Abstract
Context:
Trichilemmal (pilar) cysts are common skin lesions that often present on the scalps of mature men and women. These cysts often become inflamed when the wall of the cyst ruptures, but few reports have addressed the immunologic features of this process.
Case Report:
A 22-year-old female presented with rapidly growing nodule on her left cheek, with evidence of acute inflammation. Skin tissue for hematoxylin and eosin examination, as well as for immunohistochemical analysis was taken and reviewed. As controls, we utilized two archival, non-inflamed trichilemmal cysts. Hematoxylin and eosin staining demonstrated classic features of an inflamed trichilemmal cyst. No cytologic atypia was noted, and no significant number of mitotic figures was identified. Immunohistochemistry stains revealed that several cell cycle/tumor suppressor/apoptotic markers, antigen presenting cell markers, metalloproteinases and T cell response markers were highly expressed inside and around the disrupted cyst. The control, non-inflamed cysts were negative for the same markers. CD1a was also appreciated within the epidermis, suprajacent to the inflamed cyst.
Conclusions:
Upregulation and/or downregulation of selected cell cycle regulator and/or tumor suppressor/apoptotic markers, as well as antigen presenting cells and some protein kinases could recruit and activate T lymphocytes and other inflammatory cells to the non-disrupted cyst for unknown reasons. The immune response may be involved in the initial cyst rupture, or induced by an unknown alteration in the cyst. Larger studies are needed to address these questions.
Keywords: Trichilemmal (pilar) cyst, p27kip1, matrix metalloproteinase-9 (MMP9), BCL-10, cytokeratin A1/A3, vimentin
Introduction
A trichilemmal cyst (also known as a “wen”, “pilar cyst” or “isthmus-catagen” cyst) is a common cyst that forms from a hair follicle[1,2]. These cysts are most often found on the scalp. The cysts are externally smooth, mobile and filled with cytokeratin, a protein family found in hair, nails, and skin[1,2]. Trichilemmal cysts may present clinically in families[1,2]. Trichilemmal cysts are histologically related to rapidly multiplying trichilemmal tumors (also called proliferating trichilemmal cysts), which are benign but may grow aggressively. Proliferating trichilemmal tumours show some histologic features of a typical trichilemmal cyst, but also often display epithelial proliferation, variable cytologic atypia, and mitotic activity; they often present in elderly women as a slowly enlarging, painful subcutaneous scalp nodule[1,2].
For unknown reasons, trichilemmal cysts may become clinically tender and inflamed, and rupture[1,2]. In these cases, dermatologists and primary care physicians often excise the lesion and submit the tissue for hematoxylin and eosin (H & E) histopathology. A ruptured trichilemmal cyst must be differentiated from skin cancers, especially nodular or ‘nodulocystic’ basal cell carcinomas. In our case, H & E review demonstrated that the cyst was inflamed and anatomically intact.
Case Report
A 22-year-old female presented with an inflammatory plaque on her left cheek. The lesion was well-demarcated, skin-colored, hairless, rubbery, non-tender, and mobile over the underlying subcutaneous tissues. The lesion had grown slowly since arising over the last year. The lesional growth prompted the dermatologist to excise the area. The patient denied a history of similar lesions in herself or her family, and no personal or family history of skin cancer. A deep ellipse skin specimen was taken for pathological examination. The cyst sac was filled with a soft, whitish brown material that oozed onto the skin surface. Hematoxylin and eosin (H & E) analysis and immunohistochemistry (IHC) studies were then performed.
Our H & E examination and immunohistochemistry (IHC) analysis were performed as previously described[3–10]. We aimed to determine a specific cause for the clinical inflammation. Two archival, non-inflamed trhichilemmal cysts were utilized as controls. We tested for several antibodies that could potentially be involved in 1) alterations of the cell cycle, 2) extracellular matrix remodeling, 3) apoptosis, 4) autophagic processes and 5) antigen presenting cells within inflammation. The following antibodies were evaluated: vimentin, Cytokeratin AE1/AE3, matrix metalloproteinase 9 (MMP9), the human tissue inhibitor of metallopreinase 1 (TIMP1), alpha 1 anti-trypsin, p53, BCL-2, BCL-10, p27Kip1, BRCA1, CD68, CD1a, myeloid histoid antigen , linker for activation of T cells (LAT), zeta-chain (TCR) associated protein kinase/70kDa (Zap-70), and BAX(all from Dako, Carpinteria, California USA).
