Abstract
The abstract is available at the Clinical pancreatic disorder I: Acute pancreatitis. North Am J Med Sci 2011; 3: 316-319. doi: 10.4297/najms.2011.3316
Keywords: Operation volume and quality, pancreaticoduodenectomy, pancreatic, ampullary, distal bile duct cancer, pancreas, survival, surgeon, anesthesiologists, total pancreatectomies
High Volume Hospital
All Germany
Relationship between operation volume and quality has been an ongoing debate for years. With repetitive anonymous surveys among visceral surgeons it was tried to describe the situation in Germany over a three year period. Recent German laws require a minimum of 10 pancreatic operations per year for any institution as basis for reasonable performance of pancreatic surgery. Survey was conducted twice with questions about frequency and results of pancreatic resections in 2006, 2008 and 2009. Operations were divided in volume categories by calculating percentiles. Outcome and volume relationship was analyzed with regard to volume categories and types of institution. Return rates of surveys were 50 percent and 48 percent. Very High volume (VHV) was calculated as 32+ (2006) and 34+ (2008, 2009) operations. Overall mortality was 2.9 percent (2006), 4.0 percent (2008) and 2.6 percent (2009).Comparing all volume categories, there was no statistical difference in mortality between groups: 2006, 2008, and 2009. Although mortality decreases with increasing volume, no statistically significant correlation was observed. Comparing VHV centers no difference was seen compared to all other hospitals, or compared to hospitals below legal minimum. An exception in latter comparison was observed in 2008. Proportion of operations increased in university hospitals from 38 percent (2006) to 50 percent (2009) and decreased in teaching hospitals from 52 percent to 42 percent. The percentage of hospitals without pancreatic resection rose from 16 percent to 32 percent and 31 percent. Overall number of pancreatic surgeons increased from 2.4/hospital to 2.6/hospital. Strongest increase was seen at university hospitals from 5.2/hospital to 5.8/hospital. Increasing number of surgeons per hospital did not increase mortality. It was concluded that hospital volume does not seem to influence mortality in pancreatic surgery in Germany. Even in very low volume hospitals good quality is possible. Pancreatic operations are shifted towards university hospitals at the expense of other hospitals. Training in pancreatic surgery is feasible without increased mortality[1].
Rostock, Germany
The benefit of surgical center formation has become a focus of interest in Germany over the past years. It is well documented that a high case load of complex gastrointestinal resections in so called “high volume” centers significantly improves morbidity and mortality. In one analysis it was aimed to analyze this effect on the results of pancreatic surgery performed. A total of 265 consecutive patients underwent open pancreatic surgery between 2003 and 2010. There were 152 patients with pancreatic/ampullary/distal bile duct cancer, 90 patients with chronic pancreatitis, and 23 patients with benign or borderline tumors. A pylorus-preserving pancreatoduodenectomy was performed in 153 (58 %), a classical Whipple's procedure in 35 (13 %), and a duodenum preserving pancreatic head resection (DPPHR) in 35 (13 %) patients. Twenty-eight (11 %) patients underwent a distal resection, 5 patients a total pancreatoduodenectomy, and 5 patients other types of pancreatic resections. This corresponds to an average of 38 resections per year. For classical Whipple, DPPHR, and distal resection the mortality was 6 (3.1 %), 0, and 1 (3.6 %). The authors concluded that their results met the “high volume” quality standards of a center for pancreatic surgery with regard to the number of resections per year, morbidity, and mortality[2].
