Abstract
Acquired immunity depends on proliferation and differentiation of antigen (Ag)-specific B cells in germinal centers (GCs) of lymphoid follicles in response to T cell-dependent Ags. Here, we studied the function of GC-associated nuclear protein that is selectively up-regulated in GC-B cells. B cell-specific ganp-deficient mice were compromised in affinity maturation of hapten-specific antibodies against T cell-dependent Ags with retarded development of GCs. B cell numbers and development, serum Ig levels, mitogen-induced B cell proliferation in vitro, and responses to T cell-independent Ag were nearly normal; however, the mutant B cells showed a decrease in anti-CD40-induced proliferation and an increased susceptibility to B cell apoptosis in vitro and in vivo. B cell-specific ganp-deficient mice showed a decreased frequency of variable-region somatic mutations, especially of the high-affinity type (W33 → L) in the VH186.2 region against nitrophenyl-chicken gamma globulin, whereas the class switching was normal. We conclude that GC-associated nuclear protein is necessary for generation or maintenance of B cells with high-affinity B cell Ag receptors during the maturation in GCs.
Naïve B cells express B cell antigen receptor (BCR) determined by the expression of variable (V) regions that are created by random recombinations of Ig genes as VH-DH-JH (variable heavy-diversity heavy-joining heavy chain) rearrangements for heavy (H) chain and VL-JL (variable light-joining light chain) rearrangement for light (L) chain during early B cell differentiation in the fetal liver or in the bone marrow microenvironments (1–4). The binding of antigen (Ag) to the BCR initiates the activation of Ag-specific B cells for the proliferation and differentiation into Ab-secreting plasma cells. For efficient B cell activation to T cell-dependent Ags (TD-Ags), Ag-driven B cells gather in the germinal center (GC) follicular region of peripheral lymphoid organs and proliferate as centroblasts at an enormous rate in the dark zone (4–6). During the proliferation and differentiation of Ag-driven B cells in GCs, B cells undergo somatic hypermutation (SHM) of Ig V regions and class-switch recombination (CSR) of Ig-H.
SHM is induced by the stimulation of BCR complexes containing BCR, CD19, and CD21 and by costimulatory signals through CD40 and CD154 (7, 8). These stimuli provided in GCs probably up-regulate molecules required for SHM. An RNA-editing molecule, activation-induced cytidine deaminase, expressed specifically in GCs is necessary for generation of SHM and CSR (9–11). Uracil DNA-glycosylase is up-regulated in GCs and is also involved in generation of SHM in a B cell line in vitro (12). The initial modification of DNA by activation-induced cytidine deaminase is probably followed by uracil DNA-glycosylase action, which may account for the generation of SHM in V regions during the immune response (12, 13). Various DNA polymerases were shown to be involved in generation of SHM in Ig V regions by experiments using cell lines of various DNA polymerases (14–18).
Because the mutations generated by these molecular events are probably random, it is not clear how the high-affinity B cells are predominantly generated and selected during immune responses. This process is mostly undertaken in Ag-driven B cells in GCs, and the B cells with high-affinity BCRs are presumably selected for the higher binding activity to the Ags presented by the immune complex through CR1 expressed on the surface of follicular dendritic cells. Such B cells might undergo clonal expansion and differentiation into Ab-producing cells with the help of costimulatory activities provided by T helper 2 cells through cognate interactions such as CD40/CD154 (CD40L) (19) and CD80/CD28 (20) or mediated by cytokines such as IL-4 (21), IL-6 (22), BAFF/Blys (23), and lymphotoxin-α (24). B cells with low-affinity, irrelevant, and self-reactive BCRs are eliminated or not stimulated for subsequent proliferation (25, 26). However, B cells with high-affinity BCRs are rescued from death in GCs by secreted or cell-bound antiapoptotic factors, such as CD40/CD154 and CD80/CD28, provided by the T cells (27). To understand further how high-affinity B cells accumulate, it is helpful to study the functions of specific molecules that appear selectively in GC-B cells during the immune response.
Here, we focus on the function of GC-associated nuclear protein (GANP) that appears in GC-B cells (28–30). GANP is up-regulated selectively in GC-B cells surrounded by a follicular dendritic cell network (28). GANP is a 210-kDa nuclear protein with a homology to Saccharomyces Sac3, characterized as a suppressor of actin formation, but its detailed immunological function remains undetermined. Stimulation of B cells with anti-μ Ab and anti-CD40 mAb in vitro induced not only the up-regulation of GANP expression but also the phosphorylation of a specific serine residue (S502, the 502nd amino acid), which is probably a key reaction for RNA-primase activity of GANP (30). The N-terminal side RNA-primase domain contains the serine residue whose phosphorylation is catalyzed in vitro by Cdk2. By the C-terminal side domain, GANP binds to the MCM3 replication-licensing factor (28, 29), a component of the MCM complex that is involved in DNA replication through DNA-helicase activity (31). An alternatively spliced transcript of ganp gene encodes a 80-kDa protein named Map80/MCM3AP, which is supposed to be involved in the association with MCM3 and possess an acetylating activity on MCM3 (32). Northern blot analysis with mRNAs of various organs and tissues of mouse and human demonstrated exclusively a 7-kb band of ganp gene transcript rather than the short-form map80/mcm3ap transcript (28, 29), suggesting that the Map80 region of GANP plays an important role in DNA replication or DNA repair mechanisms. Therefore, we addressed the question whether GANP is involved in the immune response. By preparing ganp-knockout (B-Ganp–/–) mice whose ganp gene expression is conditionally targeted in B cells, we show that GANP plays a critical role in generation of Ag-specific and high-affinity B cells during the immune response.
