Abstract
Object
Supratentorial cortical ependymomas (CE) are rare, with 7 cases reported. The lesions, typically occurring in the superficial cortex in young adults and associated with a history of seizures, are not fully characterized. Furthermore, their relationship with the recently described angiocentric glioma (AG) is still being debated. This study was undertaken to summarize the authors’ experience with CEs.
Methods
Between 1997 and 2009, 202 cases of ependymoma were surgically treated at the Mayo Clinic, 49 of which were supratentorial. Among these, 9 CE cases were retrospectively identified. Clinical, imaging, and pathological features of each case were reviewed.
Results
Tumors arose from the frontal (5 cases), parietal (3), and occipital (1) lobes. No tumor occurred in the temporal lobe, despite its reported association with seizures. The mean age at presentation was 27 ± 19 years (± SD) and age at resection was 36 ± 16 years. The mean size of the lesion was 16 ± 14 cm3. Seizures were the presenting symptom in 78%. Cross-sectional imaging in 8 cases was characterized by a heterogeneous mass with multiple cystlike areas and enhancement of the soft-tissue component. Gross-total resection was achieved in 8 of 9 tumors. Pathologically, 6 were low-grade (WHO Grade II) and 3 were anaplastic (WHO Grade III) ependymomas. All tumors exhibited the focal presence of perivascular pseudorosettes, but only 1 (11 %) exhibited the focal presence of a true rosette. A bipolar spindle cell component resembling AG was present in 3 (33%) and “Schwannian-like” nodules in 2 (22%). Subpial aggregation and peripheral infiltration were present in 4 cases (44%}. With a mean postsurgery follow-up of 62 ± 38 months, only 2 lesions recurred locally after imaging-confirmed gross-total resection, both being Grade III. In 5 (71 %) of 7 patients presenting with seizures an Engel Class I outcome was achieved.
Conclusions
Cortical ependymomas represent a rare type of ependymoma occurring superficially in the cortex. Morphologically, these tumors are protean, varying from classic to epithelioid, clear cell, and tanycytic. Some also exhibited features typical of AG. Most tumors were low grade and cured with resection. Anaplastic tumors occur and may recur locally despite provision of radiation therapy. Cortical ependymomas frequently, but not always, present with seizures, but despite their high association with epilepsy, none occurred in the temporal lobe in any of the authors’ 9 patients. Overall, CEs appear to have a relatively favorable prognosis compared with other supratentorial ependymomas.
Keywords: ependymoma, epilepsy, cortical ependymoma, outcome
Ependymomas represent 2%–9% of all neuroepithelial tumors and most frequently affect children and young adults.17 Commonly involving the cervicothoracic segment of the spinal central canal and fourth ventricle, they can occur in any site of the craniospinal axis, even outside the ventricular system. The current WHO classification of CNS tumors divides ependymomas into low-grade (Grade II) and high-grade or anaplastic (Grade III) lesions, reserving Grade I for myxopapillary ependymoma, a variant of the filum that has an indolent biological behavior.15 Tumor location, patient age at diagnosis, and extent of resection are the most important prognostic factors. 18,19,23 It has been suggested that ependymal tumors are perhaps less aggressive the further they occur primarily from the fourth ventricle.18
Cortical ependymomas—supratentorial ependymomas that selectively involve cerebral cortex—are rare. Only 7 cases have reported to date.14,16,22,24,32 In all published cases, the patients have presented with seizures, have undergone complete surgical removal, and tumors have not recurred.24 Recently (2007), the AG—a seizure-associated tumor characterized by an angiocentric pattern of growth, monomorphous bipolar cells, and features of ependymal differentiation—has been added as a new entity to the WHO classification. Angiocentric gliomas appear to encompass cases previously described as monomorphous angiocentric gliomas and angiocentric neuroepithelial tumors.2,12 Debate is ongoing as to whether AGs and CEs represent distinct entities and if CEs truly exist or rather represent examples of misdiagnosed AGs or vice versa.12–14 In a recent case series, Lehman13 suggests that AGs and CEs may both represent entities within a spectrum of “clinically low grade” tumors with ependymal differentiation. Currently there appears to be only 7 published cases of CEs (accounting for 2 papers by Lehman with 1 patient being reported on in both articles) and approximately 26 cases of AGs.1,7,13,16,24,27,28,32 This study reporting on 9 patients with CEs was undertaken to further characterize and understand the lesion and its relationship to the AG.
Methods
Following approval by the Mayo Clinic internal review board, we searched the surgical, pathology, and clinical patient database at the Mayo Clinic in Rochester, Minnesota, for ependymomas diagnosed between January 1997 and January 2009, We identified 202 cases of ependymoma surgically treated at our institution, 49 (24%) being supratentorial. Nine tumors (4%) were located superficially in the cortex, All cases were reviewed pathologically by 2 neuropathologists (C.G, and P.C.B.) and the diagnosis of ependymoma was confirmed. We then retrospectively reviewed in detail the presenting symptoms, radiographic features, extent of resection, and outcomes in this cohort of patients. Seizure outcome was assessed at last follow-up based on the Engel classification system.6 Engel classes were designated as Class I, completely seizure free since surgery; Class II, rare disabling and non-disabling seizures occurring within 2 years of follow-up as well as nocturnal seizures; Class III, worthwhile seizure reduction according to patient self-reporting; and Class IV, no appreciable change or seizures worse.