North Carolina
Pancreaticoduodenectomy (PD) for pancreatic cancer carries a significant morbidity and mortality. Current evidence suggests that hospitals with a high annual PD volume provide improved outcomes after PD for cancer and regionalization to specialty centers is advocated. While better outcomes at high-volume centers (HVCs) are accepted, a rapid regionalization process could also have detrimental effects. For example, HVCs must be equipped to accommodate increased volume without compromising outcomes. North Carolina (NC) is unique in that there are multiple HVCs within the state to accommodate regionalization. Upon noting changes in referral patterns for surgical evaluation since 2007, it was began investigation into regionalization of PD and its effects upon outcomes during this transition period within NC. The North Carolina Hospital Discharge Database (2004-09) was queried by ICD-9 code for all PD performed in NC during two time periods: 2004-06 and 2007-09. Hospitals were categorized by overall PD volume into three groups: Low (1-9 PD/yr), medium (10-19 PD/yr), and high (< 20 PD/yr) volume. Regionalization and operative mortality and major morbidity of PD performed for pancreatic cancer were assessed by comparing volume groups across time periods. The total number of PD performed for cancer in NC increased across 3-year time frames (271 to 350) while increasing by 91 percent (129 to 246 cases) at HVCs and decreasing at low-volume (62 to 58 cases) and medium-volume (80 to 46 cases) centers. The percentage of PD for pancreatic cancer performed at HVCs increased significantly (48 % to 70 %), while decreasing for low-volume (23 % to 17 %) and medium-volume (30 % to 13 %) centers. Mortality was significantly less at HVCs (2.8 %) compared to low-volume centers (10.3 %) for the 2007-09 timeframe, and was not different across time periods for any group. Mortality for all PD performed for pancreatic cancer in NC decreased from 6.6 percent to 4.6 percent across time periods. Major morbidity was not significantly different between volume groups within either time period (33 %, 20 %, and 29 % for low-, medium-, and high-volume centers respectively in 2007-09). There was a significant increase in major morbidity at low-volume centers across time periods (15 % to 33 %). It was thus found that regionalization of PD for pancreatic cancer is occurring in NC, with a near doubling of PD performed at HVCs across these time periods. Mortality was significantly lower at HVCs during the most recent period, and importantly, this rapid and substantial regionalization has only served to enhance outcomes at HVCs. The low mortality rate at HVCs despite a major morbidity rate comparable to low- and medium volume centers is an intriguing finding that likely indicates higher case complexity with early complication recognition and management to “rescue” these patients. The increasing number of PD performed for cancer in NC across this time period is encouraging as it likely indicates increasing utilization of surgical resection rather than an increasing incidence of pancreatic cancer[3].
Anaestesiology
Recent data demonstrates that short- and long-term survival is superior when pancreaticoduodenectomy (PD) for PDAC is performed at a high-volume center. Prior review of data has consistently demonstrated that patients who experience a prolonged hospital stay (>11 days) have a diminished cancer-related survival, suggesting that potentially correctable perioperative events or treatment practices are the cause. It was hypothesized that many of these events or factors are preventable. The aim of one study was to evaluate intraoperative factors and events that negatively influence long-term survival and to identify preventable factors. A prospectively maintained pancreatic cancer database was retrospectively reviewed to identify patients who received PD for PDAC. Demographic, clinical, and transfusion records were evaluated. Operative records were examined to identify teams between surgeon and anesthesiologists (>10 cases performed with a single anesthesiologist and surgeon) and low- and high-volume anesthesiologists (>20 cases for PD). Univariate and multivariate analyses were performed to evaluate the impact of clinicopathologic and intraoperative variables on survival. It was identified 350 patients undergoing PD performed at a high-volume center between 2000 and 2008; median follow-up was 35 months with a median survival of 14 months. Median estimated blood loss was 700 mL (range 100–6000) and total fluid resuscitation was 5100 mL (range 550–33125). Intraoperative treatment factors predicting poor outcome included the experience of the anesthesiologist and the need for blood transfusion. 167 patients (48 %) received intraoperative blood transfusion. The median survival was 25 months versus 36 months compared to those who did not receive transfusion. Patients were more likely to receive transfusions based on their preoperative hemoglobin, age, and EBL > 700 mL. Both high- and low-volume anesthesiologists were involved in nearly half of the cases requiring blood transfusions, but low volume anesthesiologists were more likely to give less resuscitative fluid. It was concluded that this analysis of pancreaticoduodenectomy performed at a high-volume center found that the events and non-surgical personnel within the operating room influence long-term survival of patients receiving surgery for pancreatic cancer[4].