Materials and Methods
Establishment of B-Ganp–/– Mice. The targeting vector was prepared by the insertion of the neomycin-resistance gene (neo) in the downstream of the ganp exon II. LoxP sites were introduced in the downstream of neo gene and the intron between ganp exons I and II. TT2 embryonic stem cells (33) were transfected. Homologous embryonic stem recombinants were screened by neo2 primer (5′-GCCTGCTTGCCGAATATCATGGTGGAAAAT-3′) and CGK3′-2 primer (5′-GGCACCAAGCATGCACGGAGTACACAGA-3′). The ganp flox/+ mice were backcrossed to C57BL/6 at least 10 times. To delete the ganp gene in B cells, we crossed ganp-floxed mice with CD19-Cre knock-in mice (34).
mAbs. Biotinylated mAbs against IgM, CD43, and IgD and phycoerythrin-conjugated mAbs against B220 and IgM were purchased (Pharmingen).
RT-PCR. Total RNAs from spleens or spleen B cells were amplified by RT-PCR using two primers, ganp 1-5′ and ganp 1-3′, described above (28), and analyzed. A β-actin transcript was used as control (29). The primer sets for bcl-2 family genes were as described (35).
In Vitro Proliferation Assay. Reagents were affinity-purified goat anti-μ-chain-specific Ab (10 μg/ml) [F(ab′)2] (ICN), rat anti-mCD40 mAb (LB429, 10 μg/ml) (36), and lipopolysaccharide (LPS; Sigma L4005, 10 μg/ml).
Ag and Immunization. Trinitrophenyl-keyhole limpet hemocyanin (TNP-KLH), TNP-Ficoll, and nitrophenyl (NP)-chicken gamma globulin (CG) (23 NP groups per molecule) were purchased from Biosearch. Twenty to 100 μg of TNP-KLH and NP-CG precipitated by alum (Pierce) or 25 μg of TNP-Ficoll in PBS was injected i.p.
Measurement of Ag-Specific Ab Production. At days 10 or 14 after immunization, sera were collected and measured on ELISA plates with 5 μg of TNP-BSA per well (Biosearch). To estimate the affinity of NP-binding Ab in the sera, ratios of NP2-binding Ab to and NP25-binding Ab were calculated by using NP2-BSA and NP25-BSA (Biosearch).
Detection of Apoptotic Cells. B cells from Cre-flox/+ and B-Ganp–/– mice were stimulated for 40 h as described (37). For the activation-induced cell death (AICD), anti-μ Ab was immobilized on a 24-well culture dish. Purified B cells were incubated with stimulants for 48 h, followed with anti-Fas mAb (Jo2, Pharmingen) for 4 h (38). Apoptotic cells were calculated (percent) as sub-G1 area by FACScan.
Terminal Deoxynucleotidyltransferase-Mediated dUTP-Biotin Nick-End Labeling (TUNEL) Assay. Spleen sections from Cre-flox/+ and B-Ganp–/– mice immunized with sheep red blood cells were processed with MEBSTAIN Apoptosis Kit II (Medical and Biological Laboratories, Nagoya, Japan) and counterstained by propidium iodide. For other experiments together with the TUNEL assay, the sections were stained with anti-IgG1 mAb (Pharmingen) with Alexa546-goat anti-rat IgG Ab (Molecular Probes).
Results
Immune Response of B-Ganp–/– Mice. GANP expression is selectively up-regulated in GC-B cells (28); therefore, we studied the function of GANP by generating mutant mice (B-Ganp–/– mice), in which the ganp gene is specifically targeted in CD19+ B cells by using the Cre-loxP system (Fig. 3 A and B, which is published as supporting information on the PNAS web site). The B cells of the B-Ganp–/– mice, created by mating of the floxed mice with CD19-Cre knock-in mice (34), lost nearly 90% of the ganp gene (Fig. 3C), had a marked decrease of ganp mRNA expression (Fig. 3D), and did not show the induction of GANP protein by immunohistochemical staining (Fig. 3E). These B-Ganp–/– mice, however, grew normally and had normal numbers of lymphoid cells in the bone marrow, spleen, thymus, and lymph nodes (data not shown). The B-Ganp–/– mice showed normal surface markers on the lymphoid cells of the bone marrow, spleen, and lymph nodes by flow cytometric analyses (Fig. 3F). We observed that B-Ganp–/– mice showed a slight decrease of sIgDhigh+ B cells in the lymph nodes (from 6.4% to 4.8%).
The B cells from B-Ganp–/– mice underwent normal proliferative responses in comparison with CD19-Cre-ganp flox/+ (Creflox/+) littermates after stimulation with anti-μ Ab, anti-μ Ab + anti-CD40 mAb, or LPS in vitro. For comparison of B-Ganp–/– mice, we used the controls of Cre-flox/+ littermates and WT C57BL/6 mice (data not shown) in the experiments, in which both controls showed similar results. The mice showed a decreased response to anti-CD40 stimulation (LB429) at 5 and 10 μg/ml (Fig. 3G), demonstrating that B cell proliferation of B-Ganp–/– mice was slightly impaired in response to CD40/CD154 interaction; however, the proliferative potential of B cells was not impaired in B-Ganp–/– mice as observed in response to LPS stimulation. Serum Ig titers were normal in all the tested IgM, IgG1, IgG2a, IgG2b, and IgG3 classes (Fig. 3H).
