Abstract
Introduction:
Urinary tract squamous cell carcinoma and transitional cell carcinoma with squamous differentiation are rare entities. To characterize tumour biology, prognosis, and therapy, we reviewed our data with squamous cell carcinoma (SCC) and transitional cell carcinoma (TCC/SCC).
Materials and Methods:
We performed a retrospective single-center analysis of 53 patients with SCC and TCC/SCC treated at our urology department from 30.05.1989 to 30.09.2004.
Results:
SCC was found in 2% (42/1573) of bladder carcinoma and 7% (11/130) of renal pelvis specimen. Stage pT3 was present in 55% of our patients, indicating a tendency to deep muscular invasion. Nodal and distant metastases appeared in 26%. The overall 5-year survival rate was 26% (tumor specific 46%), with a median survival of 10.5 months. We found that three of four patients with pT2N0 bladder carcinoma could be cured by cystectomy. Lymphnode status was identified as a significant prognostic parameter. For renal pelvis carcinoma, median survival was 7.35 months, with an overall 5-year-survival of 30%. Adjuvant therapy modalities were only performed in a minority of cases, although a therapeutic response was often noticed.
Conclusions:
SCC is characterized by poor prognosis and individual tumor biology. Survival is related to local tumor extension, indicating the necessity of an early radical surgery. To adequately discuss the role of adjuvant therapy on SCC and TCC/SCC further trials are needed.
Keywords: Bladder carcinoma, renal pelvis, squamous cell carcinoma, squamous differentiation
INTRODUCTION
In western countries, squamous cell carcinoma (SCC) and transitional carcinoma (TCC) with squamous differentiation are rare tumor entities of the urinary tract. Bilharzial-associated squamous cell carcinoma of the bladder is the most common urothelial neoplasm in countries with epidemic schistosomiasis and offers individual tumor characteristics.[1] Natural history, treatment, and outcome of non-bilharzial bladder squamous cell carcinoma have been studied in several retrospective analyses.
In analogy to transitional cell carcinoma, upper-tract squamous cell carcinoma is rarely seen and only limited published data is available to evaluate treatment and prognosis of patients.
We systematically reviewed our experience with squamous cell carcinoma and squamous differentiated transitional cell carcinoma of the lower and upper urinary tract within a period of 15 years in order to study treatment modalities, patient outcome, and to evaluate the tumor biology of those lesions.
MATERIALS AND METHODS
We performed a retrospective analysis of 53 patients with pure SCC and TCC with squamous differentiation treated at our urology department to 30.09.2004. Patients were identified by an analysis of histopathologic databases and pathology reports were reviewed to classify the lesions into pure SCC and TCC with squamous differentiation. For the identified cases, clinical history, treatment, histology, and postoperative follow-up were recorded. A primary squamous carcinoma of the ureter was not identified in our patient collective. None of the patients suffered from schistosomiasis.
Survival analysis was performed using the Kaplan-Meier method. For group comparisons, a formal nonparametric log rank test was used.
RESULTS
Bladder carcinoma
Of 1573 patients with bladder cancer, 31 had pure squamous cell carcinoma and 11 had transitional cell carcinoma with squamous differentiation, accounting for 2% and 0.7%, respectively. In our collective, a male predominance with a ratio of 1.9:1 was observed. The average age at diagnosis was 68.3 years.
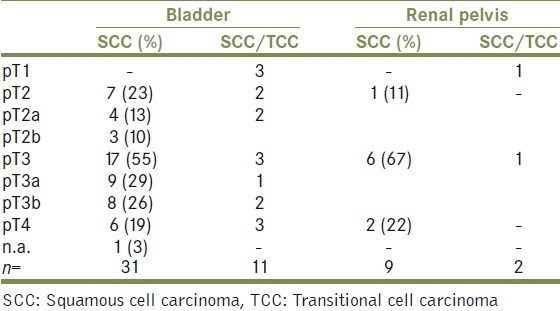
The review of TNM-staging showed that no lesion ≤pT1 was present in pure SCC and squamous differentiated TCC. All pure SCCs were muscle invasive carcinoma with a staging of at least pT2.