Trends in Selection of Patients for Surgery
To evaluate changes in the indications and outcomes of pancreaticoduodenectomy (PD) and total pancreatectomies (TP) over time in the largest single-institution series data from 3880 consecutive patients who underwent PD or TP between 1980 and 2010 was used from a prospectively collected Institutional database. The proportion of patients >80 years and patients with a Charlson comorbidity score of >7 increased significantly over time. However there was no change in the overall 30-, 60- and 90-day mortality while the median length of stay decreased significantly. The proportion of older patients and patients with multiple comorbidities has continued to increase over time. Despite operating upon older and sicker patients, the unadjusted 30-day operative mortality has remained unchanged while the length of stay has decreased[5].
Fig. 1.
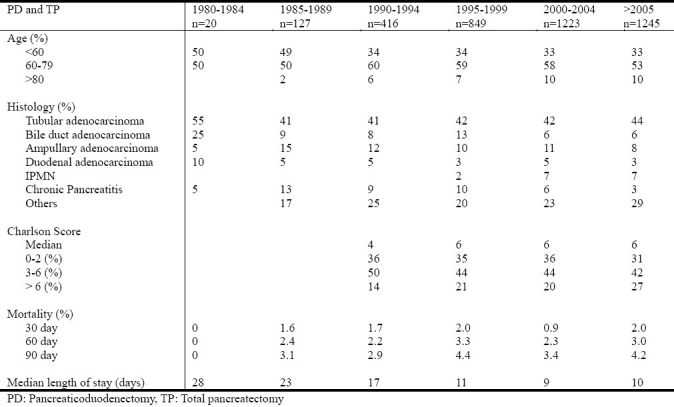
The indications and outcomes of pancreaticoduodenectomy and total pancreatectomy from 3880 consecutive patients
Changes in perioperative management and morbidity associated with resection for patients with pancreatic adenocarcinoma (PAC) remain poorly characterized. It was sought to define the utilization patterns of perioperative and operative procedures for patients with PAC, as well as evaluate population-based temporal trends in morbidity and mortality. Using Surveillance, Epidemiology and End Results (SEER)-Medicare linked data, it was identified 2,461 patients with PAC who underwent pancreatic resection from 1991-2005. Trends in preoperative comorbidity indices (Elixhauser), perioperative management, type of surgical procedures performed, as well as changes in morbidity and mortality were examined. Preoperative evaluation included ERCP (59 %), CT (92 %), MRI (1 4%) and PET (2 %) with a significant temporal increase in the use of all four. The proportion of patients who underwent total pancreatectomy (n=28; 1%) or pancreaticoduodenectomy (n=1945; 79 %) did not change whereas distal pancreatectomy (n=333; 14 %) increased over the study period. There was a temporal increase in median patient age and number of patients with multiple preoperative comorbidities. Despite the increase in patient age and comorbidities over time, perioperative morbidity (53 %) did not change during the study period. The most common postoperative complications were bleeding and need for re-exploration, both of which decreased over time (9 % to 4 % and 11 % to 7 %, respectively). In contrast, there was a significant temporal increase in the number of percutaneous interventional procedures (8 % to 12 %). Perioperative mortality decreased by half over the study period (1991-1996: 6 % vs. 2003-2005: 3 %). Overall 1-and 5-year survival was 53 percent and 13 percent with a modest but statistically significant improvement in median survival. It was concluded that mortality associated with pancreatic resection for PAC has decreased by one-half. Despite surgical resection for PAC being offered to older patients with more preoperative comorbidities, the incidence of perioperative complications remained stable. Resection for PAC in an aging population with more medical comorbidities can be performed safely; however, further progress is necessary to decrease morbidity[6].