Mean data are presented ± SD.
Results
Patient Characteristics
Four patients were male and 5 were female. The mean age at presentation was 27 ± 19 years and that at resection was 36 ± 16 years. Four tumors occurred in the left hemisphere and 5 in the right. The tumors were located in the following lobes: frontal (5 cases), parietal (3 cases), and occipital (l case), None of occurred in the temporal lobe. The mean tumor volume at presentation was 16.3 ± 14.4 cm3. Table 1 summarizes this data combined with data from previously reported cases.
TABLE 1.
Summary of present CE cases and CE cases reported in the literature*
| Case No. | Sex | WHO Grade | Lobar Involvement | Age (yrs) at Resection† | Type of Resection | Recurrence | Presentation (yrs w/ seizures) | Radiation Therapy (cGy)‡ | Follow-Up (mos) | Disease Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | II | lt parietal | 32 | GTR | no | incidental | NA | 59 | NED |
| 2 | F | II | lt frontal, insular | 43 | STR | stable | seizures (25) | 5580 | 60 | AWD |
| 3 | M | III | rt parietal | 12 | GTR | yes | seizures (2) | 5520 | 101 | NED |
| 4 | F | II | rt frontal | 40 | GTR | no | seizures (23) | NA | 131 | NED |
| 5 | M | III | rt frontal | 25 | GTR | no | seizures (2) | 5940 | 80 | NED |
| 6 | M | II | rt occipital | 26 | GTR | no | seizures (8) | NA | 6 | NED |
| 7 | F | II | lt parietal | 59 | GTR | no | incidental | NA | 31 | NED |
| 8 | M | III | rt frontal | 59 | GTR | yes | seizures (2) | 5580 | 47 | AWD |
| 9 | F | II | lt frontal | 25 | GTR | no | seizures (9) | NA | 39 | NED |
| 10§ | F | II | lt parietal | 63 | GTR | no | seizures | 5600 | 14 | NED |
| 11¶ | M | II | rt frontal | 10 | GTR | no | seizures | NA | 84 | NED |
| 12¶ | F | III | rt frontal | 1 | GTR | no | seizures | NA | 48 | NED |
| 13** | M | II | lt frontal | 52 | GTR | no | seizures | 5500 | 130 | NED |
| 14** | M | II | lt temporal | 34 | GTR | no | seizures | NA | 100 | NED |
| 15** | F | II | rt parietal | 7 | GTR | no | seizures | NA | 48 | NED |
| 16†† | M | II | lt frontal | 15 | STR | stable | seizures | NA | 20 | AWD |
AWD = alive with disease; NA = not applicable; NED = no evidence of disease.
The mean age of the 16 patients is 31 years.
No patient received chemotherapy.
Saito et al., 1999.
Lehman et al., 2003.
Roncaroli et al., 2005.
Yadav et al., 2009.
Presenting Symptoms
Seven patients presented with medically intractable epilepsy. In 2 patients the tumor was found incidentally during workup for headaches and was not thought to account for the patient's symptoms.
Radiological Features
Radiological data are summarized in Table 2. Pre-operative MR imaging studies were available for review in all cases. All tumors, by definition, were located in the cerebral cortex and exhibited a mixed heterogeneous solid cystlike appearance with hypointensity on T1-weighted sequences and heterogeneous hyperintensity on T2-weighted sequences (Fig. 1.2A) compared with normal white matter. Imaging revealed a multicystic appearance in 5 cases, a predominantly cystlike appearance in 2, and a solid appearance in only 1. Two patients underwent CT scanning: one mass was mildly hyperattenuated with scattered calcifications whereas the other mass was hypoattenuated without calcifications. Both T1- and T2-weighted MR imaging scattered hypointense areas in one mass, suggesting calcification or hemorrhage, but no CT study was available for correlation. In one case there was evidence of exophytic extension from the underlying cortex (Fig. 1.1). One tumor had a moderate amount of associated vasogenic edema (Fig. 1.3), whereas another had a minimal amount of surrounding T2 hyperintensity, likely reflective of either gliosis or edema. Seven patients underwent Gd-enhanced imaging, and all but 1 tumor exhibited at least some enhancement with the soft-tissue components often enhancing intensely while the cystlike areas did not enhance. The combination of these features produced a “popcorn” appearance on the postcontrast images in 2 cases (Fig. 1.2B and C). Progression in tumor size was noted in 3 of the masses.
TABLE 2.