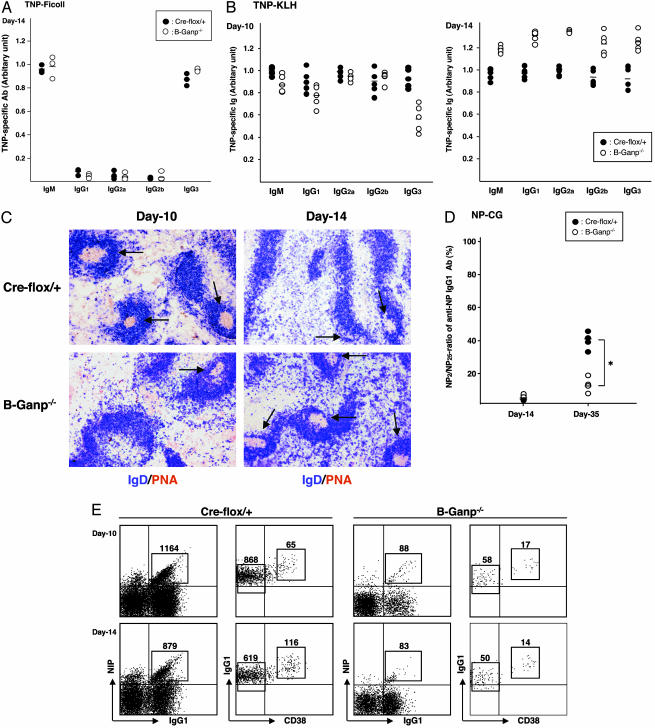
Next, we examined the immune responses of B-Ganp–/– mice against T cell-independent (TI)-II Ag and TD-Ag after Ag immunization. A type II TI-Ag, TNP-Ficoll, induced similar responses in B-Ganp–/– and Cre-flox/+ mice at day 14 after immunization (Fig. 1A). No alteration occurred in isotype switching. Mutant mice showed similar or even higher Ab responses to the TD-Ag, TNP-KLH, than Cre-flox/+ controls at days 10 and 14 (Fig. 1B). The Ag-specific IgG3 response was slightly decreased at day 10; however, the Ab responses of various isotypes were increased at day 14 in the mutant mice. The overall Ab responses were not impaired in B-Ganp–/– mice; even the responses to TD-Ag were augmented. However, the mutant mice showed a delayed GC formation in response to the TD-Ag compared with Cre-flox/+ controls. B-Ganp–/– mice had few GCs until day 10, but a gradual increase and enlargement of GCs occurred at day 14 after immunization with TNP-KLH (Fig. 1C). The peak response of GC formation was retarded compared with Cre-flox/+ mice, but the B-Ganp–/– mice developed large and mature GCs stained by PNA at day 14. This finding was observed by the repeated experiments, which showed that the B-Ganp–/– mice could not induce an immediate GC formation after immunization with TD-Ags.
Fig. 1.
Ag-specific responses in B-Ganp–/– mice. (A) TI-II-specific Ab in B-Ganp–/– mice. Sera from 8-week-old mice immunized with 25 μg of TNP-Ficoll were collected at day 14 for TNP-specific Abs. Circles indicate values from Cre-flox/+ mice (•) and B-Ganp–/– mice (○). (B) TD-specific Ab in B-Ganp–/– mice. Sera were collected at days 10 and 14 after immunization with TNP-KLH for Ag-specific Abs. (C) Time course of GC formation in B-Ganp–/– mice. Spleen sections after immunization with TNP-KLH were immunostained doubly by PNA (brown) and IgD (blue) at days 10 and 14. PNA-positive GCs are indicated by arrows. (D) Relative affinity of serum Abs in B-Ganp–/– mice. Sera from Cre-flox/+ and B-Ganp–/– mice immunized with NP-CG were collected at days 14 and 35. The NP2/NP25 ratios of anti-NP IgG1 were measured by ELISA. *, P < 0.05. (E) Decreased number of NP-binding GC-B cells in B-Ganp–/– mice. Cre-flox/+ and B-Ganp–/– mice were immunized with 100 μg of NP-CG, and the spleen cells were prepared on days 10 and 14. NP-binding IgG1dullCD38low B cells were analyzed as GC-B cells by flow cytometry. NIP, (4-hydroxy-5-iodo-3-nitrophenyl)acetyl. The results shown are representative of four independent pairs.
