Abstract
Within the B cell antigen receptor (BCR), the cytoplasmic tails of both Igα and Igβ are required for normal B cell development and maturation. To dissect the mechanisms by which each tail contributes to development in vivo, Igβ–/– mice were reconstituted with retroviruses encoding either wild-type Igβ, an Igβ molecule lacking a cytoplasmic tail (IgβΔC) or one in which the cytoplasmic tail was derived from Igα (IgβCα). All constructs rescued B cell development and generated immature B cell populations in the bone marrow with similar expression levels of both Igβ and membrane-bound IgM. In the periphery, receptor-surface density was inversely proportional to the number of Igα tails in the BCR. Although peripheral-surface-receptor levels differed, splenic B cells expressing either Igβ or IgβCα responded similarly to stimulation through the BCR. Analysis of membrane-bound IgM and Igβ expression revealed that peripheral-receptor expression was primarily determined by positive selection between the bone marrow and peripheral immature B cell populations. These data indicate that B cells are selected into the periphery on the basis of a common level of antigen responsiveness.
Keywords: signal transduction, B cell differentiation, CD antigen, tyrosine kinase
Signals through the B cell antigen receptor (BCR) or its surrogates are required for B cell development, central and peripheral tolerance, and responses to antigen (1, 2). The earliest stage at which BCR signaling is required is the pro- to pre-B cell transition. At this stage the Igα-Igβ heterodimer initiates allelic exclusion, clonal expansion, and light-chain rearrangement (3–5). The cell fate of immature B cells, which express heavy and light chains associated with Igα-Igβ, is determined by ligand-mediated receptor aggregation (6, 7). Cells that survive negative selection transit to the periphery, where they mature.
In addition to negative selection, recent evidence indicates that developing B cells may also undergo positive selection. Antigen-based positive selection has been conclusively demonstrated in peritoneal B-1 B cells (8) and has been suggested in marginal zone B cells (9). Evidence for positive selection of conventional B-2 cells is more circumstantial. Analysis of light-chain rearrangements in HSA+ and HSA– splenic B cells indicates that the repertoire shifts during maturation (10). One limitation of this study is that the analyzed HSA+ population may also contain mature marginal zone B cells, which have a restricted repertoire. Thus, the evidence for positive selection in follicular B cells remains inconclusive.
In vitro studies indicate that Igα and Igβ have unique signaling capacities, with Igα being the primary activator of tyrosine kinases (11–13). Complementary in vivo studies have demonstrated that the cytosolic tail of Igα is more important for early B cell development, whereas the tails of both Igα and Igβ are required for peripheral B cell maturation (14–16). These studies demonstrate the relative requirements for Igα and Igβ at discrete stages of B cell development and maturation. However, they do not provide insights into the processes evoked by each chain to establish peripheral B cell populations.
To examine how the different cytoplasmic domains of Igα and Igβ contribute to B cell development and selection, we used a bicistronic, GFP-expressing retroviral vector to reconstitute Igβ–/– bone marrow with surface antigen receptors containing either one Igα tail, one Igα and one Igβ tail, or two Igα tails. As expected, the Igα tail was more potent than the Igβ tail in reconstituting B cell development. Analysis of GFP expression, BCR surface densities, and receptor-mediated proliferation indicated that B lymphocytes were positively selected into the periphery based on both the intrinsic functional capacity of the BCR and the surface density. These data indicate that surviving negative selection is insufficient for residence in the peripheral B cell pool; a threshold of antigen responsiveness is also required.
Materials and Methods
Construction of Igβ and Variants. Igβ (b29), Igα (mb-1), and derivative mutants were cloned from BALB/c splenocyte cDNA by using PCR. All PCR products were ligated into pGEM-T (Stratagene), sequenced, and then subcloned into MIG-R1 (17).
Cell Culture and DNA Transfections. Phoenix, NIH 3T3, mouse AtT20 endocrine cell lines (18), and BOSC fibroblast cells were grown in DMEM supplemented with 10% FCS, glutamine, penicillin, and streptomycin.
Phoenix cells were transiently transfected by using FuGENE6 (Roche Applied Science). Forty-four hours after transfection, viral supernatants were harvested, filtered, flash-frozen, and aliquots were titrated on NIH 3T3 cells. BOSC and AtT20 cells were transfected with calcium phosphate (19). For stable clones, cells were cotransfected with pWLZBlast and selected with blasticidin (2 μg/ml, Invitrogen) (20).
Cell Lysis. Cell aliquots were solubilized in either 1% Triton X-100 (21) or 1% digitonin lysis buffer (22). Detergent-insoluble material was removed by centrifugation, and protein concentrations were determined by using the bicinchoninic acid protein assay (Pierce).