Microscopic Description
Examination of the H&E tissue sections demonstrated a cystic structure within the dermis. The cyst lining displayed trichilemmal keratinization. The cyst lumen contained dense, compact keratin debris. There were multiple, irregularly-shaped cytokeratin lobules and smaller aggregates of squamous epithelium within the cyst that exhibited trichilemmal keratinization. The surrounding stroma was hyalinized with focal calcifications (Figures 1–3). Of interest in our immunohistochemical staining, stained areas with round shapes that could be interpreted as procedural artifact represent, in our opinion, genuine staining. Specifically, we have noticed these patterns of IHC staining in multiple cysts. Several areas of the cyst were inflamed; a mixed inflammatory infiltrate was noted, especially at the deep base of the cyst. Lesional mitotic figures were rare. The cyst appeared free of the specimen margins in the sections examined. To our surprise, several cell cycle regulator, extracellular matrix and inflammatory markers were positive on IHC review. Some markers displayed positive patch staining inside the cyst, some outside the cyst, and others within the inflammatory infiltrate and/or in dermal tissue adjacent to the cyst. We observed positive expression of p27Kip1 and MMP9 inside the cyst; focal positivity of LAT and BCL-10 were also found in this area. In addition, some antigen presenting cell markers such CD68 and myeloid/histoid antigen were noticed either 1) closely adjacent to the non-inflamed external cyst wall, and/or 2) inside the inflammatory infiltrate. Significantly, CD1a was noted in the epidermis, suprajacent to the inflamed cyst (Figures 1–3). Our control cysts stained negative for these markers.
Fig. 1.
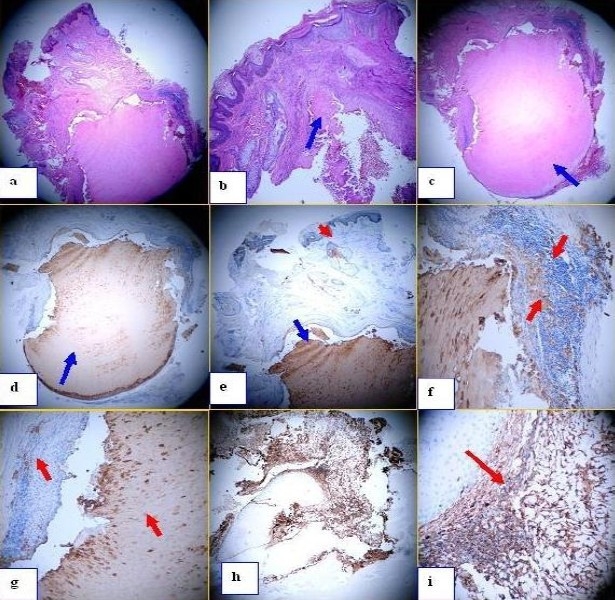
a. H & E staining shows the series of cyst in the epidermis at 40x magnification. b similar but at 100x, and in this case one of the cyst is broken (blue arrow). c. Similar but in this case the lower part of the cyst is observed (blue arrow). d. IHC staining using Cytokeratin AE1/AE3 antibody shows the positive staining inside the cyst (brown stain) (blue arrow). e. Same antibody as d, but the stain is not only in the cyst but also in some of the “apparently normal pilosebaceous units” around the cyst (red arrows). f. and g similar that e, at higher magnification. Please note that some of the areas around the cyst are also positive with the Cytokeratin AE1/AE3 antibody (red arrow). h and i, IHC using vimentin. Please note some compartamentalization of this antibody around the cyst (red arrow).
Fig. 3.
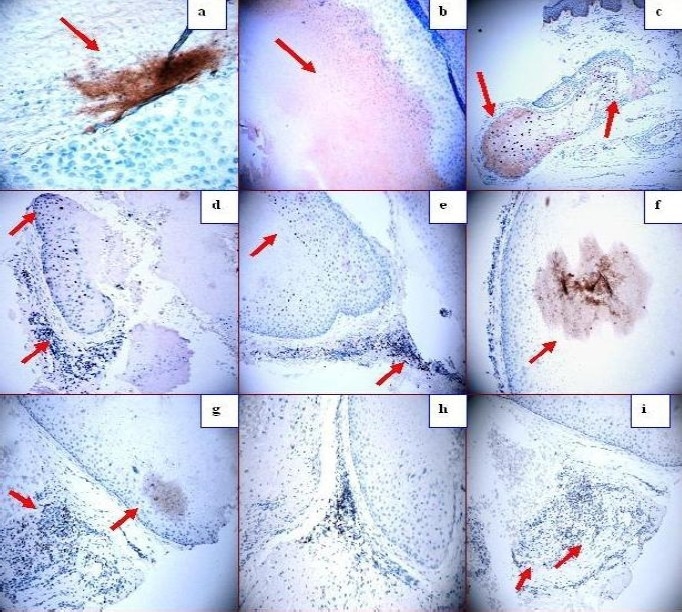
a. Zap-70 positive staining in some spots inside the cyst (dark staining; red arrows). b. Positive staining in some spots inside the cyst using TIMP1 antibody (dark staining; red arrows). c, d, and e. p27 positive staining in areas of pilosebaceous units that are apparently “normal” by histology (brown staining; red arrows). p27 stains around other partially damaged cysts, and inside the sebaceous glands (red arrows). f. Positive staining inside the cyst in some patches with alpha 1 anti-trypsin (red arrow). g. BAX positive staining in a patch inside the cyst, and also in the inflammatory infiltrate outside the cyst (dark staining; red arrows). h. BAX positive staining in the inflammatory infiltrate around the cyst (red arrow). i. BCL-10 positive staining in the inflammatory infiltrate around the cyst (red arrow).