Three patients who showed lamina propria invasive (pT1) disease, all had TCC and squamous differentiation. Stage distribution for all patients is shown in Table 1. Overall, a tendency to wide local spread is documented by more than 70% of bladder carcinomas being staged pT3 or higher. The incidence of a tumor-grading G3 was highest among pure SCC (18/31, 58%) and TCC/SCC lesions (9/11, 82%). Nine patients (29%) with pure SCC had a positive nodal status and two patients had distant metastases at preoperative staging. Two of 11 Patients with TCC and a squamous fraction showed lymphnode metastases.
Table 1.
Stage distribution
Radical cystectomy was treatment of choice in patients with muscle invasive disease that were eligible to undergo surgical therapy. Symptomatic treatment for patients with a reduced general state of health consisted of transurethral resection (five patients) and partial bladder resection (one patient).
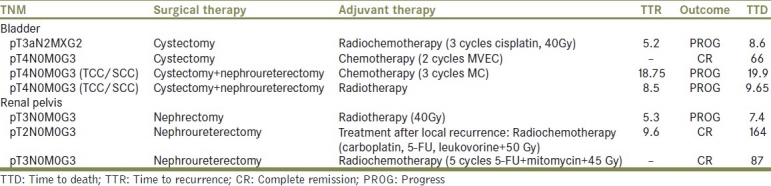
Adjuvant treatment was conducted in four patients (two pure SCC/and two TCC/SCC) administering either postoperative local irradiation, three cycles of methotrexate/cisplatin, two cycles of methotrexate, vinblastine, epirubicine, cisplatin (MVEC) or a combined radio-chemotherapy with three cycles of Cisplatin and pelvic irradiation of 40 Gy [Table 2]. One patient with histological pT1G2 TCC/SCC underwent maintenance BCG-instillation.
Table 2.
Adjuvant therapies
The median overall survival for patients with pure SCC was 10.5 months and 19.9 months for TCC/SCC, respectively. Kaplan-Meier survival analysis revealed a 1-year survival rate of 45% and 5-year survival of 26% (46% tumor specific) for pure SCC patients, while the TCC/SCC collective survived 54% and 27%, respectively [Figure 1]. The difference in overall survival between the two subgroups was not statistically significant (P=0.86). In our patient selection, staging (P=0.24) and grading (P=0.93) were not significantly associated with survival. Only nodal status (P=0.009) was identified as a significant prognostic factor for patient outcome. Despite that fact, three out of four patients with pT2N0 bladder carcinoma could be cured by radical cystectomy. Poor prognosis was observed for individuals not undergoing radical surgery.
Figure 1.
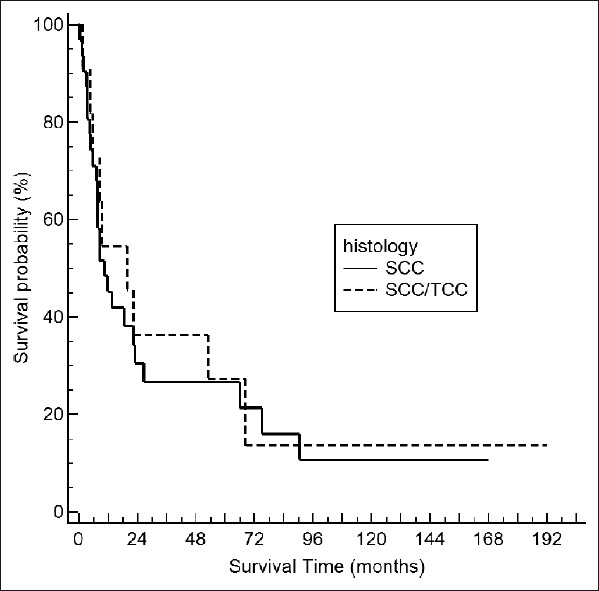
Kaplan–Meier survival curves for bladder SCC and SCC/TCC (P=0.86, log rank test)
Table 2 demonstrates the effect of adjuvant therapy forms on patient outcomes. In summary, radiotherapy alone was not sufficient to improve survival. Complete remissions could however be obtained with radio-chemotherapy and chemotherapy.