Deglycosylation. N-linked carbohydrates were removed by digesting 50-μg detergent cell extracts suspended in 2× PNGase F digestion buffer (74 mM NaPO4, pH 7.2/10 mM EDTA/0.4% SDS/0.04% 2-mercaptoethanol) with 5 units of PNGase F for 18 h at 37°C.
SDS/PAGE and Immunoblotting. Protein-normalized cell lysate samples were resolved on SDS-12% polyacrylamide gels and transferred to nitrocellulose filters (VWR Scientific). The filters were blocked in tris-buffered saline/0.05% Tween 20 containing either 5% skim milk or BSA. Filters were incubated in primary antibodies, washed and then incubated in a horseradish peroxidase-conjugated secondary reagent and washed. Filters were developed by using enhanced chemiluminescence (Amersham Pharmacia Biosciences).
Retroviral Infection of Progenitor-Enriched Cultures and Bone Marrow Transfer. Infection of bone marrow cells from Igβ–/– BALB/c mice (23) with retroviral supernatants and adoptive transfer of these cells into lethally irradiated (900 rad) 6- to 8-week-old RAG-2–/– BALB/c mice (The Jackson Laboratory) was performed as described (24) with the modification that IL-7 (20 ng/ml) was added to the ex vivo cultures.
Flow Cytometric Analysis of Bone Marrow and Splenic B Cells. Six to eight weeks after adoptive transfer, bone marrow and splenocytes were harvested and erythrocyte-depleted. Bone marrow cells were stained for cell-surface marker expression by using anti-CD19-biotin followed by PerCP-streptavidin, anti-IgM-Cy5, and anti-CD43-PE (Pharmingen). GFP+, B220+ or GFP+, Thy-1– splenocytes were sorted on a MoFlo flow cytometer (Cytomation Industries, Fort Collins, CO).
Proliferation Assays. GFP+, B220+ splenocytes were MoFlosorted and plated out at a concentration of 2 × 105 cells per well in 96-well plates with 100 ng/ml IL-4 and 5–15 μg/ml stimulating antibody (anti-IgM/IgG (H+L) F(ab′)2, Jackson Immunologicals, West Grove, PA). Cells were stimulated with lipopolysaccharide (70 μg/ml, Sigma) as a positive control. After 60 h, cells were treated with tritiated thymidine (5 μCi/ml) for 12 h. Wells were harvested onto glass-fiber filters and β-emission was quantitated.
Results
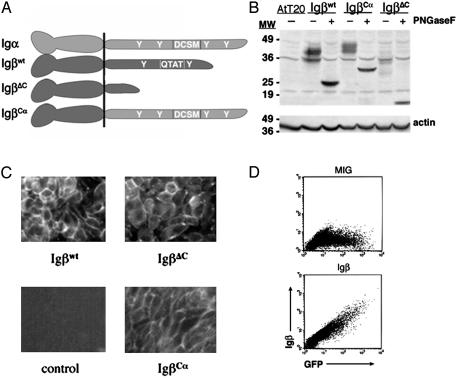
Retroviral Expression of Igβwt Rescues Cell-Surface BCR Expression. In vitro studies have demonstrated that Igα and Igβ have significantly different signaling capacities (13, 22, 25–27). To assess the roles of these domains in vivo, cDNAs encoding the cytoplasmic tail of Igα or Igβ fused to the extracellular and transmembrane domains of Igβ were constructed and cloned into the retroviral vector MIG-R1 (Fig. 1). The Igβwt construct contains the ORF of the b29 gene cDNA, whereas the IgβΔC cDNA encodes an Igβ molecule in which the cytoplasmic tail is truncated at residue 194 (28). The IgβCα plasmid encodes the carboxy-terminal 60 amino acids of Igα grafted onto the extracellular and transmembrane domains (amino acids 1–180) of Igβ. These three constructs, IgβΔC, Igβwt, and IgβCα, were designed to generate B cell receptors containing one Igα tail only, one Igα and one Igβ tail, or two Igα tails, respectively (Fig. 1 A).
Fig. 1.