Underpriviliged Groups
Prior literature has identified significant demographic differences in surgically treated patients with pancreas cancer (PC). Therefore, it was hypothesized that disparities in socio-demographics continue to exist in critical parameters along the continuum of PC care. It was compiled a retrospective cohort of patients with AJCC Stage I-IV pancreatic cancer using the California Cancer Registry from 1994 to 2008. Using logistic regression methods, it was examined the impact of socio-demographic factors in predicting resectability at diagnosis or treatment (defined as absence of pre- or post-operative features of advanced or metastatic disease), undergoing pancreatectomy, and receipt of adjuvant chemotherapy (with/without radiotherapy) after surgery, while adjusting for confounders. Among 20,312 patients, only 7585 (37 %) presented with resectable disease. Less than half (40 %) of the patients who met this definition received pancreatectomy (n=3153). At multivariate analysis, black patients were just as likely as white patients to present with resectable disease. Yet, black patients were significantly less likely to undergo pancreatectomy or receive adjuvant chemotherapy with/without radiation. In comparison, white patients were 1.5 times as likely as black patients to undergo pancreatectomy and, following surgery, were more likely to receive chemotherapy with or without radiotherapy. Black patients were significantly more likely to carry Medicaid than white patients (10 % vs. 5 %). Compared to those with other insurances, Medicaid recipients showed lower odds of undergoing pancreatectomy and those without insurance were less likely to receive adjuvant therapy. It was concluded that black race and suboptimal insurance represent barriers in the continuum of pancreatic cancer care. Though black patients appear to present with comparable rates of resectability, they continue to receive care that deviates from current guidelines[7].
Numerous studies demonstrate that inequities in treatment result in worse outcomes for selected patients. The objective of one study was to determine if there were differences in surgical therapy and subsequent survival in select racial and ethnic groups from a large metropolitan region. Patients diagnosed with local and regional pancreatic cancer in Los Angeles County between 1988 and 2006 were identified from the California Cancer Surveillance Program (CSP). Patients were grouped according to receipt of surgery (yes vs. no) and by race/ethnicity (white, black, Asian, and Hispanic). Survival after curative-intent surgical resection was assessed by Kaplan-Meier method and compared among the racial/ethnic groups. Query of the CSP registry identified 4039 patients with local or regional pancreatic adenocarcinoma; 62 percent (n=2424) were white, 13 percent (n=518) were black, 10 percent (n=390) were Asian, and 20 percent (n=707) were Hispanic. Overall, 24 percent (n=981) of patients underwent curative-intent surgical intervention while 76 percent (n=3058) did not. When racial/ethnic groups were compared, Hispanic patients (27 %) were most likely to undergo surgery whereas black patients (19 %) were least likely to undergo surgery. Among the surgically resected racial/ethnic groups, Hispanic and Asian patients had improved overall survival compared to black patients (median overall survival 14 mo vs. 12 mo; and 15 mo vs. 12 mo; p=0.044). From this large metropolitan population, it was observed disparities in administration of curative-intent surgical resection among select racial/ethnic groups. Furthermore, even though black patients underwent disproportionately lower rates of curative-intent surgical resection, the black patients that did receive surgery had lower survival compared to other racial/ethnic groups. These results highlight the need to increase efforts to provide appropriate therapy to all patients, but also to better understand the biologic factors that underlie racial disparities in outcomes[8].