Affinity Maturation of Anti-NP-Specific B Cells in B-Ganp–/– Mice. To study the immune response of B-Ganp–/– mice, we measured affinity maturation of NP-specific serum Igs after immunization with 100 μg of alum-precipitated NP-CG. The differential ELISA method using hapten/protein conjugates of NP with different molecular numbers on BSA showed a low-affinity Ab response (13%) in B-Ganp–/– mice against the pauci-hapten NP2-BSA conjugate in comparison with the response to the multiple-hapten NP25-BSA conjugate. This response is markedly reduced in comparison with the responses of Cre-flox/+ (42%) mice at day 35 (Fig. 1D). To characterize the lower affinity of the Ab response in B-Ganp–/– mice, we analyzed the responding B cells by focusing on the Ag-specific IgG1+ cells. Ag-specific IgG1 GC-B cells (NIP+IgG1+) were examined after immunization with NP-CG (Fig. 1E). The NP-specific IgG1dull B cells were markedly decreased in B-Ganp–/– mice at day 10 (88 cells per 106 cells in B-Ganp–/– mice vs. 1,164 cells in Cre-flox/+ mice) and at day 14 (83 cells vs. 879 cells). This tendency was also observed at day 20 (data not shown). The NP-specific IgG1+ B cells were further separated as CD38high memory B cells and CD38low GC-B cells. The decrease was in the GC-B cell and memory B cell populations. These results suggest that the GANP-null mutation caused a defect in generation of high-affinity BCR+ B cells in vivo.
Decrease in Accumulation of High-Affinity Type Mutation in VH186.2 in B-Ganp–/– Mice. To confirm the decrease of affinity maturation of the Abs in B-Ganp–/– mice, we studied the sequence of the VH186.2 region of the spleen B cells after immunization with NP-CG. The NP response has been extensively investigated, and it is known that the VH186.2 locus shows a peculiar pattern of SHM for high-affinity IgG (γ1λ1) NP responses in C57BL/6 mice (39). SHM was found to be decreased in B-Ganp–/– mice at VH186.2 of IgM Abs (50% of control Cre-flox/+ mice; data not shown). Therefore, we purified the NP-binding IgG1 B cells as shown in Fig. 1E and compared the sequences of their VH186.2 (Fig. 4A, which is published as supporting information on the PNAS web site). Overall mutation in VH186.2 of IgG1 was decreased in B-GANP–/– mice (14 × 10–3 mutations at V region) compared with Cre-flox/+ mice (21 × 10–3 mutations at V region) (Fig. 4 A and C). More strikingly, the high-affinity type mutation of W33 to L was severely decreased in the mutant mice (2 per 15 V regions, 13%) in comparison with Cre-flox/+ mice (10 per 14 V regions, 71%) (Fig. 4D). These results were consistent with the decreased affinity maturation of the serum Ig against the hapten NP as measured by ELISA (Fig. 1D). (See Fig. 4 and Supporting Materials and Methods, which are published as supporting information on the PNAS web site.)
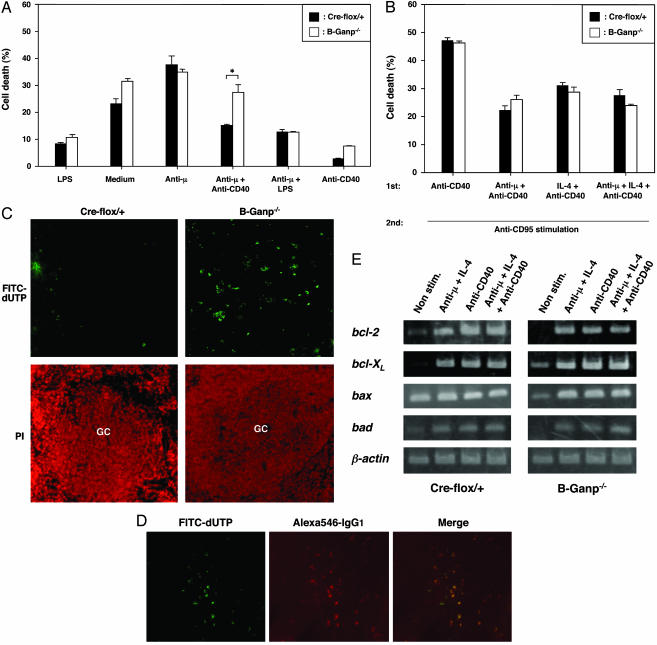
Impairment in the CD40-Mediated Prevention of Apoptosis in B-Ganp–/– Mice. The impairment in generating high-affinity Abs in B-Ganp–/– mice might be due to the instability of B cells after Ag stimulation, potentially regulated by CD40/CD154 interaction. To examine the susceptibility of B cells, we investigated the B cell apoptosis in vitro. Strong cross-linkage of BCR induces AICD responses in normal spleen B cells in vitro that are prevented by stimulation by CD40 (37). B-Ganp–/– B cells are similarly susceptible to the stimulation of AICD (Fig. 2A). However, the B cells are rescued less by anti-CD40-mediated prevention of apoptosis than by Cre-flox/+ control B cells, suggesting that B-Ganp–/– mice are impaired in the protection of Ag-reactive B cells during the development of GCs.
Fig. 2.
