Abstract
Background:
Atherosclerosis remains one of the leading causes of death all over the world. Flax, pumpkin and purslane seeds are rich sources of unsaturated fatty acids, antioxidants and fibers, known to have antiatherogenic activities.
Aims:
This study was to examine the efficiency of using either flax/pumpkin or purslane/pumpkin seed mixture (components of ω-3 and ω-6) on hyperlipidemia, kidney function and as immunomodulators in rats fed high cholesterol diets.
Materials and Methods:
40 male albino rats were divided into four groups: control group, hypercholesterolemic rats, fed the balanced diet supplemented with cholesterol at a dose level of 2 g/100 g diet; the other two groups of animals fed the same previous hypercholesterolemic diet supplemented with either flax/pumpkin seed mixture or pumpkin/purslane seed mixture at ratio of (5/1) (ω-3 and ω-6).
Results:
The present study showed that 2% cholesterol administration caused a significant increase in total cholesterol, total lipids, and triacylglycerol in both serum and liver. Serum phospholipids, LDL-C, and atherogenic index AI also significantly increased compared to control group. Cholesterol-enriched diet significantly increased serum urea, creatinine, sodium and potassium levels as well as significantly increased serum IgG and IgM compared to healthy control. Consumption of flax/pumpkin or purslane/pumpkin seed mixtures by hypercholesterolemic rats resulted in a significantly decrement in lipid parameters and significant improvement in IgG and IgM levels as compared with hypercholesterolemic rats.
Conclusion:
Our results suggests that both flax/pumpkin and purslane/pumpkin seed mixtures had anti-atherogenic hypolipidemic and immunmodulator effects which were probably mediated by unsaturated fatty acids (including alpha linolenic acid) present in seed mixture.
Keywords: Hypercholesterolemia, flax, pumpkin, purslane seeds, kidney, IgG, IgM
Introduction
Abnormal lipid metabolism is a main cause of dyslipidemia, which is a major risk factor for cardiovascular disease, obesity, cholesthiasis and overall mortality[1]. The concentration of plasma cholesterol can be regulated by cholesterol biosynthesis, removal of cholesterol from the circulation, absorption of dietary cholesterol and excretion of cholesterol via bile and feces[2]. In liver, such lipid accumulation initially results in fatty liver that develops fatty infiltration and in chronic stages results in damage of hepatocytes, that causes gross fatty infiltration in parenchyma cells of liver. It is well known that diet plays an important role in the control of cholesterol homeostasis. In this context, it has been reported that herbs have been used as food and for medicinal purpose for hyperlipidemia that may be useful in reducing the risk of cardiovascular disease and alterations in liver metabolism[3].
It is important to have a balance of omega-3 and omega-6 in the diet. The typical American diet tends to contain 14-25 times more omega-6 fatty acids than omega-3 fatty acids. The Mediterranean diet, on the other hand, has a healthier balance between omega-3 and omega-6 fatty acids. Recent studies have demonstrated that ingestion of polyunsaturated fatty acids (ω-3 and ω-6) including alpha linolenic acid (ALA), present in vegetable oils, is inversely related to the incidence of heart disease by decreasing cholesterol and triacylglycerol plasmatic levels[4]. Flaxseed (Linum usitatissimum), also known as linseed, contains 32–45% of its mass as oil of which 51–55% is alpha-linolenic acid (ALA) (18:3 n-3 Omega-3 fatty acid), a precursor to eicosapentanoic acid EPA, as well as being a good source of dietary fibers and lignans. Flaxseed oil (FO) is readily available in the diet as flaxseed is incorporated into many commonly consumed foods such as breads, muffins and cereals. FO is one of the vegetable sources of ALA and its content ranges from approximately 40% to 60% of the total fatty acids. Clinical conditions such as cardiovascular disease, blood pressure, cancer, skin diseases and immune disorders such as renal failure, rheumatoid arthritis and multiple sclerosis may be prevented by ALA in flaxseed oil[5].