Igβ constructs and their expression. (A) The Igβwt construct contains the ORF of the b29 gene and encodes wild-type Igβ.IgβΔC encodes a version of Igβ that is truncated at amino acid 194 and therefore lacks an immunoreceptor tyrosine-based activation motif. IgβCα consists of the cytoplasmic tail of Igα grafted onto the transmembrane and extracellular domains of Igβ.(B) The R142 cell line was stably infected with retroviruses encoding Igβwt, IgβΔC, or IgβCα. These cells were lysed, deglycosylated with PNGase F, and immunoblotted with antibodies to the extracellular domain of Igβ.(C) R142 cells stably expressing the indicated Igβ molecules were stained with rhodamine-conjugated anti-IgM antibodies and visualized by fluorescence microscopy. (D) Transiently transfected Phoenix cells were fixed, permeabilized, and stained intracellularly with antibodies to Igβ. Shown are representative fluorescence-activated cell sorter profiles for cells transfected with empty vector (MIG, Upper) or Igβwt (Lower).
To examine the fidelity of each encoded protein, we established stable transfectants of the AtT20 cell line expressing Igα, μ heavy chain, and λ light chain. This cell line, designated R142, was transfected with retroviral plasmid encoding each Igβ molecule. Lysates from each line were resolved by SDS/PAGE and immunoblotted with anti-Igβ antibodies as above. As seen in Fig. 1B, each Igβ molecule migrated as multiple bands with the distribution of relative molecular weights observed for Igβwt, approximating that observed for endogenous Igβ (29). Digestion with PNGase F, which removes N-linked glycosyl residues, resulted in core proteins with the predicted relative molecular weights of ≈24,000 (Igβwt), 30,000 (IgβCα), and 17,000 (IgβΔC) (Fig. 1B).
We next determined whether each Igβ molecule could productively assemble with the other components of the BCR. As seen in Fig. 1C, all the Igβ molecules were capable of rescuing cell-surface expression of IgM. We also verified that GFP expression was an accurate surrogate of Igβ expression by staining permeabilized transfected Phoenix cells for intracellular Igβ (Fig. 1D).
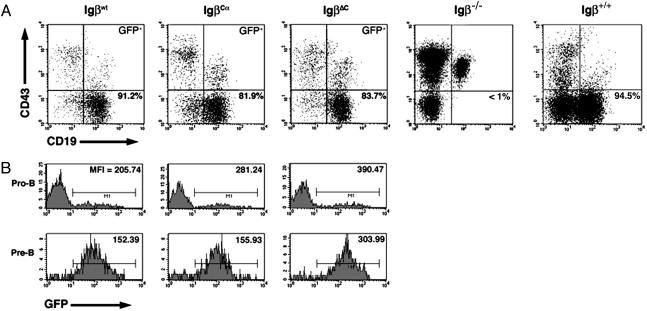
Rescue of Early B Cell Development. Bone marrow precursors from 6- to 8-week-old 5-fluorouracil-treated BALB/c Igβ–/– mice were retrovirally transduced with Igβwt, IgβCα, or IgβΔC. After infection, precursors were adoptively transferred into irradiated age-matched BALB/c RAG-2–/– recipients by retroorbital injection. After 6–8 weeks, the mice were killed, and bone marrow aspirates were analyzed by multiparameter flow cytometry.
Bone marrow from reconstituted mice was stained for the pan-B cell marker CD19 and the pro-B cell marker CD43, and GFP+ cells were analyzed by flow cytometry. As demonstrated in Fig. 2A, all three Igβ molecules efficiently rescued pre-B cell development. In Fig. 2B, we also analyzed the distribution of GFP expression in the pro- and pre-B cell populations. Typically, 10–20% of the CD19+, CD43+ pro-B cells from Igβ–/– mice reconstituted with Igβwt, IgβΔC, or IgβCα were GFP+. Because no requirement exists for Igβ during the pro-B cell stage, these values reflect the efficiency of infection and suggest that the different retroviruses had similar infection efficiencies. In contrast, almost all the CD19+, CD43– pre-B cells were GFP-positive (bottom row), confirming that expression of Igβ was required for early B cell development. Comparison of the upper and lower histograms in Fig. 2B revealed that the mean fluorescence index (MFI) of GFP+ pre-B cells was uniformly lower than the MFI of GFP+ pro-B cells. This difference was primarily due to a paucity of GFPhigh pre-B cells. pre-B cells expressing IgβΔC had higher levels of GFP, and by extension higher levels of IgβΔC, than pre-B cells expressing either IgβCα or Igβwt.
Fig. 2.
B cell development in reconstituted mice. Bone marrow cells from mice reconstituted with each construct were harvested and analyzed by multiparameter flow cytometry. (A) Bone marrow lymphocytes were stained with antibodies to CD19 and CD43 and gated on GFP expression. Igβ–/– and BALB/c control bone marrow stains are shown to the right. Percentages indicate the proportion of CD19+ CD43– pre-B cells relative to total number of CD19+ cells. (B) GFP expression in CD19+ CD43+ pro-B cells (Upper) and CD19+ CD43– pre-B cells (Lower) in lymphocyte-gated bone marrows reconstituted with the Igβ constructs identified at the top of each column. The proportion of GFP+ pro-B cells is between 10% and 12% for all three constructs, whereas the proportion of GFP+ pre-B cells is >90% in all cases. GFP mean fluorescence indices are listed.