Radiographic features of CEs
| MR Imaging Sequence |
||||||
|---|---|---|---|---|---|---|
| Case No. | T1-Weighted | T2-Weighted | T1-Weighted & Gd | Size Progression | Tumor Volume (cm3) | Cystic Nature |
| 1 | isointense | solid isointense, cystic hyperintense | enhancing | none | 4.3 | multicystic |
| 2 | isointense | solid isointense, cystic hyperintense | enhancing | yes | 63.4 | multicystic |
| 3 | isointense | solid isointense, cystic hyperintense | enhancing | none | 16.0 | multicystic |
| 4 | hypointense | solid isointense, cystic hyperintense | none given | none | 1.2 | single cyst |
| 5 | isointense | solid isointense, cystic hyperintense | enhancing | none | 7.8 | multicystic |
| 6 | isointense | solid isointense, cystic hyperintense | enhancing | none | 2.9 | single cyst |
| 7 | isointense | solid isointense | enhancing | yes | 12.9 | none |
| 8 | isointense | hyperintense | enhancing | yes | 34.0 | none |
| 9 | isointense | solid isointense, cystic hyperintense | enhancing | none | 4.3 | small cysts, mostly solid |
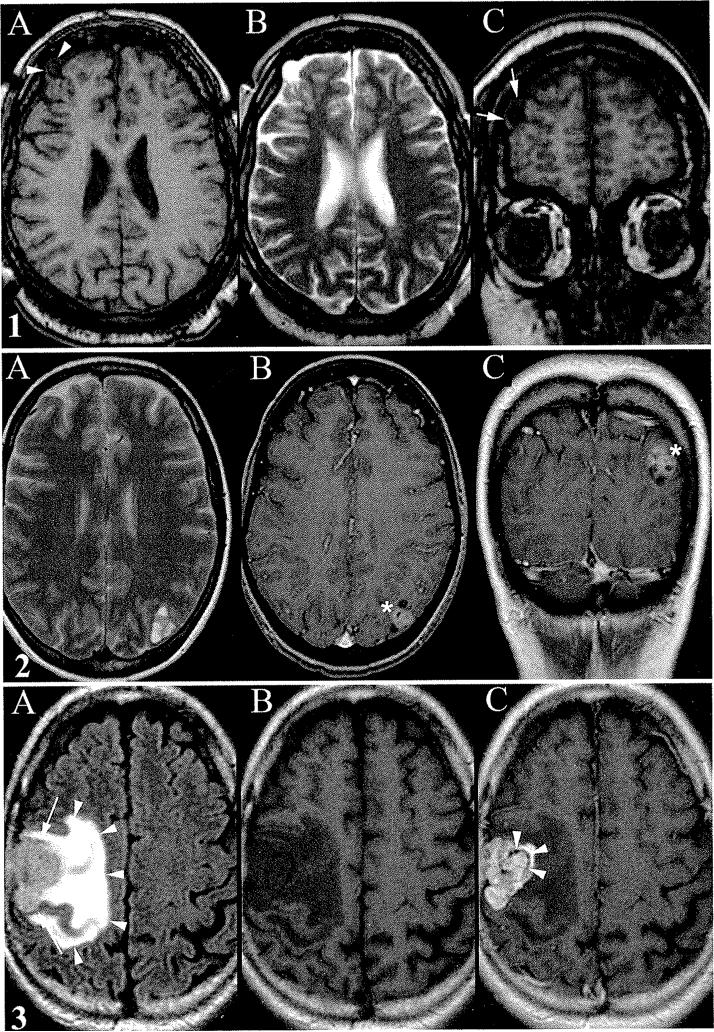
Fig. 1.
1.1. Case 5. A-C: Small exophytic CE. Noncontrast axial T1-weighted image showing small focal exophytic cystlike mass extending from cortical margin of right frontal lobe (arrowheads, A), axial T2-weighted image revealing hyperintensity of the mass similar to fluid signal intensity (B), and noncontrast coronal T1-weighted image demonstrating a small rim of cortex around the margin of the mass (arrows, C). 1.2. Case 7. A-C: Left parietal CE. Axial T2-weighted image revealing a bubbly appearance reminiscent of dysembryoplastic neuroepithelial tumor (multicystic) (A), postcontrast axial T1-weighted image demonstrating enhancement (asterisk, B), and postcontrast coronal T1-weighted image demonstrating popcorn enhancement (asterisk, C). 1.3. Case 3. A-C: Cortical ependymoma with surrounding edema. Noncontrast axial T1-weighted image showing hypointense mass (arrows) and surrounding edema (arrowheads, A), axial FLAIR image highlights surrounding vasogenic edema (B), and postcontrast axial T1-weighted image showing intense enhancement of the mass with scattered curvilinear nonenhancing areas (arrowheads) along the medial margin, which may represent calcification (C).
