Abstract
Background:
Allergic diseases substantially affect human health and social economy. The pathogenesis is to be further understood. The effect of current therapeutic remedies on allergic diseases is not satisfactory.
Aims:
This study aimed to inhibit allergic rhinitis in a mouse model with a Chinese traditional medical prescription, Bu-Zhong-Yi-Qi-Tang.
Material and Methods:
A mouse AR model was developed with ovalbumin (OVA) plus adjuvant alum. The AR clinical symptoms and immune pathology in the nasal mucosa were assessed with the AR mouse model. Some mice were treated with Bu-Zhong-Yi-Qi-Tang via gavage-fed. The immune tolerance status in the nasal mucosa was evaluated by counting the numbers of tolerogenic dendritic cells (DC) and regulatory T cells (Treg).
Results:
After exposure to the specific antigen, OVA, the sensitized mice had AR-like symptoms including nasal itch and sneeze. The frequency of mast cells, levels of IgE/IL-4 in nasal mucosa was markedly higher in sensitized mice than naïve controls; while the levels of integration alphavbeta6 (avb6), the number of tolerogenic DCs and Tregs in nasal mucosa were significantly lower than naïve control mice. The AR-like symptoms and immune pathology and immune tolerance status in the AR nasal mucosa were substantially improved by administration with Bu-Zhong-Yi-Qi-Tang.
Conclusions:
The immune tolerance status is impaired in the AR nasal mucosa that can be improved by administering with Bu-Zhong-Yi-Qi-Tang.
Keywords: Airways, nose, allergic rhinitis, Chinese traditional medicine, immune tolerance
Introduction
The prevalence of allergic diseases is more than 20% population in the world that was increased tremendously in recent years. Allergic diseases can be suffered by almost every organ in the body such as asthma, allergic rhinitis, and allergic dermatitis. Currently we do not have efficient specific remedies for the treatment of allergic diseases. In fact, allergic diseases are great negative impact on human health and social economy. Thus, fresh ideas and novel therapeutic approaches for allergic diseases are imperatively wanted.
Bu-Zhong-Yi-Qi-Tang (BZYQT) is a prescription of Chinese traditional medicine. It is composed of 9 herbs that are finely processed according to the procedures in the Pharmacopeia of Chinese traditional medicine. BZYQT is widely used in China, Korea and Japan for the treatment of a number of chronic disorders, such as chronic fatigue[1], airway injury[2], airway allergy[3], male infertility[4], allergic dermatitis[3], etc. Oral administration with BZYQT can adjust immune cells’ function[5] and suppress serum IgE levels in animal models of allergy[6].
Immune tolerance indicates a condition that immune cells do not respond to stimuli that they are supposed to react[7]. It is usually induced by pre-exposure to the specific antigens[7]. Immune tolerance plays a critical role in the maintenance of homeostasis in the body. A number of immune disorders are related to the breakdown of immune tolerance, such as allergic asthma and diabetes[8]. It is proposed that CD4+ Foxp3+ regulatory T cells (Treg) play a central role in immune tolerance[9]. Tolerogenic dendritic cells (DC) are required in the development of Tregs[3]. Epithelial cell-derived integrin alpha v beta 6 (avb6) plays an important role in activating the immune suppressive molecule, transforming growth factor (TGF)-β[10]. Based on the information above, we hypothesize that administration with BZYQT can regulate the development of tolerogenic DCs and Tregs to reverse the compromised immune tolerance in the body, so that inhibits allergic disorders. In this study, we developed a mouse model of allergic rhinitis (AR) and observed that administration with BZYQT increased the number of tolerogenic DCs and Tregs in the nasal mucosa and inhibited AR symptoms and immune pathology in the nasal mucosa.
Materials and Methods
Reagents
Antibodies of ALDH1/2 (H-8), Foxp3 (F-9), CD11c (F-20), integrin β6 (H-110) were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). ELISA kits of IL-4 (sensitivity = 2 pg/ml) and IFNγ (sensitivity = 4 pg/ml) were purchased from R&D Systems (Shanghai, China). OVA-specific IgE ELISA kit was purchased from AbD Serotec (Shanghai, China).
