Abstract
Aim:
The aim of our study was to evaluate the treatment outcomes of medical and surgical management of urinary tract endometriosis.
Materials and Methods:
Urinary tract endometriosis patients enrolled between Jan 2006 and May 2010 were retrospectively reviewed. Preoperative datas (mode of presentation, diagnosis, imaging), intraoperative findings (location and size of lesion), postoperative histopathology and follow-up were recorded and results were analyzed and the success rate of different modalities of treatment was calculated.
Results:
In our study, of nineteen patients, nine had vesical involvement and ten had ureteric involvement. Among the vesical group, the success rate of transurethral resection followed by injection leuproide was 60% (3/5), while among the partial cystectomy group, the success rate was 100%. Among patients with ureteric involvement, success rate of distal ureterectomy and reimplantation was 100%, laparoscopic ureterolysis with Double J stenting followed by injection leuprolide was 75% while that of Gonadotropin- releasing hormone (GnRh) analogue alone was 67%.
Conclusion:
One should have a high index of suspicion with irritative voiding symptoms with or without hematuria, with negative urine culture, in all premenopausal women to diagnose urinary tract endometriosis. Partial cystectomy is a better alternative to transurethral resection followed by GnRh analogue in vesical endometriosis. Approach to the ureter must be individualised depending upon the severity of disease and dilatation of the upper tract to maximise the preservation of renal function.
Keywords: Laparoscopic ureterolysis, partial cystectomy, reimplantation, transurethral resection, ureteric endometriosis, vesical endometriosis
INTRODUCTION
Endometriosis is characterized by the presence of endometrial tissue in the ectopic foci outside the uterus. Most commonly, it affects organs like ovaries, uterine ligaments, fallopian tubes, rectum and cervico-vaginal regions, but involvement of the urinary tract is rare (1–2%),[1] among which 84% involves the bladder.[2] We report our experience of 19 patients of urinary tract endometriosis.
MATERIALS AND METHODS
Patients with urinary tract endometriosis between Jan 2006 and May 2010 were retrospectively reviewed. From the records, age of patients, mode of presentation, location and size of lesion, history of prior surgery, reproductive wishes, how they were diagnosed, what radiological imaging they had undergone and what treatment they had received were analyzed. Patients underwent evaluation by urinalysis, including cytology, urine culture and hematological and biochemical parameters. Ultrasonography-whole abdomen and contrast-enhanced computed tomography (CT)-pelvis were performed in each case. Each patient underwent cystoscopy to locate the site and size of the lesion and to look for ureteric involvement. Intraoperative retrograde ureteropyelography confirmed the level and degree of obstruction. In case of ureteral polypoidal mass, ureteroscopic biopsy confirmed the lesion. Thorough search for endometriosis in other pelvic organs was made by per speculum examination, bimanual examination and diagnostic laparoscopy.
Success rate, defined by total improvement of symptoms and no recurrence of lesion after the initial treatment, was reported.
RESULTS
Mean age of the patients was 30.16 (range: 27–38) years. Others parameters including the site and the size of lesion, prior surgery, reproductive wishes, mode of presentation, treatment received and follow up is illustrated in [Table 1]. In our study, nine patients had vesical involvement and ten had ureteric involvement. Among patients who had vesical involvement, two of them underwent open partial cystectomy and another two underwent laparoscopic partial cystectomy, while the remaining five underwent transurethral resection followed by injection leuprolide. Two patients who initially underwent transurethral resection developed recurrence at the previously resected site for which they underwent open partial cystectomy.