The majority of patients with pancreatic cancer have metastatic disease at their initial presentation. Treatment disparities in racial and ethnic groups have been observed in previous studies. The objective was to evaluate the management of patients with metastatic pancreatic cancer among different racial and ethnic groups using a population-based cancer registry. Patients with metastatic pancreatic cancer treated in Los Angeles County from 1988 to 2006 were identified using the Los Angeles County Cancer Surveillance Program. Patients were evaluated by race/ethnicity and by receipt of chemotherapy (yes vs. no). Overall survival differences among racial/ethnic groups were assessed by Kaplan-Meier method and significance was determined using log-rank test. Of 5669 patients with metastatic pancreatic cancer, 59 percent (n=3323) were white, 18 percent Hispanics, 15 percent blacks, and 8 percent Asians; <1 percent had unknown race/ethnicity. Overall, 34 percent of patients received chemotherapy, but there were varying rates among the racial/ethnic groups (37 % of whites compared to 32 % of Asians, 31 % of Hispanics, and 27 % of blacks). In 289 patients, chemotherapy was futile with patients surviving lt;1 month from initiation of therapy. The majority of these patients were white (62 %) followed next by Hispanics (19 %), blacks (13 %), and Asians (6 %). We then examined median overall survival (2 mo, entire cohort; 6 mo, patients who received chemotherapy; and 1 mo, no therapy). Overall survival was significantly higher for Asians in the total cohort (3 mo vs. 2 mo), but lower for blacks in the chemotherapy cohort (6 mo vs. 5 mo). It was concluded that racial disparities exist in the management of patients with metastatic pancreatic cancer in LA County. It was observed a lower rate of chemotherapy administration in black patients which correlated with a generally lower survival. Since effective chemotherapy regimens are now available, appropriate steps must be taken to ensure equal administration of these therapies to all eligible patients[9].
Register Research
The importance of an electronic medical record (EMR) has been highlighted for both clinical care and research. In the current era, data warehouses and repositories have been established to serve the dual function of patient care and investigation. The aim of one study was to compare a newly developed institutional clinical data warehouse, linked with the hospital information system (HIS), to a prospectively maintained departmental database. A novel HIS-linked institutional clinical data warehouse was queried for 9 primary and secondary ICD-9-CM discharge diagnosis codes for pancreatic cancer. The database captures inpatient and outpatient clinical and billing information from a pool of over 2 million patients evaluated at an academic medical institution and its affiliates, since 1995. A cohort was identified; following Institutional Review Board approval, demographic and clinical data was obtained. This was compared to a manually entered and prospectively maintained surgical oncology database of the same institution, tracking 394 patients since 1999. Duplicated patients, and those unique to either dataset, were flagged. Patients with diagnosis dates prior to 1999 were excluded to allow comparison over the same time period. For validation purposes, a 10 percent random sample of remaining patients unique to each dataset underwent manual review of medical records including clinic notes, admission and discharge notes, diagnostic imaging, and pathology reports. 1107 patients were identified from the HIS-linked dataset with pancreatic neoplasm-associated diagnosis codes dating from 1999 to 2009. Of these, 254 (23 %) were captured in both datasets, while 853 (77 %) were only in the HIS-linked dataset. Manual review of the 10 percent subset of the HIS-only group demonstrated that 56 percent of patients were without identifiable pancreatic pathology, suggesting miscoding, while 32 percent had diagnoses consistent with pancreatic neoplasm, and 13 percent with pseudocyst or pancreatitis. Of the 394 patients tracked by surgical oncology, 254 (65 %) were captured in both datasets, while 140 (36 %) had not been captured in the HIS-linked dataset. Manual review of the 10 percent subset of the non-captured patients demonstrated 93 percent with pancreatic neoplasm and 7 percent with pseudocyst or pancreatitis. Lastly, a review of the 10 percent subset of the 254 patient overlap demonstrated that 88 percent of patients were with pancreatic neoplasm, 8 percent with pseudocyst or pancreatitis, and 4 percent without pancreatic pathology. Thus, while technological advances provide a powerful means to automate institutional-level cohort identification and data collection, a high degree of misclassification may be present if queries are based solely on ICD-9-CM discharge codes. For that reason, careful validation and data cleaning are critical steps prior to research use. These results also suggest cautious interpretation of national-level administrative data utilizing ICD-9-CM diagnosis codes. The findings suggest that the current state-of-the-art data warehouses continue to require clinical correlation and validation through traditional retrospective mechanisms[10].