Modified BZYQT
The prescription of modified BZYQT was adopted from the Pharmacopeia of Chinese traditional medicine. Components of BZYQT included 9 Chinese herbs: Astragali: 35%; ginseng: 6%; atractylodes macrocephala: 12%; prepared Radix Glycyrrhizae: 6%; black cohosh root: 6%; bupleuri, radix: 6%; aurantii nobilis pericarpium: 14%; finger citron: 9%; Chinese magnoliavine fruit: 6%. The herbs were refined and formulated to powder by the Pharmaceutical Department at Hunan University of Chinese Traditional Medicine. The powder 10 g was dissolved in 100 ml distilled water and filtered and autoclaved. The solution was centrifuged for 10 min at 12000 ×g and stored at 4°C up to 3 days.
AR Mouse Model
Balb/c mice (6-8 week old) were purchased from the Experimental Animal Center at Zhongshan University. Mice were subcutaneously injected with ovalbumin (OVA, 20 μg/mouse) in 0.3 ml alum on day 0 and day 11. From day 14, mice were challenged by nasal drop of OVA solution (5% OVA in PBS, 30 μg/mouse) daily for 18 days. The clinical symptoms of “nasal itch” and “sneeze” were observed for 15 min after the OVA challenge. Control mice were received saline instead of the OVA solution.
Administration of BZYQT
Two groups of mice were receiving BZYQT solution treatment by gavage-fed with the solution 0.3 ml daily. Group I (GI) was a prevention group and was treated with BZYQT from day 14 until sacrifice. Group II (GII) was a treatment group and was treated with BZYQT from day 23 until sacrifice. Mice were sacrificed on day 33.
Mast Cell Counting In The Nasal Mucosa
After sacrifice, a piece of nasal mucosa was excised and processed for paraffin section. The sections were stained with 0.5% toluidine blue for 2 min, washed with PBS, dehydration with graded alcohol, mounted with cover slips and observed under a light microscope. Mast cells were stained in purple and counted in 20 fields (×200) per mouse. The observer was not aware of the tissue type to avoid the observer-bias.
Immunohistochemistry
A piece of nasal mucosa was snapped frozen and prepared for cryosections. The sections were stained with antibodies of CD11c (1:100) and ALDH (1:50). After washing, the sections were stained with fluorescence-labeled second antibodies (1:300). The nuclei were stained with propidium iodide. The double positively stained cells were regarded as tolerogenic DCs and counted under a confocal microscope. Twenty fields (×200) were counted per mouse. The observer was not aware of the tissue type to avoid the observer-bias.
Western Blotting
A piece of nasal mucosa was processed for protein extraction. Protein extracts were subjected to electrophoresis on a 10% SDS-polyacrylamide electrophoresis gel. The proteins were transferred to a nitrocellulose membrane, which was then blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 h. The blot was then incubated with the primary antibody (1:500) overnight at 4°C in 5% BSA/TBST. The blot was washed with TBST and further incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Subsequently, the blot was washed 3 times with TBST for 1 h, developed with SuperSignal chemiluminescence reagent, and exposed to x-ray film to visualize the proteins.
Statistics
Data were presented as mean ± SD. The means were analyzed by student t test between 2 groups or ANOVA if more than 2 groups. P<0.05 was set as the significant criteria.
Results
Allergic Responses Decrease The Number Of Tregs In The Nasal Mucosa
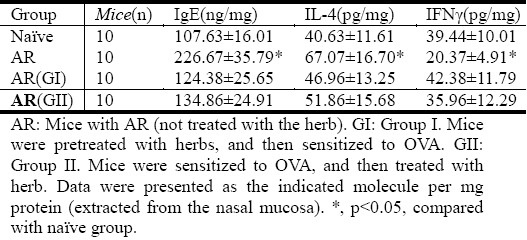
In this study, we developed a mouse model of AR. The AR mice had high frequencies of mast cells in the nasal mucosa (Table 1) and high levels of OVA-specific IgE, IL-4 and IFNγ in the nasal tissue protein extracts (Table 2). When challenged the nasal mucosa with a specific antigen, OVA, all the mice had similar symptoms to the “nasal itch” and “sneeze” in human with AR attack occurred within 15 min after antigen challenge.
Table 1.