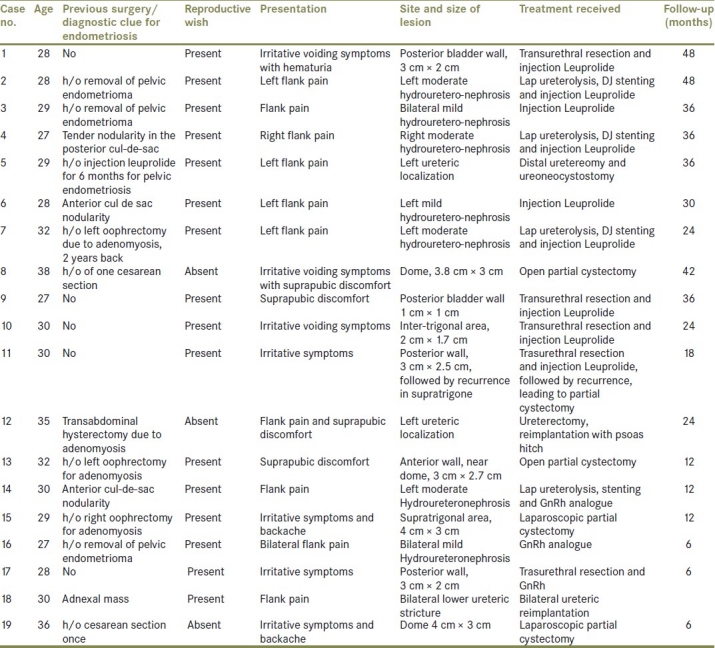
Table 1.
Patient profile and treatment received
Partial cystectomy, either open or laparoscopic, was performed in those cases where the lesion was either big or involved the dome or anterior wall of the bladder. Via an infraumblical midline extaperitoneal approach, open partial cystectomy was performed. The retropubic space was entered and the bladder was mobilised carefully. A small anterior cystotomy was made to inspect the endometrioma and, after applying stay suture in the margin of the resection, the endometrioma was excised and the bladder was closed in one layer using polyglactin 2-0 suture. A pelvic drain was inserted.
When laparoscopic partial cystectomy was planned, first via cystoscopy, bilateral ureteric catheterisation was done and a 16 F Foley catheter was inserted and was connected to the intravenous set with saline for controlling bladder volume intraoperatively. The patient was put in a supine position with 20 degree trendelenburg tilt. Laparoscopic partial cystectomy was performed with four ports–one supraumbilical 10 mm camera port, one 10 mm working port at the right lateral edge of the rectus in the midclavicular line, other 5 mm port at the left lateral edge of the rectus in the midclavicular line, each 1.5 cm below the umbilicus, and the fourth port (5 mm) was placed midway between the umbilicus and the pubic symphysis. Intraoperative cystoscopy helps in localisation of the lesion and in determining the probable line of cystotomy. Cystotomy is made by electrocautery. Once the edge of the lesion was seen, it was later completely excised. Bladder defect was closed with a continuous 2-0 polyglactin suture. The bladder was distended to reveal any leak. The suture line was reinforced with omentum. The specimen was delivered by enlarging the 10 mm port. The drain was inserted through one of the lateral ports. The drain was taken out after 48-72 hours. Cystogram was performed on the seventh postop day and the catheter was removed on the eighth day.
Among patients with ureteric involvement, three patients underwent distal ureterectomy and ureteric re-implantation by the modified Leich gregor technique. Two patients developed silent loss of renal function; one had diffuse pelvic endometriosis and bilateral hydrouretero-nephrosis with serum creatinine 3.2 mg%, who was initially treated with left Double J (DJ) stenting, right-sided per cutaneous nephrostomy because of inability to pass DJ stent on the right side. Once her creatinine dropped to normal, she underwent total abdominal hysterectomy with bilateral salpingo-oophrectomy. On further evaluation, she was found to have total ureteric obstruction for about 4 cm, about 1 cm from the Ureterovesical junction (UVJ), confirmed by antegrade nephrostogram. The Guide wire also could not be negotiated 1 cm beyond Vesicoureteric junction (VUJ), right-sided distal ureterectomy and ureteric reimplantation with psoas hitch was performed. Another patient presented with bilateral lower ureteric stricture with serum creatinine 1.6 mg%. This patient had right-sided lower ureteric stricture, 3 cm in length, about 2 cm above the UVJ, and left-sided lower ureteric stricture, 2.5 cm in length, about 1.5 cm from the UVJ, on retrograde pyelography. She underwent bilateral distal ureterectomy and ureteric reimplantation. The third patient presented with left flank pain with severe hydrouretero-nephrosis and left lower ureteric polypoidal mass, measuring 3 cm × 1 cm on CT scan. She initially underwent ureteroscopic biopsy, which revealed the presence of endometriod epithelium and endometrial stroma. Afterwards, she underwent resection of mass with distal ureterectomy and ureteric reimplantation.