Pathological Features
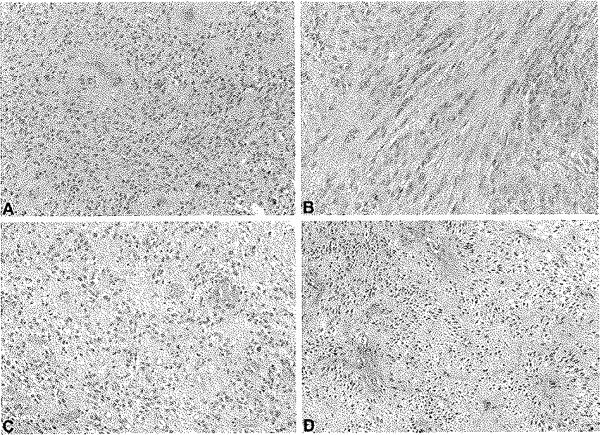
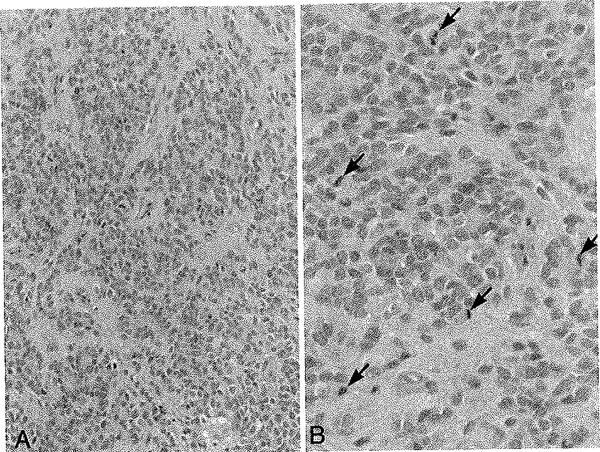
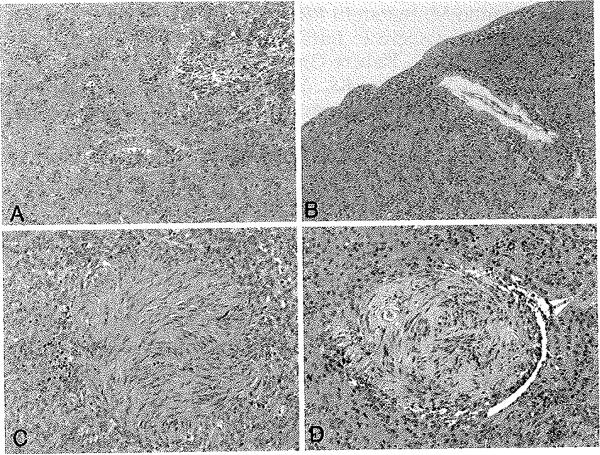
Morphological features of the tumors are illustrated in Figs. 2–5 and summarized in Table 3. Histologically, the tumors had a protean appearance, Although classic ependymal features, with at least focal perivascular pseudorosette formation, were seen in all cases (Fig. 2A), only 1 (11%) showed true rosette formation (Fig. 3F). Unusual morphological features included spindly bipolar elements resembling tanycytes (Fig. 2B), as well as epithelioid (Fig. 2C) and clear cell (Fig. 2D) features. The tanycytic and clear cell features were only focal and we did not consider the lesions to be either tanycytic or clear cell ependymomas. A bipolar spindle cell component resembling AG was present in 3 (33%) and “Schwannian-like” nodules were present in 2 (22%) (Fig. 4). Subpial aggregation and peripheral infiltration were noted in 4 (44%) of the cases (Fig. 4). According to the WHO classification system, 6 tumors were low grade (Grade II), and 3 were anaplastic (Grade III), largely based on more mitoses (> 6 × 10 hpf) (Fig. 5).
Fig. 2.
Photomicrographs illustrating the spectrum of CE morphologies, varying from classic with pseudorosette formation as seen in Case 1 (A), to tanycytic as seen in Case 2 (B), and to epithelioid (C) and clear cell (D) in Case 7. H & E, original magnification × 20.
Fig. 5.
Case 3. Anaplastic ependymoma. Left: Low-power H & E-stained photomicrograph demonstrating classic features of ependymoma, Original magnification × 20. Right: High-power H & E-stained photomicrograph showing high mitotic activity (mitotic figures indicated by arrows) supporting the diagnosis of anaplastic ependymoma (WHO Grade III). Original magnification × 40.
TABLE 3.