Renal pelvis carcinoma
Out of 130 individuals with upper-tract cancer, 11 with pure SCC (9) and TCC/SCC (2) could be identified in our patient cohort. The incidence in comparison to lower-tract SCC is higher, with 6.9%.
All tumors were located in the renal pelvis. There was no presence of a primary ureteral tumor.
Nephroureterectomy was favored as primary treatment. In two patients, nephrectomy and ureterectomy were performed as staged procedure. Adjuvant therapeutic approaches were performed in two cases and consisted of adjuvant radiotherapy and an aggressive adjuvant approach of radio-chemotherapy with five cycles of mitomycin-c and 5-FU plus 45 Gy radiation dose.
Overall median survival for renal pelvis SCC was 7.25 months. The observed 5-year survival rate was 18%, while the tumor specific survival was 30% in five years [Table 3].
Table 3.
Survival
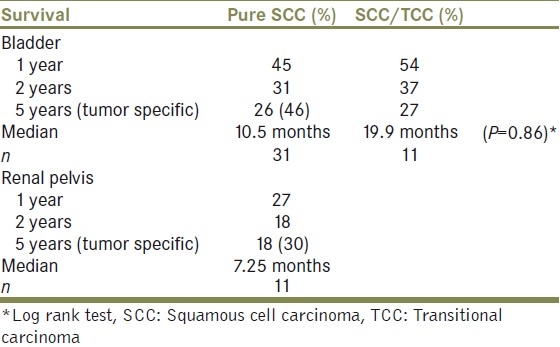
Both patients with mixed tumor differentiation (pT3G3, pT1G3) had unfavorable outcomes with a TTD of 1.1 and 4.7 months.
DISCUSSION
The possibilities to evaluate natural history and prognosis of SCC are limited because of its low incidence. Schistosoma-associated SCC is to be regarded as an independent pathology with distinct clinical and prognostic parameters.[1]
Approximately 2-5% of bladder cancers are SCC.[2–6] In our patient cohort, pure SCC accounts for 2% of all malignant lesions of the bladder whereas renal pelvis SCC occurred in 6.9% of all upper-tract carcinoma. No pT1 lesion was observed in the pure SCC group. Seventy percent of bladder SCC were staged pT3 or higher, indicating their locally aggressive, invasive growth.