In bilateral ureteric involvement, we used midline extraperitoneal infraumblical incision while in unilateral involvement, Gibson incision was used. Detrussor was incised over the postero-lateral aspect using electrocautery followed by opening of the bladder mocosa at the distal-most part of the incision by scissors. The ureter was spatulated and uretero-vesical anastomosis was performed using a 5-0 vicryl suture over the DJ stent. The edges of the detrussor muscle were approximated to create a submucosal tunnel. The repair was tested by bladder filling and visualisation of any leakage. A pelvic drain was used in all cases. The drain was removed when the drain output was less than 50 cc. DJ stent was removed after 6 weeks in each case. Urethral catheter was removed on the seventh postoperative day. We used this technique in our patients because it is easier to perform, there is no need to open the bladder and the procedure comprises lesser morbidity.
Three patients presented with flank pain with mild hydro-ureteronephrosis (two bilateral, one unilateral) received injection Leuprolide for 6 months, among whom one patient (unilateral disease) again became symptomatic 6 months after stopping the injection. This patient was evaluated by Intravenous urography (IVU) and intraoperative retrograde ureteropyelography, and was found to have a 2-cm-long lower ureteric stricture, 1 cm proximal to the UVJ, and was managed by the Leich Gregor technique.
Four patients, three left-sided moderate hydro-ureteronephrosis and one right-sided moderate hydro-ureteronephrosis, underwent laparoscopic ureterolysis and DJ stenting and received injection Leuprolide. DJ stent in all was removed after 2 months. One patient, 1 month after removing DJ stent, again became symptomatic.
Patients in whom contrast-enhanced CT showed soft tissue density mass around the ureter at the level of stenosis and significant back-pressure changes in the upper tract underwent laparoscopic ureterolysis. Preoperatively, all these patients had undergone DJ stenting. Although retroperitoneoscopy is frequently used for ureteral lesion, transperitoneal laparoscopy has an advantage for resection of ectopic endometriosis surrounding the ureter.
We used the transperitoneal laparoscopic ureterolysis approach. Four ports were placed–one paraumblical camera port, two secondary ports (10 mm and 5 mm) in the midclavicular line and a fourth port (5 mm) from the corresponding iliac fossa. After mobilising the colon, the first step consisted of opening the peritoneum overlying the ureter and then carefully blunt dissection was started where the ureter was clearly visible (above the level of the plaque and without the adhesions) and progressed in the direction of uterosacral ligaments until insertion into the bladder. At the end of the dissection, the ureter was completely mobilized and visible from the pelvic brim to its insertion into the bladder. In two patients, blueberry spots were clearly observed on the pelvic brim, the fibrous plaque surrounding the ureter was removed with peritoneal bleeding spots and sent for histopathology. After releasing the ureter completely, omentum was mobilised and wrapped around the ureter and the wrapped omentum was transfixed. DJ stent was removed after 2 months.
In our study, eight patients were infertile before treatment and five became pregnant. None of the patients developed any surgical complication, including bowel-related complications like prolonged ileus, wound infection and urinary tract infection.
Histopathology of vesical endometriosis showed endometrial glands and stroma within the bladder musculature. The excised strictured segments were consistent with intrinsic endometriosis. The resected ureteral polypoidal mass showed endometroid epithelium and stromal cells beneath the urothelium with no atypia. Endometriotic nature of all excised fibrotic plaques was confirmed histologically.
All operated patients were followed-up after 2 weeks and 1 month of surgery to assess subjective well-being. Afterwards, they were followed up 6 monthly during the first 2 years and annually thereafter, with clinical examination and transvaginal and transabdominal ultrasound scanning. Patients of vesical endometriosis also underwent cystoscopy 6 monthly during the first 2 years. The mean duration of follow-up in each group is shown in Table 2. None of the patients developed any deterioration of the upper tract. Among patients with vesical involvement who initially underwent transurethral resection, one patient developed recurrence at 6 months and another at 9 months, both of which were managed by open partial cystectomy. On follow-up, all patients with vesical endometriosis showed fairly good bladder capacity.