Pumpkins belong to the family of Cucurbitaceae. Pumpkin seeds are a popular snack food in several countries among of which is Greece. They are consumed either raw or roasted (salted or not) and used in cooking and baking as an ingredient of bread, cereals, salads and cakes. Moreover, pumpkin seed oil nowadays gains wide acceptance not only as edible oil but as a nutraceutical, too[6]. Pumpkin seed and seed oil have been implicated in providing many health benefits, which are attributed to their macro- and micro-constituent composition. They are a rich natural source of proteins, phytosterols , polyunsaturated fatty acids , antioxidant vitamins such as carotenoids and tocopherol[7] and trace elements, such as zinc[8]. It is also contains 40.4-55.6% of linolenic acid: LA; 18:2 n-6, ω-6 fatty acid[9].
Portulaca oleracea (Portulacaceae family), also referred to the common purslane, is listed in the World Health Organization as one of the most used medicinal plants and it has been given the term ‘Global Panacea’. The purslane contains many compounds, including alkaloids, omega-3 fatty acids, vitamins (mainly vitamin A, vitamin C, and some vitamin B and carotenoids), as well as dietary minerals, such as magnesium, calcium, potassium and iron. It is also rich in coumarins, flavonoids, polysaccharide, cardiacglycosides, and anthraquinone glycosides[10]. Many studies have demonstrated various pharmacological effects of this plant including hypoglycaemic, hypocholesterolemic and antioxidant effects[11].
In this study, pumpkin seed were used as a source of ω-6 fatty acids, while purslane or flax seeds were used as sources of ω-3 fatty acids. The objective of the current study was to examine the efficiency of using either flax/ pumpkin or purslane/pumpkin seed mixture (components of ω-3 and ω-6) on hyperlipidemia , kidney function and as immunomodulators in rats fed high cholesterol diets .
Materials and Methods
Animals
This study was carried out on healthy adult male albino rats (Sprague-Dawley) strain weighing 120 + 5 g, supplied from the breeding unit of the Egyptian Organization for Biological Products and Vaccines (Helwan, Egypt). Animals were maintained on a natural light/dark cycle and given food and tap water ad libitum.
Experimental Design
Flax, pumpkin and purslane seeds were purchased from local market, crushed at ambient temperature and stored at 4 °C prior to use. It is well known that, flax seeds and purslane seeds were used as ω-3 fatty acids rich sources, while pumpkin seeds used as ω-6 fatty acids rich sources. Seed mixtures of flax, pumpkin and purslane rich in ω-3 and ω-6 in was prepared in a ratio of 5/1 as recommended by the WHO and according to Makni et al.[9].
Animals randomly enrolled into four groups of ten animals each and treated as following: Group (1) G1: control rats; fed basal diet according to Reeves et al.[12]; Group (2) G2: hypercholesterolemic rats (HC), fed the balanced diet supplemented with cholesterol at a dose level of 2 g/100 g diet; Group (3) G3: hypercholesterolemic diet supplemented with flax/pumpkin seed mixture (F/P); and Group (4) G4: hypercholesterolemic diet supplemented with puslane/pumpkin seed mixture (P/P).
At the end of experimental period (6 weeks), the final body weight for each rat was recorded. Animals were fasted overnight then scarified under ether anesthesia and blood samples were collected from hepatic portal vein in centrifuge tubes. The serum was separated by allowing blood samples left for 15 minutes at temperature of 25 °C then centrifuged at 4000 r.p.m for 20 minutes, then kept in plastic vials at –20°C until analysis. Rat organs (liver, kidney, hearts) were immediately removed, rinsed with ice cold saline, and blotted dry, weighed separately and the relative weight were calculated.
Biochemical Analysis
Serum was analyzed for the following biochemical parameter: total cholesterol by the method of Allian et al.[13] , HDL-cholesterol by Burstein et al.[14], triacylglycerol by Fossati and Prencipe[15], total lipids by Zollner and Kirsch[16] and phospholipids by Takayama et al.[17]. Calculation of LDL-Cholesterol fraction and atherogenic index (AI) and HTR ration involves an equations developed by Friedewald et al.[18]. Kidney functions tests: Urea, creatinine were determined according to Patton and Crouch[19], Bonsens and Taussky[20], respectively, sodium, potassium and phosphorous according to Trinder[21] Sunderman[22], El-Merzabani et al.[23], respectively.