Fig. 2.
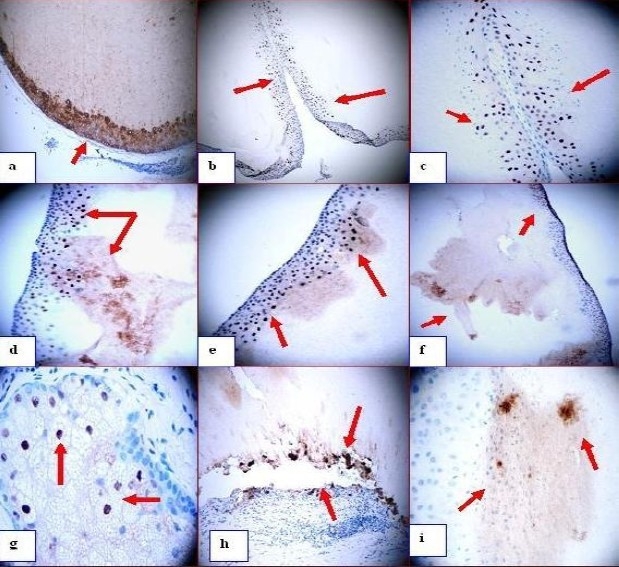
a Positive staining with the antibody to Cytokeratin A1/A3 antibody around the wall of the cyst and inside in (red arrow). b through e positive staining with p27 antibody in the wall of the cyst as well as in some spots inside the cyst (red arrows). f. IHC using MMP9 showing some positive staining in the wall of the cyst as well as inside it (red arrows). g. MMP9 (positive in some cells of the sebaceous glands (red arrows) (dark staining). h, MMP9 stain showing how some parts of the cyst are being separated from the adjacent matrix and staining positive for MMP9 on both sites of the gap (red arrow) (dark brown stain). i. Positive staining in some spots inside the cyst using TIMP1 antibody (dark staining, red arrows).
A trichilemmal cyst (also known as a “wen”, “pilar cyst” or “isthmus-catagen cyst”) is a common cyst that forms from a hair follicle[1,2]. These cysts are most often found on the scalp. The cysts are externally smooth, mobile and filled with cytokeratin, a protein family found in hair, nails, and skin[1,2]. Trichilemmal cysts may present clinically in families[1,2]. Trichilemmal cysts are histologically related to rapidly multiplying trichilemmal tumors (also called proliferating trichilemmal cysts), which are benign but may grow aggressively. Proliferating trichilemmal tumors show some histologic features of a typical trichilemmal cyst, but also often display epithelial proliferation, variable cytologic atypia, and mitotic activity; they often present in elderly women as a slowly enlarging, painful subcutaneous scalp nodule[1,2].
Discussion
The trichilemmal cyst (also known as a pilar cyst or wen) may be clinically indistinguishable from the epidermoid, or epidermal inclusion cyst[1,2]. An overlying punctum is usually absent[1,2]. Trichilemmal cysts are thought to originate via budding of the external root sheath of the hair follicle, secondary to a genetically determined structural aberration. They arise preferentially in areas of high hair follicle concentration; thus, 90% of cases occur on the scalp. They are solitary in 30% of cases, and multiple in 70%[1,2]. Histologically, areas of squamous proliferation are found in some cysts, as observed in this case. In rare cases, this process leads to formation of a proliferating trichilemmal cyst; these cysts may have dyskeratotic cells, mitotic figures and nuclear atypia. These features can be misleading, and a misdiagnosis of squamous cell carcinoma should be avoided. Small trichilemmal cysts (e.g., less than 5mm diameter) do not usually need treatment, but can often be removed by a minor surgical procedure. Larger trichilemmal cysts are often removed, either for cosmetic reasons or because they are inflamed. Trichilemmal cysts are classically removed by creating a small surgical opening on the skin and removing the cyst sac (excisional biopsy). The procedure is often performed under local anesthesia and may require stitches for surgical closure[1–3].