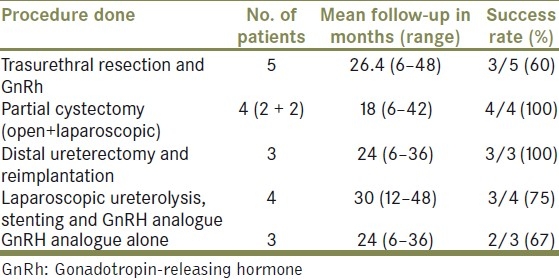
Table 2.
Follow-up and success rate in each group
DISCUSSION
Endometriosis usually occurs between menarche and menopause because of fluctuating levels of estrogen and progesterone required for the stimulation and propagation of endometrial proliferation. Urinary tract endometriosis is rare, and bladder is the most common site of involvement (84%), with the ureter being involved in 15–20%. In contrast to the literature, in our study, the bladder was involved in 9/19 (47%) cases while the ureter was involved in 10/19 (53%) cases. The peak incidence is around 30–40 years, and the patient is either nulliparous in over 50% or has had one or at most two children several years previously.
Several theories have been proposed to explain the pathogenesis of endometriosis – coelomic metaplastic, embryologic and migratory theories. No single theory can account for the location of the ectopic endometrium in all cases of endometriosis. Most cases can be explained by the migratory theory proposed by Sampson.[3] Retrograde menstruation of viable endometrial tissue through the fallopian tubes during menstruation can allow the endometrial glands to reach an ectopic position, e.g. bladder and ureter. Recently, Jaques Donnez et al.[4] reported that primary bladder endometriosis must be considered as a retroperitoneal adenomyotic nodule, which is the consequence of metaplasia of the Müllerian rests.
The classical presentations are cyclical urgency, frequency, suprapubic pain with or without hematuria and dyspareunia. Endometriosis should be suspected with such presentation with no documented infection in child-bearing age. Symptoms are commonly exaggerated during menses, because blood is contained within the involved organ or affected tissue, distending it and the surrounding peritoneum. Vesical endometriosis may present with variable symptoms and subtle onset, often mimicking recurrent cystitis. Although cyclical gross hematuria is pathognomonic for vesical endometriosis, it is only present in 20% of the patients.[5]
The literature revealed two distinct forms of vesical endometriosis – spontaneous and postoperative. In the former case, the bladder lesion is a manifestation of a generalized pelvic disease, whereas after iatrogenic dissemination, growth of the ectopic endometrium is usually limited to the bladder wall.
The ureter may be involved in 15–20% of the urinary tract cases. The left side is more commonly affected, and bilateral disease has been reported in up to 20–25% of the cases. Ureteric involvement may be either intrinsic or extrinsic. Extrinsic disease is more common than intrinsic disease. Patients with intrinsic endometriosis are more often symptomatic than those with extrinsic disease. Patients with ureteric endometriosis can present with loin pain, symptoms of “cystitis” and pelvic discomfort.[6] A significant proportion of patients with ureteral endometriosis do not have genitourinary symptoms, and may present with silent loss of renal function (25–43%).[7]
Although involvement of the ureter by endometriosis occurs in the setting of widespread pelvic disease, it may also occur without evidence of endometriosis elsewhere.[8] Clue to the presence of ureteral endometriosis includes non-specific flank pain or hematuria present or exaggerated at the time of menses, prior pelvic or abdominal surgery and pelvic mass and nulliparity.[9] Intraoperative retrograde ureteropyelography confirmed the level and degree of obstruction. In our study, involved areas were all located 1–2 cm from the uretero-vesical junction (UVJ), and were short to medium (2.5–4 cm) in length. Narrowed areas had a predilection for that segment of the pelvic ureter projecting within 3 cm of the inferior aspect of the sacroiliac joint, corresponding to the approximate location of posterior attachment of uterosacral ligament, and correlating well with known propensity of endometriosis to involve these ligaments both early and late.
Early diagnosis and treatment of urinary tract endometriosis are necessary to avoid the loss of kidney function.[10] Urinalysis with cytology examination is nonspecific for the diagnosis of urinary tract endometriosis.[11] Ultrasonography, especially endovaginal sonography, is more sensitive for diagnosis than CT or magnetic resonance imaging (MRI).[12,13]
Because of the small and intramural nature of bladder endometriosis, it is better to perform imaging like ultrasound during menstruation. Transabdominal ultrasonography was normal in non-menstruation days but it revealed a heteroechoic space occupying lesion on the posterior bladder wall during menstruation [Figure 1]. Intravenous urography, although nonspecific, is still useful to evaluate the integrity of the upper urinary tracts.[13] CT and MRI do not provide more information than ultrasonography.[14] CT-scan can provide size, depth and location of the endometrioma with varying degree of contrast enhancement [Figure 2].