Measurement of Serum Antibodies by ELISA
Serum immunoglobulin's IgG and IgM were determined by enzyme linked immunosorbent assay (ELISA), according to Erhardt et al.[24].
Extraction and Determination of Liver Lipids
Liver tissue lipids were extracted with chloroform/methanol mixture (2v/1v) according to the method of Folch et al.[25]. The dried total lipids residues were dissolved in 1 ml absolute ethanol for determination of hepatic total lipids, total cholesterol and triacylglycerol.
Statistical Analysis
The data were statistically analyzed by SPSS version 15.0 statistical packages. The results were expressed as means ±S.D, statistical differences between groups were performed using t-test. Differences considered significantly when p<0.05.
Results
Effect of seed mixture on body weight and relative organs weight
As shown in Table 1 induced hypercholesterolemia caused a significant increase in body weight gain and a significant increase in the relative organs weight as compared with healthy control G1. Administration of seed mixtures to hypercholesterolemic rats caused a significant decrease in body weight gain and in the relative organs weight to reach the level of healthy rats.
Table 1.
Effect of supplementing seed mixtures on body weight gain and relative organs weight in hypercholesterolemic rats

Effect of seed mixtures on serum and liver lipid contents
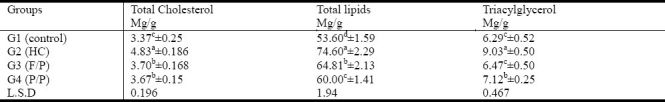
As shown in tables (2) (3) and (4), feeding rats with 2% cholesterol-enriched diet for 6-weeks resulted in a significant elevation of serum and liver total cholesterol (111.65%; 43.32%), total lipids (16.89% and 39.18%) and triacylglycerol (4.12%; 43.56%). Serum phospholipids, LDL/HDL ratio and AI were also significantly increased in G2. Seed mixtures supplementation resulted in a significant decrease in the levels of serum and liver total lipids, total cholesterol and triacylglycerol. The HTR increased by 155.13% and 165.32 while AI was significantly decreased in flax/pumpkin and Purslane/Pumpkin seed mixtures hypercholesterolemic rats respectively as compared to those of hypercholesterolemic control group. It was noticed that the lowering effect of seed mixture of G4 (P/P) on serum and hepatic total lipids was more observable than that of G3 (F/P). However, no significant difference between HDL-C levels in either G4 or G3.
Table 2.
Effect of supplementing seed mixtures on lipid parameters and atherogenic index in hypercholesterolemic rats
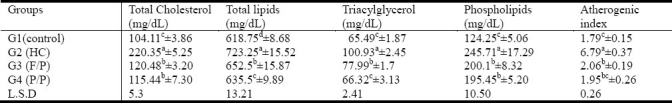
Table 3.
Effect of supplementing seed mixtures on VLDL-C, HDL-C, LDL-C, and HTR ratio in hypercholesterolemic rats

Table 4.
Effect of supplementing seed mixtures on hepatic total cholesterol, total lipids and triacylglycerols in hypercholesterolemic rats
Effect of seed mixtures on serum urea, creatinine, sodium, potassium and phosphorous
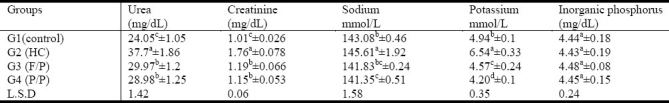
Table (5) summarizes the effect of tested seeds on serum urea, creatinine, sodium, potassium and inorganic phosphorous in rats fed high cholesterol diets. The results revealed that feeding rats with cholesterol-enriched diet caused a significant increase (P<0.05) in serum urea, creatinine, sodium and potassium by about 56.76%, 74.26%, 1.77% and 32.66% as compared with healthy control group. By contrast, administration of flax/pumpkin or purslane/pumpkin seed mixtures showed announced significant decrease (P< 0.05) in the levels of serum urea, creatinine, sodium and potassium as compared with hypercholesterolemic control group. The lowering action on urea and creatinine was more apparent in G4 fed (P/P) mixture seeds. There was no significant difference between all groups in serum phosphorous levels.