Pathological features of CEs
| Case No. | WHO Grade | Classic Ependymal Features |
Schwannian-like Nodules | Peripheral Infiltration | ||||
|---|---|---|---|---|---|---|---|---|
| Pseudorosettes | True Rosettes | Additional Feature | Spindle Bipolar Cells | Subpial Aggregates | ||||
| 1 | II | present | absent | absent | absent | present | absent | |
| 2 | II | focal | absent | tanycytic | absent | absent | absent | absent |
| 3 | III | present | absent | absent | absent | absent | absent | |
| 4 | II | present | absent | present | absent | present | present | |
| 5 | III | present | absent | absent | absent | absent | absent | |
| 6 | II | present | absent | present | present | present | present | |
| 7 | II | present | absent | epithelioid clear cell | absent | absent | absent | absent |
| 8 | III | present | absent | absent | absent | absent | absent | |
| 9 | II | present | present | present | present | present | present | |
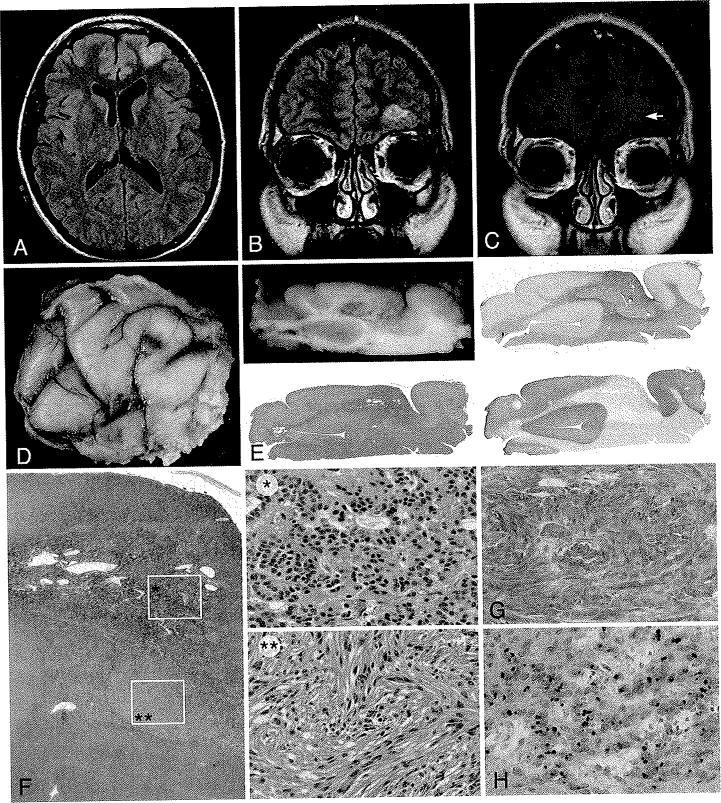
Fig. 3.
Case 9. Left-sided frontal CE. A: Axial FLAIR image showing slight gyral enlargement and flair signal change. B: Coronal FLAIR image demonstrating a hyperattenuated supraorbital abnormality. C: Postcontrast axial T1-weighted image revealing wispy enhancement (arrow) of the cortical margin of the tumor. D: Gross pathological postfixation specimen exhibiting a whitish expanded cortical area overlying the lesion associated with this tumor. E: Gross and low-power microscopic appearance of this largely cortical lesion, relatively demarcated and stained with the glial marker GFAP (upper right) but not with neuronal marker synaptophysin (lower right). F: Photomicrographs showing a solid cellular component with formation of true rosettes (single asterisk) as well as a fasciculated spindle cell component (double asterisks). H & E, original magnification × 10 (single asterisk) and × 20 (double asterisks). G: The glial marker GFAP is strongly expressed in tumor cells. Original magnification × 20. H: Epithelial membrane antigen stain highlights the presence of a typical dotlike pattern of stains, typically corresponding to cytoplasmic microlumina. Original magnification × 20.
Fig. 4.
Case 6. Photomicrographs of exophytic CE demonstrating peripheral infiltration with perivascular aggregates (A) and subpial extension (B). Two photomicrographs demonstrating examples of spindle cell “Schwannian-like” nodules (C and D). H & E, original magnification × 20.
Treatment
In all patients tumor resection was performed. In 8 of 9 cases, GTR was successfully achieved; STR was achieved in 1 patient (Table 1). After GTR, there were 2 recurrences, each in patients with WHO Grade III anaplastic tumors and each was local. These 2 patients underwent reresection, which only demonstrated local recurrence without evidence of leptomeningeal dissemination. All 3 patients with Grade III tumors and 1 patient with a subtotally resected Grade II CE received postoperative intensity-modulated radiotherapy. The mean radiation dose was 5655 cGy in 31–33 fractions. The remaining 5 patients with Grade II tumors did not undergo postoperative radiotherapy. No patients received postoperative chemotherapy.
Follow-Up
Two patients had very mild postoperative deficits that improved. After resection in one patient, a mild postoperative aphasia developed in the speech-sensitive left frontal operculum and insula, and this resolved. The second patient had pervasive mild postoperative stereoagnosis following GTR in the right parietal secondary sensory cortex. There have been no deaths to date in these patients. Seven patients are alive with no evidence of disease, and in 2 patients the disease is stable. Five of the 7 patients who presented with seizures are seizure free (Engel Class I) after surgery (71% seizure-free rate). The mean age at follow-up was 41 ± 5 years and the mean follow-up duration since surgery was 5 ± 3 years (more precisely, 62 ± 38 months). The mean follow-up duration since presentation was 14 ± 12 years (or 171 ± 143 months).