Impaired B cell survival of B-Ganp–/– mice. (A) Impairment of CD40-mediated rescue from AICD in B-Ganp–/– B cells. Purified B cells (2 × 106) from Cre-flox/+ and B-Ganp–/– mice were stimulated for 40 h on the culture plates coated with anti-μ Ab and/or various stimulants. Cell death (percent) was analyzed as marked with filled columns for Cre-flox/+ mice and open columns for B-Ganp–/– mice. The results shown are representative of four independent experiments. The difference between Cre-flox/+ mice and B-Ganp–/– mice in the stimulation with anti-μ Ab + anti-CD40 mAb was significant: *, P < 0.05. (B) No change in the anti-CD95-mediated apoptosis of B-Ganp–/– B cells. Purified B cells (2 × 106) from Cre-flox/+ and B-Ganp–/– mice were stimulated for 48 h, and then anti-CD95/Fas mAb (Jo2, 5 μg/ml) was added. (C) Apoptotic cells after immunization with TD-Ag. Spleens of Cre-flox/+ and B-Ganp–/– mice immunized with sheep red blood cells were removed at day 14 and measured by TUNEL assay. These spleens were counterstained by propidium iodide (PI) with PNA staining on the adjacent section. The results shown are representatives obtained under microscopic observations of three independent experiments, all of which showed similar results. (D) Apoptosis in GC-B cells of B-Ganp–/– mice. As secondary staining, anti-IgG1 mAb plus Alexa546-goat anti-rat IgG Ab was used. The results shown are representative. (E) Transcription of bcl-2 family genes in B-Ganp–/– B cells. B cells (2 × 106) from Cre-flox/+ and B-Ganp–/– mice were cultured for 48 h. RT-PCR was performed for bcl-2 family genes.
In GCs, B cells stimulated with Ag and CD40/CD154 interaction induce the surface expression of Fas/CD95 and become susceptible to Fas-induced apoptosis. We measured the susceptibility of the B-Ganp–/– B cells to anti-CD95 stimulation, which induces apoptosis in vitro (38). First, spleen B cells were stimulated with anti-CD40 mAb (LB429), anti-μ Ab + anti-CD40 mAb, IL-4 + anti-CD40 mAb, and anti-μ Ab + IL-4 + anti-CD40 mAb for 48 h, and then anti-CD95 mAb was added in the culture. Apoptotic responses of B-Ganp–/– B cells were similar responses of Creflox/+ B cells (Fig. 2B). The induction of CD95 expression after anti-CD40 (LB429) treatment was normal as compared with the Cre-flox/+ control B cells (data not shown).
These in vitro experiments suggested that the B-Ganp–/– B cells may be susceptible to the apoptotic stimulation normally received by GC-B cells in vivo. Therefore, the TUNEL assay was used with histological sections of the splenic GCs that were induced by immunization with TD-Ag, sheep red blood cells (Fig. 2C). TUNEL-positive cells increased in the GC area of B-Ganp–/– mice, and most of them also showed IgG1 expression as confirmed by merge image (Fig. 2D). These results demonstrated that the apoptotic cells are mostly of GC-B cells.
Substantial information is available regarding the molecules involved in CD40-mediated rescue from BCR-mediated apoptosis; and, in general, it is postulated that alterations in the ratio of proapoptotic to antiapoptotic members of the Bcl-2 family can protect against the induction or progression of apoptosis through the alteration of mitochondrial membrane permeability (40). We compared the RNA expression of Bcl-2 family members, which are generally agreed to be the molecules involved in CD40-mediated prevention of apoptosis of various malignant lymphoma cells and normal B cells (41, 42). Stimulation with anti-μ plus IL-4 induced an apparent increase of bcl-2 transcription in Cre-flox/+ B cells, and anti-CD40 mAb further up-regulated its expression (Fig. 2E). The B-Ganp–/– B cells showed the similar up-regulation of bcl-2 transcripts by anti-μ stimulation, but the response to anti-CD40 mAb (anti-CD40 mAb alone or anti-μ Ab + IL-4 + anti-CD40 mAb) was not as high as those in Cre-flox/+ B cells. The other responses, bcl-XL, bax, and bad, in mutant B cells were similar to the levels of Cre-flox/+ B cells (Fig. 2E). These observations suggest that GANP regulates the signal transduction of CD40-mediated induction of Bcl-2 expression in GC-B cells, which potentially contributes to the survival of high-affinity BCR+ B cells in vivo.
Discussion
GCs are specialized structures in which Ag-driven B cells undergo affinity maturation of BCRs by three processes. First, the BCR repertoire of Ag-stimulated B cells is diversified through the random introduction of point mutations as SHM in the V-region genes; second, the specificity and affinity of BCRs are tested by the follicular dendritic cell network (6, 27); and third, the selected B cells are maintained and differentiated into Ab-producing cells or memory B cells. Analysis of mutant B-Ganp–/– mice clearly demonstrated that GANP is involved in generation of high-affinity B cells against TD-Ag. Lack of GANP decreased the SHM frequency generated by immunization with TD-Ag in vivo, but did not abolish the SHM or prevent CSR during Ag-specific Ab responses. The results suggest that the generation of high-affinity B cells in GCs depends on GANP, and we will consider several possible mechanisms of action.
Role of GANP in B Cell Proliferation. Lack of GANP carrying the RNA-primase domain and MCM-binding region did not cause obvious impairment of B cell proliferation by stimulation with LPS in vitro. Furthermore, responses to a TI-Ag were not impaired in B-Ganp–/– mice (Fig. 1A). However, the GCs formed until day 10 after immunization with TD-Ag were apparently fewer, smaller, and less mature than GCs in Cre-flox/+ littermates, suggesting that the expression of GANP also affects the proliferation of Ag-reactive B cells in GCs. This finding was supported by the decreased proliferation of B-Ganp–/– B cells in response to anti-CD40 (LB429) stimulation in vitro (Fig. 3G).