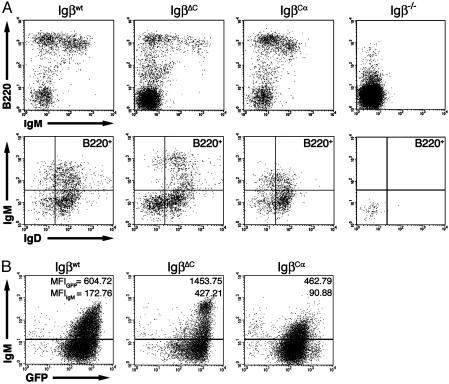
The Cytoplasmic Tails of Igα and Igβ Modulate Peripheral B Cell Development. Splenocytes were harvested from recipient mice 6–8 weeks after adoptive transfer of retrovirally transduced progenitors. Igβwt, IgβCα, and IgβΔC all reconstituted the peripheral B220+/IgM+ B cell compartment (Fig. 3A). Typically, 2–5 × 106 GFP+ splenocytes were recovered from each spleen. Igβwt and IgβCα were both efficient at rescuing peripheral B cell development, generating B220high cells totaling 57.8% and 48.1% of all GFP+ splenocytes, respectively. IgβΔC was much less efficient, with only 12.3% of GFP+ splenocytes being B220high (14, 15). Although the cells generated were predominantly B220high, mice reconstituted with IgβΔC had variable but significant populations of B220med/IgM– cells. Subsequent staining revealed these cells to be CD43+, indicating that they were B cell progenitors that had prematurely emigrated from the bone marrow (unpublished data).
Fig. 3.
Peripheral B cell development in reconstituted mice. Splenocytes from reconstituted mice were harvested 6 weeks after transfer and analyzed by multiparameter flow cytometry. (A) Splenocytes from Rag2–/– mice reconstituted with Igβwt, IgβΔC, or IgβCα were harvested and stained with antibodies to B220, IgM, and IgD. (Upper) B220 vs. IgM expression profiles for GFP+, lymphocyte-gated splenocytes. A lymphocyte-gated Igβ–/– peripheral stain is shown for comparison (far right). A population of B220-intermediate, IgM– cells was seen, most prominently in spleens from IgβΔC mice. This population is CD43+ (unpublished data) and therefore probably contains B cell progenitors that have prematurely escaped from the bone marrow. Subsequent analysis was performed on MoFlo-sorted GFP+, B220hi cells only. (Lower) IgM vs. IgD expression in B220hi splenocytes. An Igβ–/– stain is shown for comparison (far right). (B) GFP vs. IgM expression patterns for B220hi lymphocyte-gated splenocytes. The GFP MFIs of IgM+ cells (top number) closely parallel IgM MFIs (bottom number).
Further analysis was restricted to MoFlo-sorted GFP+, B220high cells. These studies revealed that on average (n > 10 for Igβwt and IgβCα and n = 6 for IgβΔC), surface IgM (sIgM) density was consistently 2.5-fold higher (representative MFIIgM = 427) on peripheral B cells from IgβΔC-reconstituted mice than on cells from mice reconstituted with Igβwt (representative MFIIgM = 173) (Fig. 3B). In contrast, splenocytes from mice reconstituted with IgβCα expressed half as much sIgM (representative MFIIgM = 91) than mice reconstituted with Igβwt. The differences in sIgM expression were paralleled by observed differences in GFP expression (Fig. 3B). A representative MFIGFP for cells reconstituted with IgβΔC was 1,454; Igβwt-reconstituted cells expressed an MFIGFP of 605, and cells transduced with IgβCα had an MFIGFP of 463. Differences in surface IgD expression in cells reconstituted with different constructs were not significant.