Table 5.
Effect of supplementing seed mixtures on kidney functions (urea, creatinine, Na+, K+ and Inorganic phosphorus) in hypercholesterolemic rats
Effect of seed mixtures on serum immunoglobulins
The current study clearly showed that the levels of IgG and IgM were significantly increased (P<0.05) in hypercholesterolemic rats as compared with healthy control. It is clear that consuming seed mixtures either F/P or P/P had positive impacts to the immunity status of hypercholesterolemic rats by significant reduction in rat serum immunoglobulines to bring them near the normal levels (Table 6).
Table 6.
Effect of supplementing seed mixtures on serum immunoglobulins in hypercholesterolemic rats

Discussion
The objective of the current study was to examine the efficiency of using either flax/pumpkin or purslane/pumpkin seed mixture (components of ω-3 and ω-6) on hyperlipidemia, kidney function and as immunomodulators in rats fed high cholesterol diets. Previous study by Lecumberri et al.[26] reported that rats fed high cholesterol diet showed significant increase in body weight and other organ weights, thus leads to secondary complications clinically. In our study, body and relative organs weight gain of hypercholesterolemic rats were decreased significantly upon treatment with flax/pumpkin or purslane/pumpkin seed mixture. The hypolipidemic and antiatherogenic effects of flax/pumpkin or purslane/pumpkin seed may be responsible for the beneficial action of these seeds on body weight gain and liver weights. Purslane/pumpkin seed mixture seems to exert a protective effect against overweight. However the pumpkin alone in each mixture may be responsible for reduction in body weight. A study by Hyounjeong et al.[27] screened various plant sources for their antiobesity activity and demonstrated that pumpkin has strong antiobesity effects in a high fat diet-induced obesity animal model. This mainly due to its effect on synthesis and degradation of lipid products in the body, also it was considered as metabolic regulator of lipogenic and lipolytic pathways and altimately as antiobesity agent.
Cholesterol- enriched diet resulted in a significant increase in total cholesterol, total lipids, phospholipids and triacylglycerol in plasma and liver this accompanied by increased serum LDL-C level, with decreased circulating HDL-C, thus providing a model for dietary hyperlipidemia[28]. The increase of lipid parameters has been shown to be a strong risk factor for coronary heart diseases in many populations .The high cholesterol level in liver and plasma may be due to increased uptake of exogenous cholesterol and subsequent deposition and decreased cholesterol catabolism as evidenced by a reduction in bile acid production and turnover of bile acids. The metabolism of free and ester cholesterol are impaired in liver, spleen and thymus tissue and the rate of turnover was specifically decreased in all tissues of hyperlipidemic rats. Lipid deposition is a major clinical complication of hyperlipidemia[29] which is consistent with the present study.
Our results indicated that both flax/pumpkin and purslane/pumpkin seed mixture rich in polyunsaturated fatty acids had a strong hypolipidemic, hypotriglyceridemic and hypocholesterolemic effects in plasma and liver of rats with a reduction of plasma LDL-C levels and an increase in HDL-C levels. Furthermore, the atherogenic index markedly decreased due to significant reduction in LDL/HDL ratio in both groups fed hypercholesterolemic diet supplemented with either flax/pumpkin or purslane/pumpkin seed mixture. Results are agreed with Feoli et al.[30] who stated that the increase in HDL-C or HTR ratio is one of the most important criteria of anti-hypercholesterolemic agent. The decrease of plasma cholesterol by administration of flaxseed was ascribed to the decrease of both free and esterified cholesterol.