Discussion
Here we have reported our experience treating 9 patients with CEs, 7 of whom were diagnosed with epilepsy-associated lesions. Unlike typical supratentorial ependymomas, CEs follow what appears to be a relatively benign clinical course and are most commonly low grade. We did have 3 anaplastic CEs in our series. Pathologically, these tumors had shown an ample spectrum of morphologies and, in some cases, were quite difficult to distinguish from ordinary diffusely infiltrative gliomas, which are certainly more frequent at these sites. Although classic ependymal features, with at least focal perivascular pseudorosette formation, were seen in all cases, true rosette formation was rare. Less typical morphological features such as tanycytic, epithelioid, and clear cell features were seen in some tumors. Three of the lesions showed an additional bipolar spindle cell component resembling that of AGs and “Schwannian-like” nodules, features that are typical of AGs, similar to the findings of Lehman.12 The presence of common features between CE and AG is well known and debated in the neuropathology literature.16 Moreover, in the largest series of its kind (8 AGs), Wang et al.31 stressed the striking pathological similarity with ependymomas as well, but it should be emphasized that the radiographic appearance (see below) and age mean/distribution are not consistent with our series.27 In his study of 6 cases, Lehman12 defined criteria to separate between the CE and the AG. Similarly to Lehman, we propose that the CE is a distinct clinical entity from an AG along a spectrum of cortically based ependymal tumors.13
The pathogenesis of cortical ependymoma remains elusive. Similar to other ectopic ependymomas, they occur in sites where a normal ependymal layer is absent, which suggests that they may originate from a cell type other than terminally differentiated ependyma.24 Hegyi et al.9 reported on an ectopic retinal ependymoma that was proposed to arise from Müller cells; this proposition may support the notion that glial cells with progenitor cell properties are the source of these ependymomas rather than terminally differentiated ependyma. Extraaxial ependymomas are also believed to derive from progenitor cells, and their occurrence in teratomas seems to support this view.3,8,21 A progenitor cell hypothesis could therefore also be suggested to explain CEs, but whether such progenitor cells result from a migration defect of subependymal neural progenitors remains to be established.
Radiographically, the combination of the multicystic appearance, peripheral cortical location, minimal associated vasogenic edema, and occasional exophytic extension of the lower-grade tumors (WHO Grade II) in our series bears a striking resemblance to that noted in the dysembryoplastic neuroepithelial tumor.10,11 Ganglioglioma, oligodendroglial tumors, and low-grade astrocytomas may also have similar imaging manifestations, and thus the differential for these lesions is typically broad preoperatively.4 These imaging features have been observed before, and again were present in our case series.14 These features are very unusual for typical ependymomas. Fortunately, pathologically, dysembryoplastic neuroepithelial tumors and CEs look extremely dissimilar. Finally, AGs appear to have distinct imaging characteristics compared with CEs in that they are usually solid, lack enhancement, and, finally, have what is believed to be a pathognomonic T2 signal “tail” connecting with the ventricle, which was seen in none of our patients.1,2,7,27,31 Therefore, it appears that radiographically, AGs and CEs are dissimilar.
Our cases represent 56% (9 of 16) of those in the extant literature (Table 1); the demographic data are comparable with those previously reported12-14,16,22,24,25,32 The largest series of ependymomas is based on the SEER (Surveillance, Epidemiology, and End Results) database, but this database tracks only death associated with tumors, and therefore it is unlikely to include further cases of CEs because no deaths due to this tumor have been documented.19 When comparing our series to the largest known series of supratentorial ependymomas by Metellus et a1.,20 it appears that our patients are younger by 8 years on average.20 Conversely, Metellus et al. reported that parenchymal tumors typically are higher grade than periventricular tumors, but this finding is not reflected by the present series of CEs. Furthermore, it is interesting to note that with a mean follow-up period of over 5 years, none of the 9 patients has died. Metellus et al. reported that the 5-year survival for these tumors was 57.1% ± 8.7%.20 Therefore, cortical ependymomas appear to have a relatively favorable prognosis compared with other ependymomas.24 However, it is unclear if their indolent behavior depends on an earlier presentation due cortical involvement, their accessibility in addition to resectability, or their intrinsic pathobiology.29
One curious finding is the distinct low frequency of CEs in the temporal lobe (Table 1). None of the 9 lesions in the present study, and only 1 of 7 in previously reported cases, was located in the temporal lobe.12-14,16,22,24,32 The 1 published case of temporal lobe CE, which did not have the typical imaging characteristics we describe in this study, was reviewed by 2 of our authors (F.R. and C.G.) and was confirmed to have morphological features consistent with others in this series.13,24 Further lending to the curious absence of these tumors from the temporal lobe is the lesion's propensity to present with epilepsy. Extratemporallobe epilepsy represents only 30% of cases in lesional epilepsy series. Cortical ependymomas’ rarity in the temporal lobe and high association with epilepsy warrant vigilance when determining the location of this tumor's origin as more cases are reported.5,30
In none of the 7 previously reported cases was the lesion reported to have recurred (Table 1), despite a similar follow-up duration to ours of 59 months.12-14,16,22,24,32 Our standard treatment approach for patients with ependymomas is to perform involved-field radiotherapy for anaplastic ependymoma (Grade III) regardless of resection status, whereas for low-grade ependymoma (Grade II) we perform radiotherapy only if GTR has not been achieved.26 In the present series, despite achieving GTR in 8 cases, unfortunately there were 2 recurrences, both in patients presenting with Grade III tumors. Only 1 case of Grade III CE has been previously reported.12 This brings to light an unreported phenomenon: there are lesions confined to the cortex that are Grade III tumors (3 in our series). Furthermore, it is possible that these tumors will recur, as was the case in 2 of our patients.12-14,16,22,24,32 Finally, it has been argued that radiotherapy is not needed for these tumors, as they are typically peripheral and may be re-resected on recurrence, However, in Grade III tumors, we believe the standard of therapy should be that postoperative radiotherapy is conducted, even in cases of GTR, because there exists up to a 30%–40% chance of leptomeningeal dissemination at recurrence.24,26 Despite radiotherapy in our 3 patients, 2 suffered recurrence.