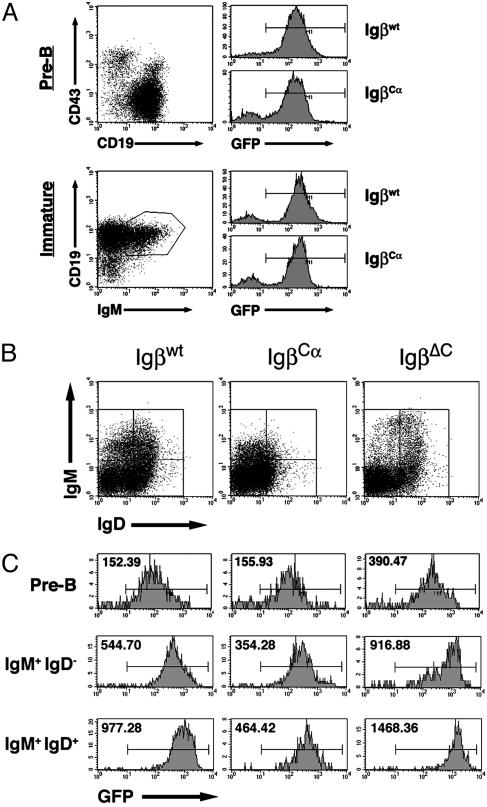
Using GFP as a marker for Igβ expression, we performed a more detailed analysis of how the expression of different Igβ molecules was regulated in the bone marrow and periphery (Fig. 4). No difference occurred in the MFIs of bone marrow immature cells (CD19+IgM+IgD–) as compared with pre-B cells, indicating that no selection occurs in the bone marrow itself (Fig. 4A). In Fig. 4B are shown the gates used to analyze peripheral IgM+/IgD–, IgMhigh/IgDhigh, and IgDhigh/IgMlo B cell populations. Fig. 4C provides the relative intensities and distribution of GFP-positive cells in the periphery as compared with the bone marrow. The MFIGFP of B220+IgM+IgD– IgβΔC splenocytes, most of which were newly immigrated transitional AA4.1+ cells (unpublished data), was 917, 3-fold higher than the MFIGFP of GFP+ CD19+ CD43– IgβΔC bone marrow pre-B cells. The MFI of the same population in Igβwt cells was 545 (a 3.6-fold increase over pre-B cells) and in IgβCα cells it was 354 (a 2.2-fold increase over the bone marrow MFI). Although the actual MFIs varied by as much as 20% from experiment to experiment, the relative change in MFI between central and peripheral compartments did not (n = 6). These data indicate that strong selection exists for increased Igβ expression between the central and peripheral immature B cell compartments.
Fig. 4.
Selection occurs between the bone marrow and periphery and between peripheral B cell compartments. (A) The GFP expression levels of bone marrow pre-B cells (CD19+, CD43–) and bone marrow immature B cells (CD19+, IgM+) were compared. (B) Splenic GFP+, B220hi lymphocytes were divided into three groups on the basis of their IgM and IgD expression patterns. (C) GFP expression was analyzed in these subpopulations as compared with bone marrow pre-B cells (Top). GFP MFIs are listed.
Selection within the peripheral B cell compartment was also evident. A consistent 1.3- to 1.6-fold increase in MFI between the T1 (A A4.1+B220+IgM+IgD–) and T2 transitional (AA4.1+B220+IgM+IgD+) stages for all three constructs was observed (n = 6) (Fig. 4 and unpublished data). Changes in GFP corresponded to differences in receptor-surface expression, indicating that particular receptor densities are selected for at each stage of maturation. The composition of the BCR complex did not influence whether peripheral cells differentiated into marginal zone B cells, because no significant differences occurred in the proportions of AA4.1–CD23loIgMhi cells in mice reconstituted with the different Igβ molecules (unpublished data).
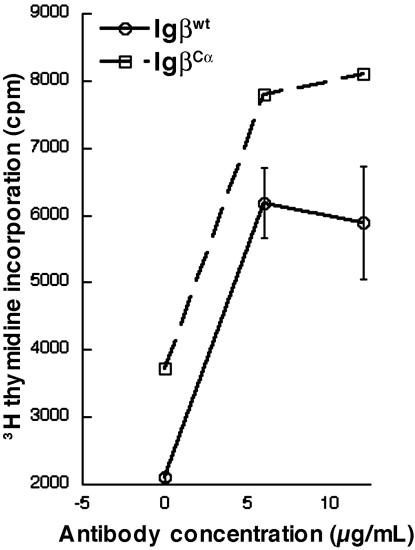
Peripheral B Cells from Reconstituted Mice Proliferate Similarly in Response to BCR Crosslinking. Cells expressing BCR complexes composed of the cytoplasmic tail of Igα alone, Igα/Igβ, or Igα/Igα established different receptor densities in the periphery, in inverse proportional relationship to the predicted signaling strength of each receptor complex. The IgMlowIgD+ surface phenotype of peripheral B cells expressing IgβCα was reminiscent of anergy (6), which could have occurred as a consequence of signaling through a receptor complex more signaling-competent than the wild type. Alternatively, these cells could be functional, expressing lower surface-receptor levels as a result of selection to maintain a certain level of cellular responsiveness to antigen. To discern between these possibilities, splenic B cells expressing either Igβwt or IgβCα were stimulated in vitro with antibodies to the B cell receptor in the presence of mIL-4 (100 ng/ml), and [3H]thymidine incorporation was assayed. As seen in Fig. 5, IgβCα cells proliferated similarly to B cells reconstituted with Igβwt. In three independent experiments, the difference between IgβCα and Igβwt proliferation amounted to one standard error and was not statistically significant (P = 0.35, 95% confidence interval for fold increase ranged from 0.91 to 1.32). This finding indicates that IgβCα cells, despite their reduced cell-surface BCR density, are not anergic.