A similar observation can be made for upper-tract SCC, where T-staging revealed an even higher amount of deep invasive growth, which is probably caused by the delayed appearance of symptoms in upper-tract cancer. According to previous reviews, distant metastatic spread is only a rare finding in bladder SCC, rates between 8-12% have been reported.[3,7–8] In our study, a comparably high number of patients (29%) had nodal metastases. This finding is in line with a recent analysis of the Netherlands cancer registry, where 21% of bladder-SCC-patients showed nodal positivity or distant metastases.[6]
The 5-year survival rate for bladder SCC in our collective was 26% with a median survival of 10.5 months. The tumor-specific 5-year survival rate was 46%. A significant difference in prognosis of pure SCC and TCC/SCC could not be observed, although median survival for mixed histology was higher, with 19.9 months. Kassouf et al. recently observed a 2-year overall survival of 47.6% in a series of 27 patients with SCC undergoing radical cystectomy, the median follow-up for survivors accounting for 15.3 months.[9] The role of radical cystectomy as the key therapeutic approach for bladder SCC was previously also described by Richie et al., who documented a 5-year survival of 48% in patients treated with cystectomy and lymphadenectomy.[10]
Neo-adjuvant irradiation has been discussed by several authors and small patient series, noticing a significant preoperative down-staging and improved overall survival by adding a possible protective effect on local tumor recurrence for SCC patients.[7,11] However, these results were not confirmed by prospective studies. For pure TCC there is no data supporting the role of preoperative irradiation before cystectomy.[12]
Squamous cell carcinoma is often described as a chemotherapy refractory disease.[1,13] In our patients, heterogeneous adjuvant chemotherapy and combination radio-chemotherapy regimens were performed in a minority of patients in advanced stages of disease. For bladder carcinoma, we could obtain a complete remission and a disease-free follow up of 66 months for one patient undergoing cystectomy and two postoperative cycles of MVEC. Other adjunctive postoperative treatments could not influence cancer progression significantly. There is a number of case reports and small patient series, where chemotherapy or radio-chemotherapy using platinum-based schemes like MVEC, cisplatin, methotrexate, bleomycin (CMB), or a combination of neo-adjuvant 5-FU/Mitomycin+external radiation was beneficial for SCC-patients. Seretta et al. analyzed 19 patients with SCC retrospectively and found six patients in complete remission after 52 months of whom four were treated with neo-adjuvant or adjuvant chemotherapy.[3,14–17]
We observed nine patients with pure SCC of the renal pelvis with a median survival of 7.25 months and a 5-year survival rate of 18%. A series of upper-tract SCC was reviewed by Nativ and coworkers in 1991. Of 44 patients, 22% survived for 2 years.[18] In their analysis of the Swedish cancer registry, Holmang and co-workers identified 65 cases of upper-tract SCC.
In comparison to advanced stage TCC, they found no significant difference in survival and the observed 5-year survival was less than 10%. According to previous reports, the incidence of SCC in the upper tract is higher than in bladder carcinoma.[19]
Primary treatment in our series was nephroureterectomy. Two individuals with pT3N0M0G3 received additional radio-chemotherapy or radiation therapy. The patient undergoing combination radio-chemotherapy survived 87 months and was disease-free at follow up. The combination regimen consisted in five cycles of 5-FU and 50 Gy of external beam radiation. Another patient successfully underwent systemic 5-FU, carboplatin and leukovorine plus 50 Gy radiation after the diagnosis of a local recurrence. Our patient data strongly suggest that urinary-tract SCC is not generally a chemotherapy refractory disease although the above-mentioned data is derived from nonrandomized retrospective analyses. To date, scientific validation for adjuvant treatment is not available and prospective randomized studies seem to be difficult to carry out, even with multi-institutional approaches. To evaluate prognostic factors and management of SCC a pooling of single centre experience or a multinational database could offer more objective data. However, our data underline the need for early radical surgery in SCC with three out of four patients with bladder confined disease being cured by radical cystectomy.
Supported by a recent stage adjusted analysis of pure TCC and SCC, it is accepted to be a malignancy with distinct pathological features.[20]
Variant histological differentiation has been identified as a risk factor for higher stage at presentation and more invasive growth in comparison to pure transitional cell cancer.[21,22] In our study, we also reviewed 11 patients with lower and upper-tract TCC and squamous differentiation. Chalasani et al. described squamous differentiation as an independent predictor of cancer specific survival.[23] There are pathogenetic models that describe the development of SCC from extensive squamous differentiation of transitional cell carcinoma and from the neoplastic transformation of primary benign lesions like leukoplakia/squamous metaplasia of the urothelium.[24] In a microarray analysis of pure SCC vs TCC, Blaveri et al. identified the parathyroid-hormone-related peptide (PTHrP) as a gene with close association to the squamous subtype. Tumors with a mixed TCC/SCC histology were classified into either the SCC or the TCC group, depending on the expression patterns of nine genes. The degree of squamous differentiation did not correlate with the classification into the subgroups. The authors conclude that morphological requirements for pure SCC features might be too stringent a criterion to distinguish TCC/SCC and pure SCC. Cancers with partial squamous differentiation may be more precisely classified and defined for therapeutic trials based on RNA expression.[25]
CONCLUSIONS
Our data underline the individuality and poor prognosis of squamous cell cancerous lesions of the urinary tract. If local surgical control is achievable, radical surgery remains the treatment of first choice with a possible, curative potential. In our collective, the application of adjuvant chemotherapy and radio-chemotherapy was successful in selected patients. The histogenesis of pure SCC lesions and their connection of TCC with squamous differentiation are not conclusively defined. Further studies are needed in fundamental, histopathological and molecular research while clinicians should undertake combined efforts to merge and analyze clinical data in order to optimize the treatment of patients with rare urological tumor entities.