Mast cell counts in the nasal mucosa
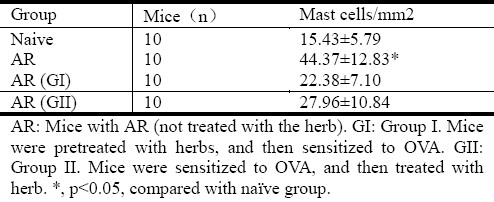
Table 2.
IgE and cytokine levels in the nasal mucosa
The breakdown of immune tolerance plays a critical role in the pathogenesis of allergy. Tregs are one of the critical components of immune tolerance. In this study, we also observed Foxp3+ Tregs in the nasal mucosa by immunohistochemistry. The results showed that significantly less Tregs were observed in mice with AR than that in naïve control mice. The suppression of Treg number was prevented or abolished by administration with BZYQT (Figure 1).
Fig. 1.
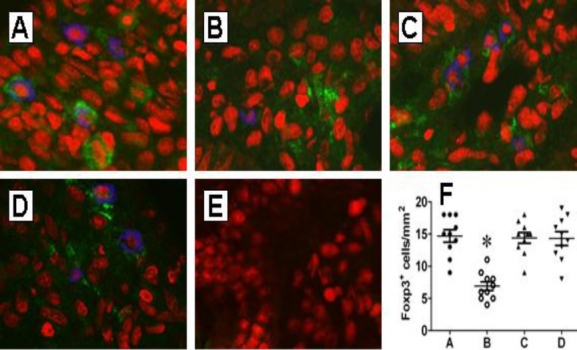
Allergic responses reduce the number of Foxp3+ Tregs in nasal mucosa. Balb/c mice (10 mice per group) were sensitized to and challenged with OVA. The nasal mucosa was taken from naïve mice (A), sensitized mice (B), mice treated with BZYQT before (C) and after (D) sensitization. The nasal mucosa was processed for immunohistochemistry. The Foxp3 was stained in blue and CD4 is stained in green. The CD4+ Foxp3+ Tregs were counted under confocal microscope. Twenty fields (×200) were counted on each sample. Panel E shows isotype IgG staining using as control. F, scatter dot plots show individual data points in panel A-D. *, p<0.05, compared with control group (A). Original magnification: ×200.
Tolerogenic DCs Are Reduced In The Nasal Mucosa Of Mice With AR
Tolerogenic DCs have the ability to induce Treg development. The finding that the number of Tregs was markedly suppressed in the AR nasal mucosa (Figure 1) implies the number of tolerogenic DCs may be reduced as well in the AR nasal mucosa. To test the hypothesis, we stained the nasal mucosal sections with antibodies against CD11c+ and ALDH+ (the markers of tolerogenic DC)[11]. Under a confocal microscope, CD11c+ ALDH+ cells could be seen in the nasal mucosa of both naïve and AR mice. The positively stained cells were counted. The results showed that the frequency of CD11c+ ALDH+ cells was markedly less in AR mice than that in naïve control mice. Treatment with BZYQT before sensitization prevented the suppression of tolerogenic DCs in the nasal mucosa. The administration with BZYQT also reversed the AR-suppressed tolerogenic DCs in nasal mucosa (Figure 2).
Fig. 2.
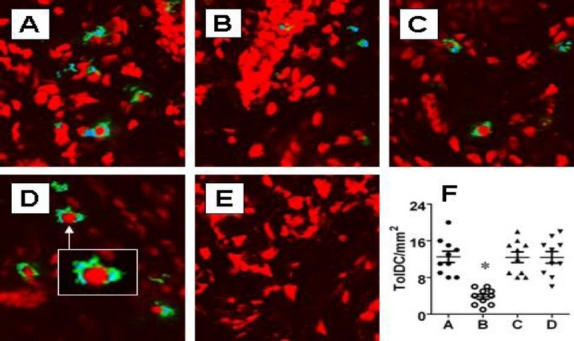
Allergic responses suppress tolerogenic DCs in the nasal mucosa. Mice were treated with the same procedures in Fig.1. Nasal mucosa was taken for immune staining with anti-CD11c and anti-ALDH antibodies. A-D, confocal images show the positive staining of CD11c (in green) and ALDH (in blue). Cells stained in both green and blue were regarded as the tolerogenic DCs and counted (20 fields per mouse) under confocal microscope. Panel D is isotype IgG control. F, scatter dot plots show individual data points in panel A-D. Original magnification: ×200. Insert: ×630.
