Abstract
Background:
Intestinal parasitic infections have been described as constituting the greatest single worldwide cause of illness and disease.
Aim:
The objective of this study is to investigate the prevalence of intestinal parasitic infections in relation to sex and age as well as seasons of the year in Benin city, Nigeria.
Materials and Methods:
The stool samples were processed using saline and iodine mounts and examined microscopically for ova and cysts of parasites.
Results:
The overall prevalence of intestinal parasitic infections was 3.9% while gender and season had no correlation with the prevalence of intestinal parasites (P = 0.548, P = 0.696). There was a significant relationship between intestinal parasitic infection and age (P < 0.033). Ascaris lumbricoides was the most prevalent (51.4%) while Entamoeba histolytica had the least prevalence (5.4%).
Conclusion:
The results of this study concluded that 36 patients were infected with various intestinal parasites and that age significantly affected the prevalence of parasitic infections. Effective treatment of infected patients and improved sanitary habits is advocated.
Keywords: Intestinal parasites, prevalence, Benin city, Nigeria
Introduction
The parasitic infections are endemic worldwide and have been described as constituting the greatest single worldwide cause of illness and disease[1]. These infections are associated with poor sanitary habits, lack of access to safe water and improper hygiene, thereby occurring wherever there is poverty[2]. The degree of each factor and the prevalence of infections vary from one region to another[3].
In spite of the prevalence of many risk factors in both rural and urban life predisposing people to parasitic agents, unfortunately, there is limited updated data about current situation of human parasitic infections in Benin City and environs.
Against this background, the present study was undertaken to investigate the prevalence of intestinal parasitic infections, its relationship with sex and age as well as seasons of the year in Benin City.
Materials and Methods
Study Area
The study was carried out in the University of Benin Teaching Hospital, Benin City, Nigeria. The hospital is a tertiary hospital and has a referral status. It is located in the South-South geographical zone of Nigeria. It serves about 6-10 states in Nigeria.
Study Population
The study was carried out within the period of March, 2009 to February, 2010 at the University of Benin Teaching Hospital. A total of 925 patients attending clinics and on admission were recruited for this study. They consisted of 430 (46.49%) males and 495 (53.51%) of females with age ranging from 0–85 years. Patients on antiparasitic agents as well as those that were HIV seropositive were excluded from this study.
Collection and Processing of Specimens
Stool samples were collected from each patient with signs and symptoms of diarrhea into a clean wide-mouthed container. The samples were examined microscopically for ova and cysts of parasites using saline and iodine mounts on grease-free slides.
Data Analysis
The frequency data were compared using the Chi square (X2) test. Odd ratios were calculated for gender and patient's status. The software INSTAT® (GraphPad, USA) was used in all statistical analyses.
Results
Of the 925 patients examined, 3.9% were infected with intestinal parasites. The prevalence of infection was similar between male and female patients (P = 0.548) and between out-patients and in-patients (P = 0.569) (Table 1). The prevalence of infection was higher in age groups ≤ 10 years (7.29%) and ≥ 51 years (7.06%). However, the prevalence of intestinal parasitic infection was age dependent (P< 0.033) (Table 1).
Table 1.
Prevalence of intestinal parasites in relation to gender, patients and age
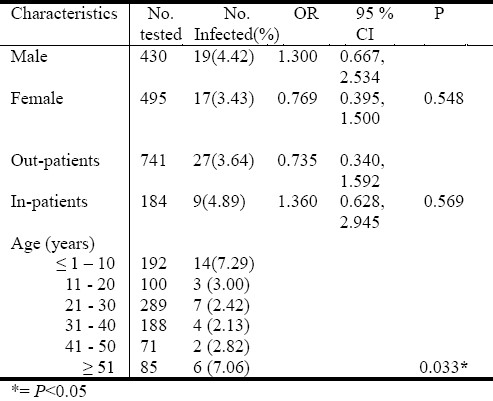
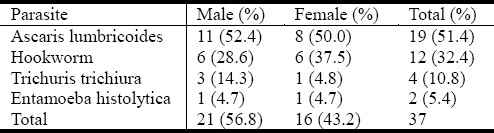
Figure 1 describes seasonal related prevalence of infection among these patients and prevalence was higher in December (dry season) while it was zero in May (rainy season). The prevalence of infection was not significantly different (P = 0.69) in relation to season Generally, the prevalence of infection was higher among male patients (56.8%) than female subjects (43.2%) (Table 2).