Footnotes
Source of Support: Nil,
Conflict of Interest: None.
REFERENCES
- 1.El-Sebaie M, Zaghloul MS, Howard G, Mokhtar A. Squamous cell carcinoma of the bilharzial and non-bilharzial urinary bladder: A review of etiological features, natural history, and management. Int J Clin Oncol. 2005;10:20–5. doi: 10.1007/s10147-004-0457-6. [DOI] [PubMed] [Google Scholar]
- 2.Tannenbaum M. Squamous cell carcinoma and urothelial tumor of bladder. Urology. 1976;7:529–30. doi: 10.1016/0090-4295(76)90200-4. [DOI] [PubMed] [Google Scholar]
- 3.Serretta V, Pomara G, Piazza F, Gange E. Pure squamous cell carcinoma of the bladder in western countries.Report on 19 consecutive cases. Eur Urol. 2000;37:85–9. doi: 10.1159/000020105. [DOI] [PubMed] [Google Scholar]
- 4.Dahm P, Gschwend JE. Malignant non-urothelial neoplasms of the urinary bladder: A review. Eur Urol. 2003;44:672–81. doi: 10.1016/s0302-2838(03)00416-0. [DOI] [PubMed] [Google Scholar]
- 5.Shokeir AA. Squamous cell carcinoma of the bladder: Pathology, diagnosis and treatment. BJU Int. 2004;93:216–20. doi: 10.1111/j.1464-410x.2004.04588.x. [DOI] [PubMed] [Google Scholar]
- 6.Ploeg M, Aben KK, Hulsbergen-van de Kaa CA, Schoenberg MP, Witjes JA, Kiemeney LA. Clinical epidemiology of nonurothelial bladder cancer: Analysis of the Netherlands Cancer Registry. J Urol. 2010;183:915–20. doi: 10.1016/j.juro.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Swanson DA, Liles A, Zagars GK. Preoperative irradiation and radical cystectomy for stages T2 and T3 squamous cell carcinoma of the bladder. J Urol. 1990;143:37–40. doi: 10.1016/s0022-5347(17)39857-9. [DOI] [PubMed] [Google Scholar]
- 8.Rundle JS, Hart AJ, McGeorge A, Smith JS, Malcolm AJ, Smith PM. Squamous cell carcinoma of bladder: A review of 114 patients. Br J Urol. 1982;54:522–6. doi: 10.1111/j.1464-410x.1982.tb13580.x. [DOI] [PubMed] [Google Scholar]
- 9.Kassouf W, Spiess PE, Siefker-Radtke A, Swanson D, Grossman HB, Kamat AM, et al. Outcome and patterns of recurrence of nonbilharzial pure squamous cell carcinoma of the bladder: A contemporary review of The University of Texas M D Anderson Cancer Center experience. Cancer. 2007;110:764–9. doi: 10.1002/cncr.22853. [DOI] [PubMed] [Google Scholar]
- 10.Richie JP, Waisman J, Skinner DG, Dretler SP. Squamous carcinoma of the bladder: Treatment by radical cystectomy. J Urol. 1976;115:670–2. doi: 10.1016/s0022-5347(17)59330-1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson DE, Schoenwald MB, Ayala AG, Miller LS. Squamous cell carcinoma of the bladder. J Urol. 1976;115:542–4. doi: 10.1016/s0022-5347(17)59272-1. [DOI] [PubMed] [Google Scholar]
- 12.Huncharek M, Muscat J, Geschwind JF. Planned preoperative radiation therapy in muscle invasive bladder cancer; Results of a meta-analysis. Anticancer Res. 1998;18:1931–4. [PubMed] [Google Scholar]