Levels of Avb6 Are Reduced In Nasal Mucosa Of Mice With AR
Our recent study indicates that integrin avb6 plays a critical role in the development of tolerogenic DCs in the intestinal mucosa. To see if the expression of avb6 was compromised in AR, we assessed the contents of avb6 in nasal mucosa. As shown by Western blotting, the contents of avb6 were significantly decreased in the AR nasal mucosa as a contrast to that in the naïve nasal mucosa that was reversed by administration with BZYQT (Figure 3).
Fig. 3.
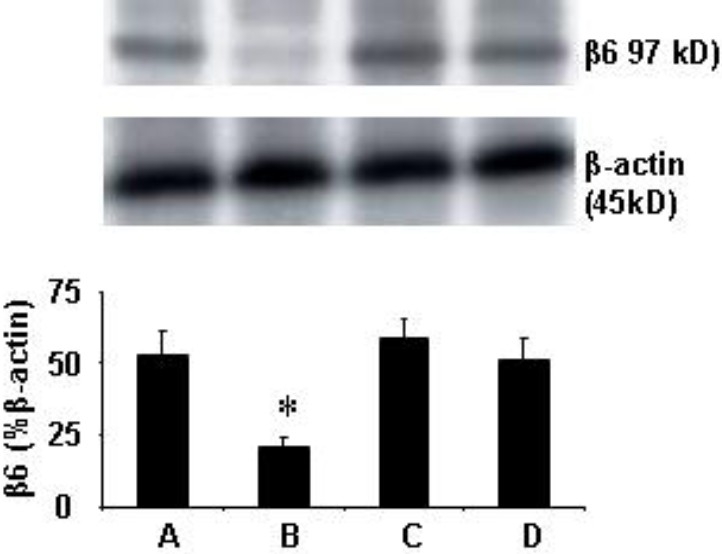
Allergic responses down regulate the expression of avb6 in nasal mucosa. Mice were treated in the same procedures in Fig.1. Nasal mucosa was taken and analyzed by Western blotting. The immune blots show avb6 levels. Bars show the summarized densitometrical data (mean ± SD from 3 experiments). *, p<0.05, compared with group A. The group annotations are the same as Fig. 1.
Discussion
This paper reports a novel set of data in which an AR mouse model shows the number of tolerogenic DCs and Tregs is markedly suppressed in the nasal mucosa that can be prevented or abolished by administration with BZYQT.
BZYQT is widely used in the treatment of a number of chronic diseases, such as chronic fatigue and immune disorders[1–3]. The pathogenesis of allergic diseases is incompletely understood. The mechanism by which BZYQT improves these diseases based on its ability in regulating the immune function in the body, such as up regulating IgA secretion[3], adjusting skewed IgE production[3], etc. The present data add novel information to this area by revealing a new aspect in BZYQT's ability. After administration with BZYQT, the clinical symptoms of AR in mice were inhibited together with the suppression of mast cell numbers in the nasal mucosa and decreased the levels of IgE and IL-4. The data demonstrate that BZYQT has the ability to suppress AR's clinical symptoms and improves the immune pathology in the nasal mucosa of AR.
Tregs have the immune regulatory function. Activation of Tregs can suppress other effector T cells’ activity. In other words, T cells’ functions are tightly regulated by integrated regulatory machinery in the body. Tregs are an important component of such regulatory machinery[12]. Dysfunction of Tregs is related to a number of immune disorders, such as experimental autoimmune encephalomyelitis[13], asthma[14] or allergic dermatitis[15]. In line with those studies, our data indicate that the number of Foxp3 Tregs is decreased in experimental AR. We also detected polarization of Th2 response in the nasal mucosa manifesting high levels of IgE, IL-4 and mastocytosis; these phenomena are evidence of the immune tolerance is breached by AR; perhaps the skewed Th2 responses are sequelae of the suppression of Tregs in the nasal mucosa because Tregs are supposed to maintain the immune tolerance[16].