Fig. 1.
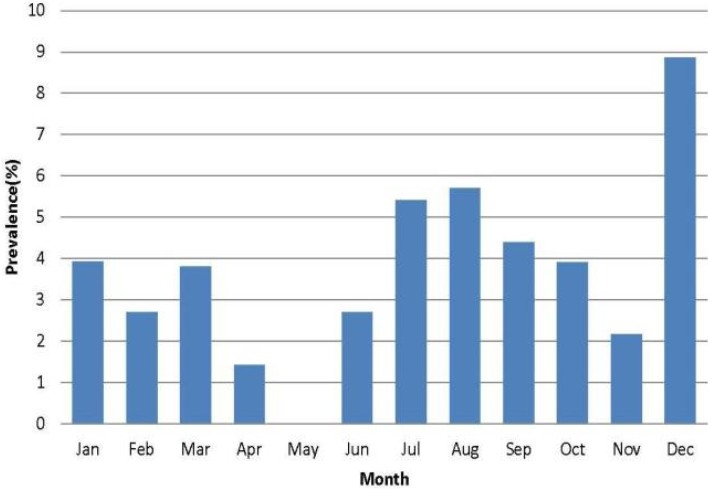
Distribution of intestinal parasites in relation to months of the year
Table 2.
Prevalence of parasitic infections in relation to sex
Discussion
Intestinal parasitic infections of humans are important threats to healthy living in developing countries[4]. The environment and the socio-cultural habits of the people could be attributable for the high prevalence of intestinal parasitic infections in the developing countries[5]. A total of 36 (3.9%) out of the 925 patients had intestinal parasitic infections in this study. This prevalence is lower than previous reports that observed 52.8%[1], 34.6 %[6], 30.6%[5], 25%[4], 6.2%[7], 10.2%[8], 6.9%[9]. The difference could be due to the type of patients used. In this study, in-patients and out-patients of a tertiary hospital with signs and symptoms of diarrhea were used. Other studies used either subjects from rural community[4–6] or those from the general population[1,7–9]. It is also important to note that our patients may have been treated at the primary and secondary healthcare levels before been referred to our tertiary hospital. This may explain the lower prevalence observed in this study.
Gender was not a risk factor for acquiring intestinal parasitic infection (P = 0.548). This finding is consistent with previous reports[1,4,7]. There was no significant difference in the prevalence of intestinal parasitic infections among out-patients and in-patients (P = 0.569). The reason for this is unclear, as all intestinal protozoa and some helminthes can be acquired nosocomially[10].
The finding that age significantly affected the prevalence of intestinal parasitic infection has been previously reported[1,6]. However, the observation in this study differs from other study. While the prevalence in this study drops from < 10 years to 41 to 50 years age group, before increasing in ≥ 51 years, other studies report increase in prevalence with increasing age and peaks between the age group of 16 to 30 years before dropping[6,9].
More infections were observed in December than other months. However, the prevalence of intestinal parasitic infections was not significantly affected by both months of the year and season.
The most prevalent intestinal parasite in this study was Ascaris lumbricoides (51.4%), followed by hookworm (32.4%), Trichuris trichiura (10.8%) while the least was Entamoeba histolytica (5.4%). This finding is consistent with previous reports[6,11–15]. Ascaris lumbricoides was the most prevalent parasite among the male (52.4%) and female (50%) patients while hookworm infection was observed more in female (37.5%). More males (14.3%) were infected with Trichuris trichiura while similar prevalence of Entamoeba histolytica (4.7%) was observed in both sexes. Effective treatment of infected patients and improved sanitary habits is advocated.