Role of GANP in Generation of High-Affinity B Cells. The production of high-affinity Ab basically depends on the selective induction of SHM in the V region, for which various molecules have been identified (14–18).
Loss of GANP expression in B cells resulted in a remarkable decrease of SHM in an individual V-region sequence. Although GANP presumably participates in post-activation-induced cytidine deaminase events required for affinity maturation of BCRs, the observation suggests that GANP might also be involved in generation of SHM in GC-B cells.
A similar abnormality was reported in transgenic mice expressing antisense DNA polymerase ζ, in which the induction of high-affinity mutations of the Ig V region was decreased (43). Ag-induced GC formation was not impaired, but the B cells specific for hapten did not gain mutations of the high-affinity type in GC-B cells and memory B cells, suggesting that the expression of DNA polymerase ζ as a non-replication-associated polymerase is important for generation of high-affinity B cells.
One particularly interesting observation in B-Ganp–/– mice was the marked increase of NP-specific B cells with VH186.2 analogues. These VH genes are used in the early stages of GC-B cell maturation after immunization with NP-CG in WT mice (44). These noncanonical V-region genes almost disappeared at day 14 after immunization in Cre-flox/+ mice; however, their utilization was much increased in B-Ganp–/– mice to almost 60% (Fig. 4B) in comparison with that of Cre-flox/+ (18%). The frequent use of VH186.2 analogues might be caused by the decrease of high-affinity NP-specific BCR+ B cells in GCs in B-Ganp–/– mice.
SHM in the VH186.2 of GANP-Mutant Mice. The base substitution pattern of the VH186.2 response of B-Ganp–/– mice after immunization with NP-CG was compared (Fig. 5, which is published as supporting information on the PNAS web site). Mutations found in the B-Ganp–/– B cells were biased to known SHM hotspots, such as RGYW, WRCY, and WA motifs; 7 of 24 mutations were found at the RGYW motif and 2 were found at the WA motif (data not shown). This proportion is similar to the frequencies generated in Cre-flox/+ littermates.
Role of GANP in CSR. GANP is probably not associated with the CSR of GC-B cells. In comparison with the activation-induced cytidine deaminase-knockout mice that bear larger GCs in the spleen after immunization (9), B-Ganp–/– mice showed a retarded and prolonged formation of GCs after immunization with TD-Ags. However, the serum Igs in B-Ganp–/– mice were similar to those in Cre-flox/+ littermates, with IgM, IgG1, IgG2a, IgG2b, and IgG3 classes after immunization with TD-Ag (Fig. 1B). We conclude that loss of GANP does not target the CSR mechanism.
Apoptotic Selection for High-Affinity B Cells in GCs. Mutations in the gene encoding CD40L (CD154) caused an immunodeficiency disease called X-linked hyper-IgM syndrome, which is characterized by normal or elevated levels of IgM but no IgG, IgA, or IgE productions and defects in GC and memory formation (45), which is similar with CD40-knockout mice (46). The B-Ganp–/– mice are quite different; no apparent abnormalities of B cell proliferation, no severe defects in GC formation, and no impairment of CSR occur. However, the B-Ganp–/– mice are impaired with respect to their production of high-affinity Ab. We consider that GANP function is involved in one of the molecular mechanisms regulated by CD40/CD154 interaction in GC-B cells and is necessary for the survival of high-affinity BCR+ B cells.
High-affinity B cells seem to resist the apoptosis-inducing signals in GCs (47), but the mechanism for their survival has remained unclear. The bcl-2 transgenic mice frequently generate self-Ag-reactive B cells, which are otherwise susceptible to the apoptotic signals but remain for a longer period without the significant increases in generation of SHM in the V region on immunization with NP-CG (48). On the contrary, CD95 mutant mice had decreased numbers of apoptotic B cells in GCs, although they showed the increased frequencies of mutations in V regions in memory B cells (49). We compared the frequencies of apoptotic cells in GCs of the B-Ganp–/– and Cre-flox/+ mice after immunization with TD-Ag. Apoptotic cells in the B-Ganp–/– mice increased, suggesting that the loss of CD40-mediated prevention of B cell apoptosis is at least partly responsible for the decrease of high-affinity B cells in GANP-mutant mice. It is suggested that GANP has a function in the antiapoptotic process in vivo, but the effect is not applied to CD40-mediated prevention of CD95-induced apoptosis in vitro. As one likely antiapoptotic mechanism for CD40-induced prevention of AICD (50), we observed decreased levels of the bcl-2 transcripts in B-Ganp–/– B cells, suggesting that regulation of Bcl-2 expression is partly involved in the regulation of apoptosis. GANP is presumably involved in a unique mechanism for survival of high-affinity BCR+ B cells.
Once GC-B cells with V-region SHM have acquired high-affinity BCRs, they should be positively selected, and further SHM of the V regions might be suppressed to retain this important characteristic. Because expression of enzymes responsible for DNA mutations might continue in GC-B cells, regulation of such enzymes could be required to maintain high-affinity BCRs. An alternative function of GANP might be involved in a regulatory mechanism after generation of high-affinity B cells. We speculate that the primary activity of GANP is presumably associated with regulation of DNA repair that is involved in generation of V-region SHM, but its molecular activity might be also necessary for survival and maintenance of B cells from various stresses that cause DNA injury during proliferation of GC-B cells. The GANP enzyme is thus shown to be a component of the apparatus that diversifies Ab responses in GCs.