Lignans, fibre and vegetable proteins present in the flaxseed could play major roles in reducing serum cholesterol in animal models and/or in humans[31]. Therefore, the effect of flaxseed in decreasing serum cholesterol does not seem to be due only to its C18:3 content, but rather to the synergistic action of its constituents. This was reported by Cintra et al.[32] who found lower serum lipid levels in normocholesterolemic and hypercholesterolemic subjects who were fed flaxseed oil. Fibers are reported to decrease plasma LDL-C levels by interrupting the cholesterol and bile acid absorption and increasing LDL receptor activity. The decline in hepatic cholesterol levels in flax/pumpkin hypercholesterolemic group indicated the possible influence of relatively higher fiber content of seed mixture. In fact, dietary fiber are known to interfere with cholesterol absorption and enterohepatic bile circulation and resulted in depletion of hepatic cholesterol pools[33]. In addition, diets rich in fibers are known to reduce triacylglycerol levels by inhibition of hepatic lipogenesis[34]. Moreover, numerous studies have demonstrated that high levels of HDL-C are associated with a lower incidence of cardiovascular diseases . The increase in HDL-C observed in our study, might be due to stimulation of pre-β HDL-C and reverse cholesterol transport as demonstrated by previous study[35]. High HDL-C levels could potential contribute to its anti-atherogenic properties, including its capacity to inhibit LDL oxidation and protect endothelial cells from the cytotoxic effects of oxidized LDL[36]. The anti-atherogenic effect of flax and pumpkin seed mixture found in our study might be due to the presence of polyunsaturated fatty acids, phytosterols, tocopherols and β-carotene[37]. The major total fatty acids present in seed mixture are unsaturated fatty acids such as oleic acid,linolenic acid and linolenic acid , which play a crucial role in reducing blood cholesterol in human and rats Movahedian et al.[38] showed that addition of purslane leaf extract to the cholesterol-enriched diet improved the hypercholesterolemia induced by a high-cholesterol diet in rabbits. The results showed that upon administration of hydroalcoholic extract of purslane for 12 weeks significantly decreased the serum total cholesterol, LDL-C and VLDL-C at doses of 200, 400 and 800 mg kg-1 bw in comparison with the hypercholesterolemic group. But serum HDL-C was elevated insignificantly in hypercholesterolemic rabbits treated with the above doses of purslane extract. Purslane treated animals also showed a decrease in the atherogenic index with respect of hypercholesterolemic groups, which is generally believed to be beneficial since the HDL level inversely correlated with coronary heart disease and reduction in this ratio is considered as an anti atherosclerotic factor.
The kidney functioning capacity was assessed in this study by measuring the levels of electrolytes, creatinine and urea in the serum of the animals. The presence of significant effect of the cholesterol-enriched diet on the serum concentrations of sodium, potassium and phosphorus of the animals suggest that, the abnormal functioning of the organ in relation to these electrolytes were affected. Kidneys remove metabolic wastes such as urea, uric acid, creatinine and ions, so optimum chemical composition of body fluids is maintained. The concentrations of the metabolites increase in blood during renal diseases or renal damage may due to high activities of xanthine oxidase, lipid peroxidation, and increased triacylglycerol and cholesterol levels[39].
Creatinine, synthesized in the liver, passes into the circulation where it is taken up almost entirely by the skeletal muscles. Its retention in the blood is an evidence of kidney impairment[40]. Therefore, the reduced levels of creatinine in serum may imply that, the seed mixture had interfered with creatinine metabolism and its eventual excretion from the blood. Urea is the main product of protein catabolism. The increase in serum urea level in hypercholesterolemic control group indicates impairment in the normal kidney function of the animal, as the mechanism of removing it from the blood might have been affected. It may also be an indication of dysfunction at the glomerular and tubular levels of the kidney, it is well known that, many biochemical and histopathological findings confirmed renal damage in hypercholesterolemia conditions[41]. Flax seed is the richest natural source of plant lignans, which are platelet-activating factor (PAF)-receptor antagonists. Platelet-activating factor plays a key role in the mediation of inflammation, mitogenesis, and alteration of glomerular permselectivity. Thus, flaxseed could potentially inhibit various mechanisms associated with the progression of renal diseases[42]. A study by Dkhill et al.[43] showed that purslane administration at 1.5mg/kg purslane aqueous juice for 12 days caused significant decrease in urea and creatinine respectively. Schaefer et al.[44] have recommended many medicinal plants used already in traditional medicine, experimental and clinical, and nephroprotective effects among them are flax and pumpkin seeds mixture rich in PUFAs and antioxidant compounds in animals.