Conclusions
Cortical ependymomas are rare ependymomas occurring within the cortical ribbon and morphologically exhibit a broad spectrum of features, The cases of CE raise the question of whether “cortical ependymoma” is a topographic description—that is, any ependymoma in the cortex, regardless of histological features—or a term implying a set of specific histological features. On one hand, it is the latter because some CEs are classic ependymomas whereas others are not. On the other hand, there may not be such a dichotomous division into classic and nonclassic ependymal tumors but rather a spectrum, with classic ependymomas at one end and a “new” WHO entity, “angiocentric glioma,” at the other. Most CEs are low grade and are cured with resection, Anaplastic (WHO Grade III) tumors occur and may recur locally despite radiation therapy.12-14,16,22,24,32 Cortical ependymomas frequently, but not always, present with seizures, and despite their high association with epilepsy, are distinctly uncommon in the temporal lobe.12-14,16,22,24,32 Overall, CEs appear to have a relatively favorable prognosis compared with other supratentorial ependymomas.
Abbreviations used in this paper
- AG
angiocentric glioma
- CE
cortical ependymoma
- GTR
gross-total resection
- STR
subtotal resection
Footnotes
This article contains some figures that are displayed in color online but in black and white in the print edition.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Van Gompel, Koeller, Meyer, Marsh, Burger, Worrell, Giannini. Acquisition of data: Van Gompel, Koeller, Giannini. Analysis and interpretation of data: Van Gompel, Koeller, Burger, Roncaroli, Giannini. Drafting the article: all authors. Critically revising the article: all authors. Reviewed final version of the manuscript and approved it for submission: all authors. Statistical analysis: Van Gompel. Administrative/technical/material support: Giannini. Study supervision: Meyer, Marsh, Giannini.
References
- 1.Arsene D, Ardeleanu C, Ogrezeanu I, Danaila L. Angiocentric glioma: presentation of two cases with dissimilar histology. Clin Neuropathol. 2008;27:391–395. doi: 10.5414/npp27391. [DOI] [PubMed] [Google Scholar]
- 2.Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO Classification of Tumours of the Central Nervous System: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 2007;17:319–324. doi: 10.1111/j.1750-3639.2007.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busse C, Nazeer T, Kanwar VS, Wolden S, LaQuag1ia MP, Rosenblum M. Sacrococcygeal immature teratoma with malignant ependymoma component. Pediatr Blood Cancer. 2009;53:680–681. doi: 10.1002/pbc.22079. [DOI] [PubMed] [Google Scholar]
- 4.Castillo M, Davis PC, Takei Y, Hoffman JC., Jr Intracranial ganglioglioma: MR, CT, and clinical findings in 18 patients. AJNR Am J Neuroradiol. 1990;11:109–114. [PMC free article] [PubMed] [Google Scholar]
- 5.Cukiert A, Buratini JA, Machado E, Sousa A, Vieira JO, Argentoni M, et al. Results of surgery in patients with refractory extratemporal epilepsy with normal or nonlocalizing magnetic resonance findings investigated with subdural grids. Epilepsia. 2001;42:889–894. doi: 10.1046/j.1528-1157.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 6.Engel J., Jr Epilepsy surgery. Curr Opin Neurol. 1994;7:140–147. doi: 10.1097/00019052-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Fulton SP, Clarke DF, Wheless JW, Ellison DW, Ogg R, Boop FA. Angiocentric glioma-induced seizures in a 2-year-old child. J Child Neurol. 2009;24:852–856. doi: 10.1177/0883073808331078. [DOI] [PubMed] [Google Scholar]
- 8.Guerrieri C, Jarlsfelt I. Ependymoma of the ovary. A case report with immunohistochemical, ultrastructural, and DNA cytometric findings, as well as histogenetic considerations. Am J Surg Pathol. 1993;17:623–632. [PubMed] [Google Scholar]
- 9.Hegyi L, Peston D, Theodorou M, Moss J, Olver J, Roncaroli F. Primary glial tumor of the retina with features of myxopapiIlary ependymoma. Am J Surg Pathol. 2005;29:1404–1410. doi: 10.1097/01.pas.0000172188.02424.d8. [DOI] [PubMed] [Google Scholar]
- 10.Koeller KK, Dillon WP. Dysembryoplastic neuroepithelial tumors: MR appearance. AJNR Am J Neuroradiol. 1992;13:1319–1325. [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroiwa T, Kishikawa T, Kato A, Ueno M, Kudo S, Tabuchi K. Dysembryoplastic neuroepithelial tumors: MR findings. J Comput Assist Tomogr. 1994;18:352–356. doi: 10.1097/00004728-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lehman NL. Central nervous system tumors with ependymal features: a broadened spectrum of primarily ependymal differentiation? J Neuropathol Exp Neurol. 2008;67:177–188. doi: 10.1097/NEN.0b013e31816543a6. [DOI] [PubMed] [Google Scholar]