Conclusion
This study concluded that 36 patients were infected with various intestinal parasites and that age significantly affected the prevalence of parasitic infections. Effective treatment of infected patients and improved sanitary habits is advocated.
Acknowledgments
We acknowledge with thanks the Management of University of Benin Teaching Hospital for permission to carry out this study.
References
- 1.Mehraj V, Hatcher J, Akhtar S, Rafique G, Beg MA. Prevalence and factors associated with intestinal parasitic infection among children in an urban slum of Karachi. PLoS One. 2008;3(11):e3680. doi: 10.1371/journal.pone.0003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steketee RW. Pregnancy, nutrition and parasitic diseases. J Nutr. 2003;133:1661S–1667S. doi: 10.1093/jn/133.5.1661S. [DOI] [PubMed] [Google Scholar]
- 3.Ogbe MG, Odudu LA. Gastro-intestinal helminthiasis in Epe L.G.A., Lagos State, Nigeria. Nigerian Journal Parasitol. 1990;9-11:95–106. [Google Scholar]
- 4.Kia EB, Hossein M, Nilforoushan MR, Meamar AR, Rezaeian M. Study of intestinal protozoan parasites in rural inhabitants of Mazandaran Province, Northern Iran. Iranian J Parasitol. 2008;3:22–25. [Google Scholar]
- 5.Mbanugo JI, Onyebuchi CJ. Prevalence of intestinal parasites in Ezinifite community, Aguata Local Government Area of Anambra State. Nigerian J. Parasitol. 2002;23:27–34. [Google Scholar]
- 6.Nduka FO, Nwaugo VO, Nwachukwu NC. Human intestinal parasite infections in Ishiagu, a leading mining Area of Abia State. Animal Research International. 2006;3(3):505–507. [Google Scholar]
- 7.Akinbo FO, Okaka CE, Machado RLD, Omoregie R, Onunu AN. Cryptospondiosis among HIV-infected patients with diarrhea in Edo State, Midwest Nigeria. Malaysian J. Microbiol. 2010;6(1):99–101. [Google Scholar]
- 8.Abu-Madi MA, Behnke JM, Doiphode SH. Changing trends in intestinal parasitic infections among long-term-residents and settled immigrants in Qatar. Parasites Vectors. 2010;3:98. doi: 10.1186/1756-3305-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamaiah I, Rohela M. Prevalence of intestinal parasites among members of the public in Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Pub Health. 2005;36:68–71. [PubMed] [Google Scholar]
- 10.Lettau LA. Nosomial transmission and infection control aspects of parasitic and ectoparastic diseases: Part I. Introduction/Enteric parasites. Infect Control Hosp Epidemiol. 1991;12(1):59–65. doi: 10.1086/646239. [DOI] [PubMed] [Google Scholar]
- 11.Wiwanitkit V. Intestinal parasitic infections in Thai HIV-infected patients with different immunity. Biomed Centr Gastroenterol. 2001;1:1–3. doi: 10.1186/1471-230X-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okodua M, Adeyeba OA, Talfeng YM, Okpala HO. Age and sex distribution of intestinal parasitic infections among HIV infected subjects in Abeokuta, Nigeria. Online J Health Allied Sci. 2003;4:3. [Google Scholar]
- 13.Mbanugo JI, Abazie OC. A comparative study of intestinal parasite infections of pregnant and non-pregnant women in Nkpor, Anambra State. Nigerian J Parasitol. 2002;23:19–26. [Google Scholar]
- 14.Oguntibeju OO. Prevalence of intestinal parasites in HIV-positive/AIDS patients. Malaysian J Med Sci. 2006;13:68–73. [PMC free article] [PubMed] [Google Scholar]
- 15.Zelalem MT, Abebe G, Mulu A. Opportunistic and other parasitic infections in AIDS patients, HIV seropositive healthy carriers and HIV sero-negative individual in southwest, Ethiopia. East Afr J Public Health. 2008;5(3):169–173. [PubMed] [Google Scholar]