The current study clearly showed that the levels of IgG and IgM were significantly increased (P<0.05) in hypercholesterolemic rats as compared with healthy control. It is clear that consuming seed mixtures either F/P or P/P had positive impacts to the immunity status of hypercholesterolemic rats by significant reduction in rat serum immunoglobulines to bring them near the normal levels. The capacity of dietary n-3 polyunsaturated fatty acids (PUFAs) suppress inflammation-associated processes, has made them attractive candidates for both the prevention and amelioration of a variety of organ-specific and systemic diseases. n-3 PUFAs suppress proinflammatory cytokine production, lymphocyte proliferation, cytotoxic T cell activity, natural killer cell activity, macrophage-mediated cytotoxicity, neutrophil/monocyte chemotaxis, and antigen presentation[45]. Evidence that these cellular effects indeed impact immune function in vivo is reflected in n-3 PUFA attenuation of mediator production, leukocyte homing, delayed-type hypersensitivity and acute inflammatory responses in experimental animals in which human inflammation and autoimmune diseases are modeled. n-3 PUFAs appear to mediate these effects via both eicosanoid-dependent and eicosanoid-independent pathways. Taken together, these anti-inflammatory and immunomodu-lating activities have led to the evaluation and application of n-3 PUFAs for prevention and treatment of inflammatory and autoimmune diseases, of particular interest here are those studies that have focused on the kidney[46]. Results by Sun et al.[47] showed that increasing the supply of linolenic acid to the small intestine of animal models linearly modulate the immunity. On the other hand, it is reported that, 100 g of fresh Purslane leaves (about 1 cup) contain 300–400 mg of alpha-linolenic acid, is important in preventing heart attacks and strengthening the immune system.
Conclusion
The current study proved the efficiency of using either flax/pumpkin or purslane/pumpkin seed mixture (components of ω-3 and ω-6) on hyperlipidemia, kidney function and as immunomodulators in rats fed high cholesterol diets.
References
- 1.Rizvi F, Iftikhar M, George J. Beneficial effects of fish liver preparations of sea bass (Lates Calcrifer) versus gemifirozil in high fat-diet-induced lipid intolerant rats. J Medicinal Food. 2003;6:123–128. doi: 10.1089/109662003322233521. [DOI] [PubMed] [Google Scholar]
- 2.Choi H, Do X, Park Y, et al. Effect of naringenin supplementation on cholesterol metabolism and antioxidant status on eats fed high cholesterol with different levels of vitamin E. Ann Nutr Metab. 2001;45:193–201. doi: 10.1159/000046729. [DOI] [PubMed] [Google Scholar]
- 3.Jayasooriya A, Sakono N, Yukizaki C, Kawano M, Yamamoto K, Fukerda N. Effect of Momordica charantia powder on serum glucose levels and various lipid parameters in rats fed with cholesterol enriched rats. J Ethnoparmacol. 2000;72:331–336. doi: 10.1016/s0378-8741(00)00259-2. [DOI] [PubMed] [Google Scholar]
- 4.Vijaimohan K, Jainu M, Sabitha K, Subramanifam S, Anandhan C, Devi C. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Science. 2006;79:448–454. doi: 10.1016/j.lfs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Prasad K. Flaxseed: a source of hypocholesterolemic and antiatherogenic agents. Drug News Perspect. 2000;13:99–102. doi: 10.1358/dnp.2000.13.2.662239. [DOI] [PubMed] [Google Scholar]
- 6.Marianna N, Tzortzis N, Elizabeth F. Antioxidant and lipoxygenase inhibitory activities of pumpkin seed extracts. Food Research International. 2009;42:641–646. [Google Scholar]
- 7.Stevenson D, Eller F, Wang L, Jane J, Wang T, Inglett G. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem. 2007;55:4005–4013. doi: 10.1021/jf0706979. [DOI] [PubMed] [Google Scholar]