Supplementary Material
Acknowledgments
We thank Drs. P. W. Kincade, T. Honjo, N. R. Klinman, and J. D. Capra for helpful comments on this work, Dr. R. C. Rickert for providing CD19-Cre knock-in mice, and S. Ishii and Y. Kumamoto for genotyping of knockout mice. This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (to N.S.) and, in part, by a grant from the Uehara Memorial Foundation (to K.K.).
Abbreviations: Ag, antigen; BCR, B cell antigen receptor; CSR, class switch recombination; NP, nitrophenyl; NP-CG, NP-chicken gamma globulin; PNA, peanut agglutinin; SHM, somatic hypermutation; TD-Ag, T cell-dependent antigen; TNP-KLH, trinitrophenyl-keyhole limpet hemocyanin; V region, variable region; AICD, activation-induced cell death; GC, germinal center; GANP, GC-associated nuclear protein; LPS, lipopolysaccharide; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling.
References
- 1.Tonegawa, S. (1983) Nature 302, 575–581. [DOI] [PubMed] [Google Scholar]
- 2.Alt, F. W., Oltz, E. M., Young, F., Gorman, J., Taccioli, G. & Chen, J. (1992) Immunol. Today 13, 306–314. [DOI] [PubMed] [Google Scholar]
- 3.Schatz, D. G., Oettinger, M. A. & Schlissel, M. S. (1992) Annu. Rev. Immunol. 10, 359–383. [DOI] [PubMed] [Google Scholar]
- 4.Rajewsky, K. (1996) Nature 381, 751–758. [DOI] [PubMed] [Google Scholar]
- 5.Kelsoe, G. (1996) Immunity 4, 107–111. [DOI] [PubMed] [Google Scholar]
- 6.Kosco-Vilbois, M. H., Bonnefoy, J. Y. & Chvatchko, Y. (1997) Immunol. Rev. 156, 127–136. [DOI] [PubMed] [Google Scholar]
- 7.Bergthorsdottir, S., Gallagher, A., Jainandunsing, S., Cockayne, D., Sutton, J., Leanderson, T. & Gray, D. (2001) J. Immunol. 166, 2228–2234. [DOI] [PubMed] [Google Scholar]
- 8.Faili, A., Aoufouchi, S., Gueranger, Q., Zober, C., Leon, A., Bertocci, B., Weill J. C. & Reynaud, C. A. (2002) Nat. Immunol. 3, 815–821. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu, M., Kinoshita, K., Fagarasan, S., Yamada, S., Shinkai, Y. & Honjo, T. (2000) Cell 102, 553–563. [DOI] [PubMed] [Google Scholar]
- 10.Revy, P., Muto, T., Levy, Y., Geissmann, F., Plebani, A., Sanal, O., Catalan, N., Forveille, M., Dufourcq-Labelouse, R., Gennery, A., et al. (2000) Cell 102, 565–575. [DOI] [PubMed] [Google Scholar]
- 11.Honjo, T., Kinoshita, K. & Muramatsu, M. (2002) Annu. Rev. Immunol. 20, 165–196. [DOI] [PubMed] [Google Scholar]
- 12.Di Noia, J. & Neuberger, M. S. (2002) Nature 419, 43–48. [DOI] [PubMed] [Google Scholar]
- 13.Storb, U. & Stavnezer, J. (2002) Curr. Biol. 12, R725–R727. [DOI] [PubMed] [Google Scholar]
- 14.Rogozin, I. B., Pavlov, Y. I., Bebenek, K., Matsuda, T. & Kunkel, T. A. (2001) Nat. Immunol. 2, 530–536. [DOI] [PubMed] [Google Scholar]
- 15.Zeng, X., Winter, D. B., Kasmer, C., Kraemer, K. H., Lehmann, A. R. & Gearhart, P. J. (2001) Nat. Immunol. 2, 537–541. [DOI] [PubMed] [Google Scholar]
- 16.Zan, H., Komori, A., Li, Z., Cerutti, A., Schaffer, A., Flajnik, M. F., Diaz, M. & Casali, P. (2001) Immunity 14, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poltoratsky, V., Woo, C. J., Tippin, B., Martin, A., Goodman, M. F. & Scharff, M. D. (2001) Proc. Natl. Acad. Sci. USA 98, 7976–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faili, A., Aoufouchi, S., Flatter, E., Gueranger, Q., Reynaud, C. A. & Weill, J. C. (2002) Nature 419, 944–947. [DOI] [PubMed] [Google Scholar]
- 19.van Kooten, C. & Banchereau, J. (2000) J. Leukocyte Biol. 67, 2–17. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson, S. E., Han, S., Kelsoe, G. & Thompson, C. B. (1996) J. Immunol. 156, 4576–4581. [PubMed] [Google Scholar]
- 21.Kuehn, R., Rajewsky, K. & Mueller, W. (1991) Science 254, 707–710. [DOI] [PubMed] [Google Scholar]
