuvant radiotherapy for uterine sarcomas spread beyond the uterus. Gynecol Oncol. 2004;94:191–196. doi: 10.1016/j.ygyno.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Wu TI, Chang TC, Hsueh S, et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100:166–172. doi: 10.1016/j.ygyno.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Maniatis T. A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- 9.Groettrup M, Khan S, Schwarz K, et al. Interferon-ν inducible exchanges of 20S proteasome active site subunits: why? Biochimie. 2001;83:367–372. doi: 10.1016/s0300-9084(01)01251-2. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima C, Uekusa Y, Iwasaki M, et al. A role of interferon-γ (IFN-γ) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-γ-deficient mice. Cancer Res. 2001;61:3399–3405. [PubMed] [Google Scholar]
- 11.Shankaran V, Ikeda H, Bruce AT, et al. IFN-γ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 12.Gaczynska M, Rock KL, Goldberg AL. Interferon-γ and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365:264–267. doi: 10.1038/365264a0. [DOI] [PubMed] [Google Scholar]
- 13.Delp K, Momburg F, Hilmes C, et al. Functional deficiencies of components of the MHC class I antigen pathway in human tumors of epithelial origin. Bone Marrow Transplant. 2000;26(Suppl 2):88–95. doi: 10.1038/sj.bmt.1702363. [DOI] [PubMed] [Google Scholar]
- 14.Dunn GP, Bruce AT, Ikeda H, et al. Cancer Immunoediting: from immuno-surveillance to tumor escape. Nature Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 15.Dunn GP, Old LT, Schreiber RD. The Immunobiology of Cancer Immuno-surveillance and Immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Van Kaer L, Ashton-Rickardt PG, Eichelberger M, et al. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Faustman DL. Development of spontaneous uterine tumors in low molecular mass polypeptide-2 knockout mice. Cancer Res. 2002;62:24–27. [PubMed] [Google Scholar]
- 18.Hayashi T, Kobayashi Y, Kohsaka S, et al. The mutation in the ATP-binding region of JAK1, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene. 2006;25:4016–4026. doi: 10.1038/sj.onc.1209434. [DOI] [PubMed] [Google Scholar]
- 19.Brucet M, Marques L, Sebastian C, et al. Regulation of murine Tap1 and Lmp2 genes in macrophages by interferon gamma is mediated by STAT1 and IRF-1. Genes and Immunity. 2004;5:25–35. doi: 10.1038/sj.gene.6364035. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi S, Shimbara N, Tamura TA. Tissue and Cell Distribution of a Mammalian Proteasomal ATPase, MSS1, and Its Complex Formation with the Basal Transcription Factors. Biochem Biophys Res Commun. 2000;279:568–573. doi: 10.1006/bbrc.2000.3969. [DOI] [PubMed] [Google Scholar]
- 21.Irina I, Lassot D, Latreille D, et al. The Proteasome Regulates HIV-1 Transcription by Both Proteolytic and Nonproteolytic Mechanisms. Molecular Cell. 2007;25:369–383. doi: 10.1016/j.molcel.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Harada H, Kitagawa M, Tanaka N, et al. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993;259:971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka N, Ishihara M, MLamphier MS, et al. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature. 1996;382:816–818. doi: 10.1038/382816a0. [DOI] [PubMed] [Google Scholar]
- 24.Hollstein M, Sidransky D, Vogelstein B, et al. p53 mutations in human cancers. Science. 1991;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 25.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 26.Koepp DM, Schaefer LK, Ye X, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 28.Liang SX, Lakshmanan Y, Woda BA, et al. A high grade primary leiomyosarcoma of the bladder in a survivor of retinoblastoma. Arch Pathol Lab Med. 2001;125:1231–1234. doi: 10.5858/2001-125-1231-AHGPLO. [DOI] [PubMed] [Google Scholar]
- 29.Venkatraman L, Goepel JR, Steele K, et al. Soft tissue, pelvic, and urinary bladder Soft tissue, pelvic, and urinary bladder hereditary retinoblastoma. J Clin Pathol. 2003;56:233–236. doi: 10.1136/jcp.56.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calo E, Quintero-Estades JA, Danielian PS, et al. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai YL, Kobayashi Y, Mori A, et al. Expression of steroid receptors, Ki-67, and p53 in uterine leiomyosarcomas. Int J Gynecol Pathol. 1999;18:20–28. doi: 10.1097/00004347-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Akhan SE, Yavuz E, Tecer A, et al. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas.A clinicopathologic study. Gynecol Oncol. 2005;99:36–42. doi: 10.1016/j.ygyno.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Wang HX, Wang HM, Li QL, Lin HY, et al. Expression of Proteasome Subunits Low Molecular Mass Polypeptide (LMP) 2 and LMP7 in the Endometrium and Placenta of Rhesus Monkey (Macaca mulatta) During Early Pregnancy. Biology Reprod. 2004;71:1317–1324. doi: 10.1095/biolreprod.104.030213. [DOI] [PubMed] [Google Scholar]
- 34.Kanamori T, Takakura K, Mandai M, et al. PEP-19 overexpression in human uterine leiomyoma. Mol Hum Reprod. 2003;9:709–717. doi: 10.1093/molehr/gag088. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Felix JC, Lee JL, et al. The proto-oncogene c-kit is expressed in leiomyosarcomas of the uterus. Gynecol Oncol. 2003;90:402–406. doi: 10.1016/s0090-8258(03)00274-9. [DOI] [PubMed] [Google Scholar]
- 36.Ylisaukko-oja SK, Kiuru M, Lehtonen HJ. Analysis of fumarate hydratase mutations in a population-based series of early onset uterine leiomyosarcoma patients. Int J Cancer. 2006;119:283–287. doi: 10.1002/ijc.21798. [DOI] [PubMed] [Google Scholar]
- 37.Horiuch A, Nikaido T, Ito K, et al. Reduced expression of Calponin h1 in Leiomyosarcoma of the Uterus. Lab Invest. 1998;78:839–846. [PubMed] [Google Scholar]
- 38.Hriuchi A, Nikaido T, Taniguchi S, et al. Possible Role of Calponin h1 as a Tumor suppressor in human uterine leiomyosarcoma. J Natl Can Inst. 1999;91:790–796. doi: 10.1093/jnci/91.9.790. [DOI] [PubMed] [Google Scholar]