Our recent study reveals that the integrin avb6 plays a critical role in the development of immune tolerance (data not shown). Avb6 is produced by epithelial cells that can be trapped by exosomes to be carried to the subepithelial region where avb6 may be captured by immature DCs. One of the functions of avb6 is to convert the precursor of TGF-β to the active form of TGF-β. The TGF-β-carrying DCs are capable of converting naïve CD4+ T cells to Foxp3+ CD4+ Tregs. This subset of DCs can be designated as tolerogenic DCs, which express ALDH; the latter has high levels of retinoic acid (RA). RA is an important molecule in the generation of Tregs[11]. Our data indicate that the number of CD11c+ ALDH+ cells is markedly less in the AR nasal mucosa; the factor might have contributed to the suppression of Treg in the nasal mucosa as we observed in the present study. The inference is supported by published data[11] that indicate that DC-derived RA is required in the development of Tregs. Therefore, based on the present data, we may envisage a scenario like this: AR responses suppress the expression of avb6 in nasal epithelial cells that results in reduction of tolerogenic DCs in the nasal mucosa, and thus the defect of Tregs is developed. The defect of Tregs leads to Th2 response polarization in the nasal mucosa. The inference is corroborated by the results of administration with BZYQT. Mice pretreated with BZYQT did not develop AR, the levels of avb6 and numbers of tolerogenic DCs and Tregs in the nasal mucosa are comparable to naïve control mice. The finding indicates that BZYQT has the ability to prevent AR. On the other hand, AR mice treated with BZYQT also showed equal amounts of avb6 and tolerogenic DCs and Tregs in the nasal mucosa to naïve mice indicating that administration with BZYQT can reverse the breached immune tolerance in the AR nasal mucosa.
Acknowledgments
This study was supported by a grant (A101129) from the Guangdong provincial scientific foundation.
References
- 1.Jong SJ, Bong HR, Jin SK, Jae WP, Won CC, Seong WY. Bojungikki-Tang for Cancer-Related Fatigue: A Pilot Randomized Clinical Trial. Integr Cancer Ther. 2010;9:331–338. doi: 10.1177/1534735410383170. [DOI] [PubMed] [Google Scholar]
- 2.Tatsumi K, Shinozuka N, Nakayama K, et al. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary DISEASE. J Am Geriatr Soc. 2009;57:169–170. doi: 10.1111/j.1532-5415.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang SH, Yu CL. Antiinflammatory effects of Bu-zhong-yi-qi-tang in patients with perennial allergic rhinitis. J Ethnopharmacol. 2008;115:104–109. doi: 10.1016/j.jep.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Furuya Y, Akashi T, Fuse H. Effect Of Bu-Zhong-Yi-Qi-Tang On Seminal Plasma Cytokine Levels In Patients With Idiopathic Male Infertility. Syst Biol Reprod Med. 2004;50:11–14. doi: 10.1080/01485010490250515. [DOI] [PubMed] [Google Scholar]
- 5.Kuroiwa A, Liou S, Yan H, Eshita A, Naitoh S, Nagayama A. Effect of a traditional Japanese herbal medicine, Hochu-ekki-to (Bu-Zhong-Yi-Qi Tang), on immunity in elderly persons. Int Immunopharmacol. 2004;4:317–324. doi: 10.1016/j.intimp.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Ishimitsu R, Nishimura H, Kawauchi H, Kawakita T, Yoshikai Y. Dichotomous effect of a traditional Japanese medicine, Bu-zhong-yi-qi-tang on allergic asthma in mice. Int Immunopharmacol. 2001;1:857–65. doi: 10.1016/s1567-5769(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 7.Mayer L, Shao L. Therapeutic potential of oral tolerance. Nat Rev Immunol. 2004;4:407–419. doi: 10.1038/nri1370. [DOI] [PubMed] [Google Scholar]
- 8.Faria AMC, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corthay A. How do Regulatory T Cells Work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 11.Coombes JL, Siddiqui KRR, Arancibia-C+írcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+CD25+CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Siegmund K, Zeis T, Kunz G, Rolink T, Schaeren-Wiemers N, Pieters J. Coronin 1-Mediated Naive T Cell Survival Is Essential for the Development of Autoimmune Encephalomyelitis. J Immunol. 2011;186:3452–3461. doi: 10.4049/jimmunol.1003491. [DOI] [PubMed] [Google Scholar]
- 14.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: Novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–968. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]