Fig. 5.
B cells from Igβwt- and IgβCα-reconstituted mice respond similarly to BCR stimulation. GFP+, B220+ splenocytes were harvested from mice reconstituted with each construct and stimulated with the indicated concentrations of antibody and then pulsed with [3H]thymidine.
Discussion
Our data demonstrate that, at many stages in B cell development, selection occurs for a window of net B cell antigen receptor responsiveness to ligand. Receptor complexes with high intrinsic responsiveness establish low receptor densities, whereas those with low intrinsic responsiveness establish high densities. Furthermore, each successive stage of B cell development, from pre-B cells to peripheral transitional cells, requires successively higher receptor densities.
Only a limited number of mechanisms could result in populations of cells expressing different and increasing levels of a retrovirally encoded protein. Cell-endogenous mechanisms are capable of silencing proviral transcription, but evidence does not show that transcription driven by the LTR can be up-regulated by endogenous lymphoid-specific transcription factors. It is possible that a retrovirally expressed protein could be regulated posttranslationally, but such a modification would not affect GFP expression. In our system sIg, a surrogate for productive Igβ expression, consistently paralleled GFP expression. Therefore, we conclude that the different levels of Igβwt, IgβCα, and IgβΔC observed in the periphery arose from the selection of cells for a particular surface-receptor density. The existence of both upper and lower thresholds for expression suggests that both negative and positive selection pressures are operative.
Previous reports have indicated that antigen-receptor density is controlled by regulating the promoters of receptor constituents and, posttranslationally, through retention in the endoplasmic reticulum (30). The relative importance of these mechanisms and the positive selection that we observed is uncertain. It is likely that normal transcriptional and posttranslational regulatory mechanisms define a narrower range of expression than that provided by the retroviral LTR. For example, retroviral expression of IgβΔC rescues peripheral development to an extent not possible in the IgβΔC knock-in mouse. However, B cells bearing targeted mutations of either CD19 or Btk, which lie along a common signaling pathway, have increased B cell receptor surface densities (31–33). Additionally, other investigators have demonstrated a relationship between receptor affinity and surface density (34–36). Therefore, it is likely that selection for receptor density is an important mechanism by which receptor threshold is controlled in vivo.
In comparison with later development, little selection occurs between the pro-B and pre-B cell pools. We did not observe a dramatic shift in mean GFP levels between the two compartments, regardless of which Igβ molecule was being expressed. Rather, GFP (and by extension Igβ) expression in pre-B cells seemed to be restricted as a result of preferential expansion of pro-B cells expressing intermediate levels. These findings corroborate observations in B cells bearing targeted truncations of either the Igα or Igβ cytosolic tail, which suggests that the signaling requirements for early development are not stringent (14, 37).
Peripheral development, in contrast, is subject to very rigorous selection. B cells recovered from the periphery had varying levels of surface IgM, depending on the composition of their B cell receptors. Cells expressing a receptor containing only one cytoplasmic tail expressed the highest levels of sIgM, whereas cells reconstituted with a receptor containing two Igα cytoplasmic tails expressed the lowest levels. Differences in IgD expression were less marked, possibly because IgD has a higher affinity for Igα-Igβ than IgM and would preferentially assemble into receptor complexes in the endoplasmic reticulum (38). Despite their differing surface Ig expression levels, splenocytes recovered from mice reconstituted with each of the Igβ molecules contained mature, functional B2 cells (CD5–, B220+, sIg+, and AA4.1–). These cells proliferated to BCR crosslinking in the presence of IL-4. Thus, the alteration of the B cell receptor composition in these cells did not affect their functionality or impede their maturation.
Upper and lower B cell receptor expression thresholds existed in the periphery, implying that both negative and positive selection pressures were active. Negative selection in B lymphocytes is well documented (6, 7). Evidence for positive selection, either dependent or independent of antigen, is much more circumstantial (8, 10, 39). Surface Ig expression is required for maintenance of peripheral B cells, defining at least a permissive requirement for receptor signaling (40). Transit into the peripheral B cell pool is associated with significant skewing of receptor repertoire, implying that antigen receptor specificity may play a role in initiating signals that determine which cells are selected (10).