- 8.Glew R, Glew R, Chuang L, Huang Y, Millson M, Constans D. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods Hum Nutr. 2006;61:51–56. doi: 10.1007/s11130-006-0010-z. [DOI] [PubMed] [Google Scholar]
- 9.Makni M, Fetoui H, Gargouri N, Jaber H, Boudawara T, Zeghal N. Hypolipidemic and hepatoprotective effects of flaxseed and pumpkin seed mixture in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food Chem Toxicol. 2008;46:3714–3720. doi: 10.1016/j.fct.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 10.Isin Y, Ismail T, Askim H, Tijen D. Salinity tolerance of (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ Exp Bot. 2007;61:49–57. [Google Scholar]
- 11.Chen Y, Shen Z, Chen X. Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. J Food Composit Anal. 2007;22:303–306. [Google Scholar]
- 12.Reeves G, Nielsen F, Fahmy G. Purified diets for laboratory rodents: Final report of the American Institute of Nutrition on the reformation of rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 13.Allian C, Poon L, Richmond W, Fu P. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 14.Burstein M, Scholnik H, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyphenols. J lipid Res. 1970;11:583–595. [PubMed] [Google Scholar]
- 15.Fossati P, Prencipe L. Serum triglycerides determination calorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2088. [PubMed] [Google Scholar]
- 16.Zollner N, Kirsch K. Microdetermination of lipids by the Sulphophospho vanillin reaction. Z Ges Exp Med. 1962;135:545–561. [Google Scholar]
- 17.Takayama M, Itoh S, Nagasaki T, Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977;79:93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- 18.Friedwald W, Levy R, Fredrichson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;226:499–502. [PubMed] [Google Scholar]
- 19.Patton C, Crouch S. Determination of serum urea. Anal Chem. 1977;49:464–469. [Google Scholar]
- 20.Bonsens K, Taussky S. Determination of serum creatinine. J Chem Inv. 1984;27:648–660. [Google Scholar]
- 21.Trinder P. A rapid method for the determination of sodium in serum. Analyst. 1951;76:596–599. [Google Scholar]
- 22.Sunderman F. Determination of serum Potassium. Am J Clin Path. 1958;29:95–103. doi: 10.1093/ajcp/29.2.95. [DOI] [PubMed] [Google Scholar]
- 23.ElMerzabani M, ElAaser A, Zakhary N. Colorimetric method for determination of serum phosphorus. J Clin Chem Biochem. 1977;15:715–718. doi: 10.1515/cclm.1977.15.1-12.715. [DOI] [PubMed] [Google Scholar]
- 24.Erhardt M, Quistop I, Vonschrummer I, et al. Development of specefid ELISA antibody assay for detection of immunoglobulin G, M and A using monoclonal antibodies. Poult Sci. 1992;71:302–310. doi: 10.3382/ps.0710302. [DOI] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 26.Lecumberri E, Goya L, Mateos R, et al. A diet rich in dietary fiber from cocoa improves lipid profile and reduced malondialdehyde in hypercholesterolemic rats. Nutrition. 2007;23:332–341. doi: 10.1016/j.nut.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Hyounjeong C, Haekwan E, Kyoungcheol P, et al. A water-soluble extract from Cucurbita moschata shows antiobesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. BBRC. 2007;359:419–425. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Moore A, Ward W. Flaxseed oil and inflammation –associated bone abnormalities in interleukin 10 Knockout mice. J Nutr Biochem. 2005;16:368–374. doi: 10.1016/j.jnutbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Shali P, Nilsson J, Cecex B. Exploiting the vascular protective effects of high density lipoprotein and its apolipoproteins: an idea whose time in testing in coming. Circulation. 2001;104:2376–2383. doi: 10.1161/hc4401.098467. [DOI] [PubMed] [Google Scholar]