Research together with clinical practice
Traditionally, clinical and research pursuits have occurred in parallel. In the current climate of reduced research funding and increased need for translation of discoveries, an integrated program is an appealing strategy. One study was designed to demonstrate research benefits of a robust clinical practice. Review of a database revealed a total of 1005 pancreatic resection for the period 1995 through 2009. The period 2006 onwards included 711 pancreatic resections and was correlated with research activities. The growth in pancreatic resections produced professional fees averaging USD 3500/case to surgery and USD 3000/case to other departments. Hospital contribution margin averaged USD 13500/case. The increased pancreatic resection volume has contributed more than USD 3 million annually to the positive hospital bottom line. Since 2006, 5 randomized clinical trials and 3 approved protocols have opened. Tissue and plasma on greater than 400 patients has been banked. Thirty-seven peer reviewed publications have resulted. The research team now numbers 23 independent researchers from the institution and 34 independent researchers from 16 collaborating institutions. Patient participation in protocols is high, from 95 percent on the tissue banking protocol to 74 percent on the recent published pancreatic anastomosis trial. It was concluded that building a robust clinical practice can drive a complementary research program. Clinicians in leadership roles in the effort can drive productivity, extensive collaboration, and high patient participation in eligible protocols[11].
References
- 1.Alsfasser G, Kittner JM, Eisold S, Klar E. Volume in pancreatic surgery – the German situation. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 2.Schwandner F, Moritz K, Alsfasser G, Rau BM, Klar E. Pancreatic surgery at the university hospital of Rostock: results of 265 consecutive pancreatic resections. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 3.Swan RZ, Sindram D, Walters A, Iannitti DA, Martinie JB. The impact of regionalization of pancreaticoduodenectomy for pancreatic cancer in North Carolina since 2004. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [PubMed] [Google Scholar]
- 4.Kwon DS, Lin H, Lichtiger B, Katz MH, Lee JE, Fleming JB. Intraoperative factors and events during pancreaticoduodenectomy influence cancer-related survival in patients with pancreatic ductal adenocarcinoma (PDAC) American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 5.Olino K, Venkat R, Cameron JL, et al. 3880 consecutive pancreaticoduodenectomies – outcomes and trends over time. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 6.Mayo SC, Gilson MM, Cameron JL, et al. Trends in management and perioperative morbidity among patients with surgically managed pancreatic adenocarcinoma: a populationbased analysis using SEER-Medicare data. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 7.Abraham A, Al-Refaie WB, Parsons HM, Dudeja V, Vickers SM, Habermann EB. The reality of pancreas cancer care from access to delivery. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 8.Le MH, Nelson R, Wiatrek R, Singh G, Garcia-Aguilar J, Kim J. Surgery for patients with local and regional pancreatic cancer: the Los Angeles county experience. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 9.Wiatrek R, Nelson R, Le M, Singh G, Garcia-Aguilar J, Kim J. Racial disparities in the management of patients with metastatic pancreatic cancer. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 10.Arous EJ, Smith JK, Ng SC, Tseng JF, McDade TP. Tales from the EMR: does a 21st-century data warehouse facilitate clinical research for pancreatic cancer? American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 11.Kennedy EP, Brody JR, Witkiewicz A, et al. Clinical practice as an engine driving a translational research program in pancreatic cancer. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 12.Truty MJ, Kim MB, Chopin X, et al. Direct tumor xenografts: a personalized in-vivo assay that predicts recurrence and survival after surgical resection of pancreatic adenocarcinoma (PDAC) American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]
- 13.Kim MP, Choi W, Kang Y, et al. Identification of a common molecular phenotype after cytotoxic therapy for pancreatic adenocarcinoma. American Pancreas Club, 45th Annual Meeting. 2011 May 6-7; [Google Scholar]