In this study, retroviral gene transduction was used to reconstitute Igβ-deficient mice with B cell antigen receptor complexes that had different functional capacities. The use of retroviral vectors allowed us to detect possible mechanisms of receptor regulation indiscernible with gene-targeting techniques. This approach allowed us to directly demonstrate that developing B cell populations are selected into the periphery based on the functional capacity of the antigen receptor expressed on the cell surface. Our data indicate that, in addition to negative selection, B cells are positively selected for a minimal receptor functional capacity. Simple neglect is not sufficient to populate the peripheral B cell pool. Therefore, we conclude that both positive and negative processes determine which B lymphocytes are selected into the periphery.
Acknowledgments
We thank Theodore Karrison and Rama Boddipalli for technical assistance; Julie Auger, Bart Eisfelder, Ryan Duggan, and James Marvin of the University of Chicago Flow Cytometry Core Facility for assistance with cytometric assays; Barbara Kee for critical reading of the manuscript and helpful discussions; and Lorrie Elliott and Stacy Gray for proofreading and moral support. This work was supported by National Institutes of Health Grant R01GM52736 (to M.R.C.), a Biomedical Sciences grant from the Arthritis Foundation (to M.R.C.), and grants from the Canadian Institutes for Health Research (to L.M.), the National Science and Engineering Council of Canada (to L.M.), and the Medical Scientist Training Program of the University of Chicago (to L.D.W.).
Abbreviations: BCR, B cell antigen receptor; MFI, mean fluorescence index; sIg, surface Ig.
References
- 1.Hardy, R. R., Li, Y. S., Allman, D., Asano, M., Gui, M. & Hayakawa, K. (2000) Immunol. Rev. 175, 23–32. [PubMed] [Google Scholar]
- 2.Meffre, E., Casellas, R. & Nussenzweig, M. C. (2000) Nat. Immunol. 1, 379–385. [DOI] [PubMed] [Google Scholar]
- 3.Papavasiliou, F., Misulovin, Z., Suh, H. & Nussenzweig, M. C. (1995) Science 268, 408–411. [DOI] [PubMed] [Google Scholar]
- 4.Cronin, F. E., Jiang, M., Abbas, A. K. & Grupp, S. A. (1998) J. Immunol. 161, 252–259. [PubMed] [Google Scholar]
- 5.Iritani, B. M., Alberola-Ila, J., Forbush, K. A. & Perlmutter, R. M. (1999) Immunity 10, 712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodnow, C. C., Crosbie, J., Adelstein, S., Lavoie, T. B., Smith-Gill, S. J., Brink, R. A., Pritchard-Briscoe, H., Wotherspoon, J. S., Loblay, R. H. & Raphael, K. (1988) Nature 334, 676–682. [DOI] [PubMed] [Google Scholar]
- 7.Nemazee, D. A. & Buerki, K. (1989) Nature 337, 562–566. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa, K., Asano, M., Shinton, S. A., Gui, M., Allman, D., Stewart, C. L., Silver, J. & Hardy, R. R. (1999) Science 285, 113–116. [DOI] [PubMed] [Google Scholar]
- 9.Li, Y., Li, H. & Weigert, M. (200) J. Exp. Med. 195, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine, M. H., Haberman, A. M., Sant'Angelo, D. B., Hannum, L. G., Cancro, M. P., Janeway, C. A. & Shlomchik, M. J. (2000) Proc. Natl. Acad. Sci. USA 97, 2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pracht, C., Gimborn, K., Reth, M. & Huber, M. (2002) Eur. J. Immunol. 32, 1614–1620. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez, M., Misulovin, Z., Burkhardt, A. L., Mahajan, S., Costa, T., Franke, R., Bolen, J. B. & Nussenzweig, M. (1993) J. Exp. Med. 178, 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luisiri, P., Lee, Y. J., Eisfelder, B. J. & Clark, M. R. (1996) J. Biol. Chem. 271, 5158–5163. [DOI] [PubMed] [Google Scholar]