- 22.Kopf, M., Herren, S., Wiles, M. V., Pepys, M. B. & Kosco-Vilbois, M. H. (1998) J. Exp. Med. 188, 1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay, F. & Browning, J. (2002) Nat. Rev. Immunol. 7, 465–475. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, M., Fu, Y. X., Molina, H. & Chaplin, D. D. (1997) Immunol. Rev. 156, 137–144. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan, I. C. M. (1994) Annu. Rev. Immunol. 12, 117–139. [DOI] [PubMed] [Google Scholar]
- 26.Liu, Y. J. & Arpin, C. (1997) Immunol. Rev. 156, 111–126. [DOI] [PubMed] [Google Scholar]
- 27.Tew, J. G., Wu, J., Qin, D., Helm, S., Burton, G. F. & Szakal, A. K. (1997) Immunol. Rev. 156, 39–52. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara, K., Yoshida, M., Kondo, E., Sakata, A., Watanabe, Y., Abe, E., Kouno, Y., Tomiyasu, S., Fujimura, S., Tokuhisa, T., et al. (2000) Blood 95, 2321–2328. [PubMed] [Google Scholar]
- 29.Abe, E., Kuwahara, K., Yoshida, M., Suzuki, M., Terasaki, H., Matsuo, Y., Takahashi, E. & Sakaguchi, N. (2000) Gene 255, 219–222. [DOI] [PubMed] [Google Scholar]
- 30.Kuwahara, K., Tomiyasu, S., Fujimura, S., Nomura, K., Xing, Y., Nishiyama, N., Ogawa, M., Imajoh-Ohmi, S., Izuta, S. & Sakaguchi, N. (2001) Proc. Natl. Acad. Sci. USA 98, 10279–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishimi, Y. (1997) J. Biol. Chem. 272, 24508–24513. [DOI] [PubMed] [Google Scholar]
- 32.Takei, Y., Swietlik, M., Tanoue, A., Tsujimoto, G., Kouzarides, T. & Laskey, R. (2001) EMBO Rep. 2, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi, T., Tokunaga, T., Furuta, Y., Nada, S., Yoshida, M., Tsukada, T., Saga, Y., Takeda, N., Ikawa, Y. & Aizawa, S. (1993) Anal. Biochem. 214, 70–76. [DOI] [PubMed] [Google Scholar]
- 34.Rickert, R. C., Roes, J. & Rajewsky, K. (1997) Nucleic Acids Res. 25, 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayanthi, S., Deng, X., Bordelon, M., McCoy, M. T. & Cadet, J. L. (2001) FASEB J. 15, 1745–1752. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, J., Inui, S., Yamasaki, K., Kataoka, S., Maeda, K., Nakanishi, K. & Sakaguchi, N. (1995) Immunol. Lett. 45, 195–203. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, N., Nomura, T., Takai, T., Chiba, T., Honjo, T. & Tsubata, T. (1998) Scand. J. Immunol. 47, 541–547. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., Koizumi, T. & Watanabe, T. (1996) J. Exp. Med. 184, 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cumano, A. & Rajewsky, K. (1985) Eur. J. Immunol. 15, 512–520. [DOI] [PubMed] [Google Scholar]
- 40.Mackus, W. J., Lens, S. M., Medema, R. H., Kwakkenbos, M. J., Evers, L. M., Oers, M. H., Lier, R. A. & Eldering, E. (2002) Int. Immunol. 14, 973–982. [DOI] [PubMed] [Google Scholar]
- 41.Kamesaki, H., Zwiebel, J. A., Reed, J. C. & Cossman, J. (1994) J. Immunol. 152, 3294–3305. [PubMed] [Google Scholar]
- 42.Tuscano, J. M., Druey, K. M., Riva, A., Pena, J., Thompson, C. B. & Kehrl, J. H. (1996) Blood 88, 1359–1364. [PubMed] [Google Scholar]
- 43.Diaz, M., Verkoczy, L. K., Flajnik, M. F. & Klinman, N. R. (2001) J. Immunol. 167, 327–335. [DOI] [PubMed] [Google Scholar]
- 44.Jacob, J., Przylepa, J., Miller, C. & Kelsoe, G. (1993) J. Exp. Med. 178, 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, J., Foy, T. M., Laman, J. D., Elliott, E. A., Dunn, J. J., Waldschmidt, T. J., Elsemore, J., Noelle, R. J. & Flavell, R. A. (1994) Immunity 1, 423–431. [DOI] [PubMed] [Google Scholar]
- 46.Kawabe, T., Naka, T., Yoshida, K., Tanaka, T., Fujiwara, H., Suematsu, S., Yoshida, N., Kishimoto, T. & Kikutani, H. (1994) Immunity 1, 167–178. [DOI] [PubMed] [Google Scholar]
- 47.Tarlinton, D. M. & Smith, K. G. (1997) Int. Rev. Immunol. 15, 53–71. [DOI] [PubMed] [Google Scholar]
- 48.Smith, K. G., Weiss, U., Rajewsky, K., Nossal, G. J. & Tarlinton, D. M. Immunity 1, 803–813. [DOI] [PubMed]
- 49.Takahashi, Y., Ohta, H. & Takemori, T. (2001) Immunity 14, 181–192. [DOI] [PubMed] [Google Scholar]
- 50.Defrance, T., Casamayor-Palleja, M. & Krammer, P. H. (2002) Adv. Cancer Res. 86, 195–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.