The pathologic changes observed in the cyst could be triggered by 1) the actual rupture of the cyst and resultant inflammation; or 2) by the balance between matrix metalloproteinases (MMPs) and their inhibitors (i.e., tissue inhibitors of metalloproteinases, or TIMPs), secreted primarily from activated macrophages. However, the precise physical and/or immunological mechanisms for these alterations have not been fully elucidated. Further, adipose tissue secretes multiple bioactive molecules, termed adipocytokines. Adipose tissue also generates anti-inflammatory molecules, such as adiponectin; potentially, adiponectin could assist in regulating inflammatory responses in the area of the cyst, where MMPs and TIMPs are abundantly expressed. Finally, the cyst pathophysiology could also be mediated via signaling pathways involving ZAP-70 and p27; we found these antibodies positively expressed in the cyst area.
The florid results we found by IHC staining in this inflamed cyst raised several questions such as whether the inflammation was as result of the cyst per se, versus whether an imbalance in dermal cell growth and/or the dermal extracellular matrix elicited the immune response; the immune response was characterized by the presence of antigen presenting cells and T cell activation markers[11–12]. Further, could an imbalance be present between dermal fibroblasts, macrophages and metalloproteinases, given the cyst expansion and disruption? We also speculate that an imbalance could be present among several checkpoint control mechanisms, and could contribute to the immune response. Larger studies would thus be of value in understanding the immunopathology of inflamed trichilemmal cysts, and could potentially lead to novel, cost effective therapeutic approaches for patients.
Acknowledgments
This study was supported by the funding from Georgia Dermatopathology Associates, Atlanta, Georgia, USA.
References
- 1.Anolik R, Firoz B, Walters RF, et al. Proliferating trichilemmal cyst with focal calcification. Dermatology Online J. 2008;14:25. [PubMed] [Google Scholar]
- 2.McKee PH, et al., editors. Pathology of the Skin with Clinical Correlations. 3rd ed. China: Mosby; 2005. Cutaneous cysts; p. 1670. [Google Scholar]
- 3.Abreu-Velez AM, Smith JG, Jr, Howard MS. IgG/IgE bullous pemphigoid with CD45 lymphocytic reactivity to dermal blood vessels, nerves and eccrine sweat glands. North Am J Med Sci. 2010;2:538–541. doi: 10.4297/najms.2010.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abreu Velez AM, Girard JG, Howard MS. IgG bullous pemphigoid with antibodies to IgD, dermal blood vessels, eccrine glands and the endomysium of monkey esophagus. Ms in press. N Dermatol Online. 2011;2:48–51. [Google Scholar]
- 5.Howard MS, Yepes MM, Maldonado-Estrada JG, et al. Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus-part 1. J Cutan Pathol. 2010;37:222–223. doi: 10.1111/j.1600-0560.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 6.Abreu Velez AM, Howard MS, Hashimoto T. Palm tissue displaying a polyclonal autoimmune response in patients affected by a new variant of endemic pemphigus foliaceus in Colombia, South America. Eur J Dermatol. 2010;20:74–78. doi: 10.1684/ejd.2010.0834. [DOI] [PubMed] [Google Scholar]
- 7.Abreu-Velez AM, Howard MS, Hashimoto T, Grossniklaus HE. Human eyelid meibomian glands and tarsal muscle are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in El-Bagre, Colombia, South America. J Am Acad Dermatol. 2010;3:437–447. doi: 10.1016/j.jaad.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abreu Velez AM, Howard MS, Hashimoto K, Hashimoto T. Autoantibodies to sweat glands detected by different methods in serum and in tissue from patients affected by a new variant of endemic pemphigus foliaceus. Arch Dermatol Res. 2009;301:711–718. doi: 10.1007/s00403-009-0972-4. [DOI] [PubMed] [Google Scholar]
- 9.Abreu-Velez AM, Howard MS, Yi H, Gao W, Hashimoto T, Grossniklaus HE. Neural system antigens are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia. J Clin Immunol. 2011;31(3):356–368. doi: 10.1007/s10875-010-9495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrèu Velez AM, Avila IC, Segovia J, Yepes MM, Bollag WB. Rare clinical form in two patients affected by a new variant of endemic pemphigus in northern Colombia. Skinmed. 2004;3:317–321. doi: 10.1111/j.1540-9740.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 11.Polyak K, Lee MH, Erdjument-Bromage H, et al. “Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals”. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 12.Lin SK, Chiang CP, Hong CY, et al. Immunolocalization of interstitial collagenase (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in radicular cysts. J Oral Pathol Med. 1997;26:458–463. doi: 10.1111/j.1600-0714.1997.tb00016.x. [DOI] [PubMed] [Google Scholar]