- 14.Reichlin, A., Hu, Y., Meffre, E., Nagaoka, H., Gong, S., Kraus, M., Rajewsky, K. & Nussenzweig, M. C. (2001) J. Exp. Med. 193, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres, R. M., Flaswinkel, H., Reth, M. & Rajewsky, K. (1996) Science 272, 1804–1808. [DOI] [PubMed] [Google Scholar]
- 16.Kraus, M., Saijo, K., Torres, R. M. & Rajewsky, K. (1999) Immunity 11, 537–545. [DOI] [PubMed] [Google Scholar]
- 17.Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. (1993) Proc. Natl. Acad. Sci. USA 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuuchi, L., Gold, M. R., Travis, A., Grosschedl, R., DeFranco, A. L. & Kelly, R. B. (1992) Proc. Natl. Acad. Sci. USA 89, 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingham, R., Santos, L., Dang-Lawson, M., Holgado-Madruga, M., Dudek, P., Maroun, C., Wong, A. J., Matsuuchi, L. & Gold, M. R. (2001) J. Biol. Chem. 276, 12257–12265. [DOI] [PubMed] [Google Scholar]
- 20.Richards, J. D., Gold, M. R., Hourihane, S. L., DeFranco, A. L. & Matsuuchi, L. (1996) J. Biol. Chem. 271. [DOI] [PubMed]
- 21.Condon, C., Hourihane, S. L., Dang-Lawson, M., Escribano, J. & Matsuuchi, L. (2000) J. Immunol. 165, 1427–1437. [DOI] [PubMed] [Google Scholar]
- 22.Clark, M. R., Johnson, S. A. & Cambier, J. C. (1994) EMBO J. 13, 1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong, S. & Nussenzweig, M. C. (1996) Science 272, 411–414. [DOI] [PubMed] [Google Scholar]
- 24.Pui, J. C., Allman, D., Xu, L., DeRocco, S., Karnell, F. G., Bakkour, S., Lee, J. Y., Kadesch, T., Hardy, R. R., Aster, J. C. & Pear, W. S. (1999) Immunity 11, 299–308. [DOI] [PubMed] [Google Scholar]
- 25.Engels, N., Wollscheid, B. & Wienands, J. (2001) Eur. J. Immunol. 31, 2126–2134. [DOI] [PubMed] [Google Scholar]
- 26.Kabak, S., Skaggs, B. J., Gold, M. R., Affolter, M., West, K. L., Foster, M. S., Siemasko, K., Chan, A. C., Aebersold, R. & Clark, M. R. (2002) Mol. Cell. Biol. 22, 2524–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, K. M., Alber, G., Weiser, P. & Reth, M. (1993) Eur. J. Immunol. 23, 911–916. [DOI] [PubMed] [Google Scholar]
- 28.Hermanson, G. G., Eisenberg, D., Kincade, P. W. & Wall, R. (1988) Proc. Natl. Acad. Sci. USA 85, 6890–6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell, K. S., Hager, E. J., Friedrich, R. J. & Cambier, J. C. (1991) Proc. Natl. Acad. Sci. USA 88, 3982–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell, S. E. & Goodnow, C. C. (1994) EMBO J. 13, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto, M., Bradney, A. P., Poe, J. C., Steeber, D. A. & Tedder, T. F. (1999) Immunity 11, 191–200. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto, M., Poe, J. C., Satterthwaite, A. B., Wahl, M. I., Witte, O. N. & Tedder, T. F. (2002) J. Immunol. 168, 5465–5476. [DOI] [PubMed] [Google Scholar]
- 33.Khan, W. N., Alt, F. W., Gerstein, R. M., Malynn, B. A., Larsson, I., Rathbun, G., Davidson, L., Muller, S., Kantor, A. B. & Herzenberg, L. A. (1995) Immunity 3, 283–299. [DOI] [PubMed] [Google Scholar]
- 34.Braun, U., Rajewsky, K. & Pelanda, R. (2000) Proc. Natl. Acad. Sci. USA 97, 7429–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heltemes, L. M. & Manser, T. (2002) J. Immunol. 169, 1283–1292. [DOI] [PubMed] [Google Scholar]
- 36.Rosado, M. M. & Freitas, A. A. (2000) Eur. J. Immunol. 30, 2181–2190. [DOI] [PubMed] [Google Scholar]
- 37.Torres, R. M. & Hafen, K. (1999) Immunity 11, 527–536. [DOI] [PubMed] [Google Scholar]
- 38.Schamel, W. W. & Reth, M. (2000) Immunity 13, 5–14. [DOI] [PubMed] [Google Scholar]
- 39.Cyster, J. G., Healy, J. I., Kishihara, K., Mak, T. W., Thomas, M. L. & Goodnow, C. C. (1996) Nature 381, 325–328. [DOI] [PubMed] [Google Scholar]
- 40.Lam, K.-P., Kuhn, R. & Rajewsky, K. (1997) Cell 90, 1073–1083. [DOI] [PubMed] [Google Scholar]