- 30.Feoli A, Roehrig C, Rotta L, et al. Serum and liver lipids in rats and chiks fed with diets containing different oils. Basic Nutrit Investig. 2003;19:789–793. doi: 10.1016/s0899-9007(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 31.Wiesenfeld P, Babu U, Collins T, et al. Flaxseed increased α-linoleic acid and eicosapentaenoic acid and decreased arachidonic acid in serum and tissues of rat dams and offspring. Food Chem Toxicol. 2003;41:841–855. doi: 10.1016/s0278-6915(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 32.Cintra D, Costa A, Peluzio M, Matta S, Silva M. Lipid profile of rats fed high fat diet based on flaxseed, peanut, trout or chicken skin. Basic Nutrit Investig. 2006;22:197–205. doi: 10.1016/j.nut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Romero A, West K, Zern T, Fernandez M. The seeds from plantago ovate lower plasma lipids by altering hepatic and bile acid metabolism in Guinea pigs. J Nutr. 2002;132:1194–1198. doi: 10.1093/jn/132.6.1194. [DOI] [PubMed] [Google Scholar]
- 34.Venkateson N, Devaraj S, Devaraj H. Increased binding of LDL and VLDL to apo B, E receptors of hepatic plasma membrane of rats treated with fibernat. Europ J Nutr. 2003;42:262–271. doi: 10.1007/s00394-003-0420-8. [DOI] [PubMed] [Google Scholar]
- 35.Daniel M. Science Publishers, Enfield, NH; 2006. Medicinal Plants: Chemistry and Properties; p. 184. [Google Scholar]
- 36.Assmann G, Nofer J. Anthropometric protective effects of high density lipoproteins. Ann Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 37.ElAdawy T, Taha K. Characteristics and composition of different seed oils and flours. Food Chem. 2001;74:47–54. doi: 10.1021/jf001117+. [DOI] [PubMed] [Google Scholar]
- 38.Movahedian A, Ghannadi A, Vashirnia M. Hypocholesterolemic effects of purslane extract on serum lipids in rabbits fed with high cholesterol levels. Int J Pharmacol. 2007;3:285–289. [Google Scholar]
- 39.Anwar M, Meki A. Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comparative Biochem Physiol. 2003;135:539–547. doi: 10.1016/s1095-6433(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 40.Wurochekke A, Anthony A, Obidah W. Biochemical effects on the liver and kidney of rats administered aqueous stem bark extract of Xemenia Americana. Afr J Biotechnol. 2008;7:2777–2780. [Google Scholar]
- 41.Stuglin C, Prasad K. Effect of flaxseed consumption on blood pressure, serum lipids, hemopoietic system and liver and kidney enzymes in healthy humans. J Cardiovasc Pharmacol Ther. 2005;10:23–27. doi: 10.1177/107424840501000103. [DOI] [PubMed] [Google Scholar]
- 42.Akpolat M, Kanter M, Topcu Y, Aydogdu N. Protective Effect of flaxseed oil on renal injury in hyperlipidemic rats: the effect of flaxseed oil on hyperlipidemia. Phytother Research. 2011;25:796–802. doi: 10.1002/ptr.3334. [DOI] [PubMed] [Google Scholar]
- 43.Dkhil1 M, Abdel Moniem A, Al-Quraishy S, Saleh R. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. J Med Plant Research. 2011;5:1589–1563. [Google Scholar]
- 44.Schaefer E, Asztalos B. Cholesteryl ester transfer protein inhibition, high-density lipoprotein metabolism and heart disease risk reduction. Curr Opin Lipidol. 2006;17:394–398. doi: 10.1097/01.mol.0000236364.63840.d8. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Pestka J. Mechanisms for suppression of interleukin-6 expression in peritoneal macrophages from docosahexaenoic acid-fed mice. J Nutr Biochem. 2009;20:358–368. doi: 10.1016/j.jnutbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James J. n-3 Polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis Prostaglandins Leukotrienes and Essential. Fatty Acids. 2010;82:251–258. doi: 10.1016/j.plefa.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun P, Wang J, Yang G, Khas E, Liu Q. Effects of different doses of free alpha-linolenic acid infused to the duodenum on the immune function of lactating dairy cows. Arch Anim Nutr. 2010;64:504–513. doi: 10.1080/1745039X.2010.511517. [DOI] [PubMed] [Google Scholar]