- 13.Logothetis CJ, Johnson DE, Chong C, Dexeus FH, Ogden S, von Eschenbach A, et al. Adjuvant chemotherapy of bladder cancer: A preliminary report. J Urol. 1988;139:1207–11. doi: 10.1016/s0022-5347(17)42861-8. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto K, Okuno H, Fukuyama T, Itoyama M, Okamoto E. A case of squamous cell carcinoma of the urinary bladder effectively responsive to combination chemotherapy. Hinyokika Kiyo. 1991;37:1045–8. [PubMed] [Google Scholar]
- 15.Matsumoto S, Nishioka T, Akiyama T, Park YC, Kurita T, Ishikawa Y. A case of squamous cell carcinoma of the bladder that was successfully treated with multidisciplinary therapies. Hinyokika Kiyo. 2001;47:43–6. [PubMed] [Google Scholar]
- 16.Inui M, Fujita K, Ueda N, Takenaka I, Kakehi Y. A case of regionally metastatic pure squamous cell carcinoma of the urinary bladder successfully treated with radical chemoradiotherapy. Hinyokika Kiyo. 2002;48:33–5. [PubMed] [Google Scholar]
- 17.Hayashi N, Asano K, Furuta A, Ikemoto I, Kishimoto K, Yamazaki H, et al. Invasive squamous cell carcinoma of the bladder: Report of 18 cases and review of literature. Nippon Hinyokika Gakkai Zasshi. 2004;95:711–7. doi: 10.5980/jpnjurol1989.95.711. [DOI] [PubMed] [Google Scholar]
- 18.Nativ O, Reiman HM, Lieber MM, Zincke H. Treatment of primary squamous cell carcinoma of the upper urinary tract. Cancer. 1991;68:2575–8. doi: 10.1002/1097-0142(19911215)68:12<2575::aid-cncr2820681208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Holmang S, Lele SM, Johansson SL. Squamous cell carcinoma of the renal pelvis and ureter: Incidence, symptoms, treatment and outcome. J Urol. 2007;178:51–6. doi: 10.1016/j.juro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Scosyrev E, Yao J, Messing E. Urothelial carcinoma versus squamous cell carcinoma of bladder: Is survival different with stage adjustment? Urology. 2009;73:822–7. doi: 10.1016/j.urology.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Billis A, Schenka AA, Ramos CC, Carneiro LT, Araujo V. Squamous and/or glandular differentiation in urothelial carcinoma: Prevalence and significance in transurethral resections of the bladder. Int Urol Nephrol. 2001;33:631–3. doi: 10.1023/a:1020597611645. [DOI] [PubMed] [Google Scholar]
- 22.Wasco MJ, Daignault S, Zhang Y, Kunju LP, Kinnaman M, Braun T, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70:69–74. doi: 10.1016/j.urology.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani V, Chin JL, Izawa JI. Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer. Can Urol Assoc J. 2009;3:S193–8. doi: 10.5489/cuaj.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu RL, Osman I, Wu XR, Lu ML, Zhang ZF, Liang FX, et al. Uroplakin II gene is expressed in transitional cell carcinoma but not in bilharzial bladder squamous cell carcinoma: Alternative pathways of bladder epithelial differentiation and tumor formation. Cancer Res. 1998;58:1291–7. [PubMed] [Google Scholar]
- 25.Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11:4044–55. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]