- 13.Lehman NL. Patterns of brain infiltration and secondary structure formation in supratentorial ependymal tumors. J Neuropathol Exp Neurol. 2008;67:900–910. doi: 10.1097/NEN.0b013e31818521cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman NL, Jorden MA, Huhn SL, Barnes PD, Nelson GB, Fisher PG, et al. Cortical ependymoma. A case report and review. Pediatr Neurosurg. 2003;39:50–54. doi: 10.1159/000070881. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. (Erratum in Acta Neuropathol 114:547, 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lum DJ, Halliday W, Watson M, Smith A, Law A. Cortical ependymoma or monomorphous angiocentric glioma? Neuropathology. 2008;28:81–86. doi: 10.1111/j.1440-1789.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 17.Massimino M, Buttarelli FR, Antonelli M, Gandola L, Modena P, Giangaspero F. Intracranial ependymoma: factors affecting outcome. Future Oncol. 2009;5:207–216. doi: 10.2217/14796694.5.2.207. [DOI] [PubMed] [Google Scholar]
- 18.McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52:65–69. doi: 10.1002/pbc.21806. [DOI] [PubMed] [Google Scholar]
- 19.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a Surveillance, Epidemiology, and End Results study. Clinical article. J Neurosurg. 2009;110:725–729. doi: 10.3171/2008.9.JNS08117. [DOI] [PubMed] [Google Scholar]
- 20.Metellus P, Figarella-Branger D, Guyotat J, Barrie M, Giorgi R, Jouvet A, et al. Supratentorial ependymomas: prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113:175–185. doi: 10.1002/cncr.23530. [DOI] [PubMed] [Google Scholar]
- 21.Mikami M, Komuro Y, Sakaiya N, Tei C, Kurahashi T, Komiyama S, et al. Primary ependymoma of the ovary, in which long-term oral etoposide (VP-16) was effective in prolonging disease-free survival. Gynecol Oncol. 2001;83:149–152. doi: 10.1006/gyno.2001.6343. [DOI] [PubMed] [Google Scholar]
- 22.Miyazawa T, Hirose T, Nakanishi K, Uozumi Y, Tsuzuki N, Shima K. Supratentorial ectopic cortical ependymoma occurring with intratumoral hemorrhage. Brain Tumor Pathol. 2007;24:35–40. doi: 10.1007/s10014-007-0215-3. [DOI] [PubMed] [Google Scholar]
- 23.Mork SJ, Loken AC. Ependymoma: a follow-up study of 101 cases. Cancer. 1977;40:907–915. doi: 10.1002/1097-0142(197708)40:2<907::aid-cncr2820400247>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Roncaroli F, Consales A, Fioravanti A, Cenacchi G. Supratentorial cortical ependymoma: report of three cases. Neurosurgery. 2005;57:EI92. doi: 10.1227/01.neu.0000164171.29292.d6. [DOI] [PubMed] [Google Scholar]
- 25.Saito T, Oki S, Mikami T, Kawamoto Y, Yamaguchi S, Kuwamoto K, et al. [Supratentorial ectopic ependymoma: a case report.] No Shinkei Geka. 1999;27:1139–1144. (Jpn) [PubMed] [Google Scholar]
- 26.Schild SE, Nisi K, Scheithauer BW, Wong WW, Lyons MK, Schomberg PJ, et al. The results of radiotherapy for ependymomas: the Mayo Clinic experience. Int J Radiat Oncol Bioi Phys. 1998;42:953–958. doi: 10.1016/s0360-3016(98)00350-2. [DOI] [PubMed] [Google Scholar]
- 27.Shakur SF, McGirt MJ, Johnson MW, Burger PC, Ahn E, Carson BS, et al. Angiocentric glioma: a case series. Clinical article. J Neurosurg Pediatr. 2009;3:197–202. doi: 10.3171/2008.11.PEDS0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun FH, Piao YS, Wang W, Chen L, Wei LF, Yang H, et al. [Brain tumors in patients with intractable epilepsy: a clinicopathologic study of 35 cases.] Zhonghua Bing Li Xue Za Ziti. 2009;38:153–157. (Chinese) [PubMed] [Google Scholar]
- 29.Van Gompel JJ, Rubio J, Cascino GD, Worrell GA, Meyer FB. Electrocorticography-guided resection of temporal caver-noma: is electrocorticography warranted and does it alter the surgical approach? Clinical article. J Neurosurg. 2009;110:1179–1185. doi: 10.3171/2008.10.JNS08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 2008;63:498–506. doi: 10.1227/01.NEU.0000324996.37228.F8. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Tihan T, Rojiani AM, Bodhireddy SR, Prayson RA, Iacuone JJ, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 2005;64:875–881. doi: 10.1097/01.jnen.0000182981.02355.10. [DOI] [PubMed] [Google Scholar]
- 32.Yadav YR, Neha, Chandrakar SK. Pure cortical supratentorial extraventricular ependymoma. Neurol India. 2009;57:213–215. doi: 10.4103/0028-3886.51301. [DOI] [PubMed] [Google Scholar]