Figure 1.
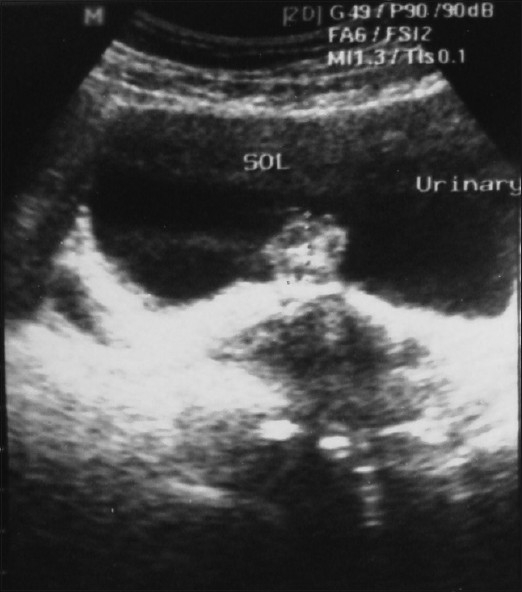
Ultrasonography showing heteroechoic space occupying lesion from bladder
Figure 2.
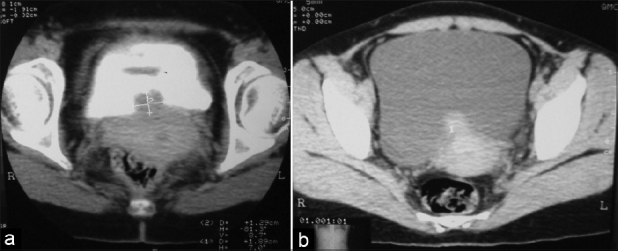
(a) Non contrast CT: Bladder mass with ill defined margin (b) Contrast enhanced CT: Bladder mass showing contrast enhancement
Cystoscopy and biopsy are the most valuable diagnostic procedures with vesical endometriosis.[15] Bladder endometriosis varies both cystoscopically and histologically during the menstrual cycle and diagnosis can therefore be difficult.[15] Cystoscopy performed during menses made lesions more evident. Premenstrually, there may be an increased elevation over the area of tumor surrounded by a congested, edematous mucosal membrane. As endometrial tumor increases in size during menstruation, cystic areas develop an intensified bluish hue [Figure 3]. The size of the mass or cysts and the color of the lesions increase prior to and during menstruation, only to regress in mid-cycle.
Figure 3.
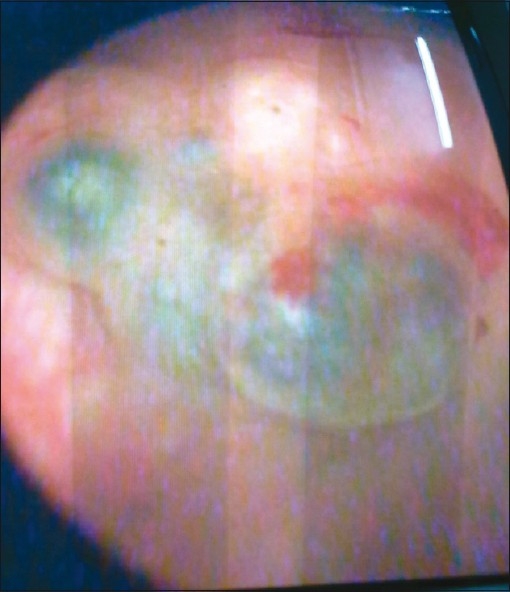
Cystoscopic appearance of bladder endometrioma showing bluish cystic areas over the tumor
The final diagnosis is made by the pathologist, who can confirm endometrial glands and stroma in the specimen [Figure 4]. The presence of typical endometrial stroma surrounding the glandular spaces is characteristic, but its absence does not rule out diagnosis. Histopathology sometimes poses difficulty in diagnosis.
Figure 4.
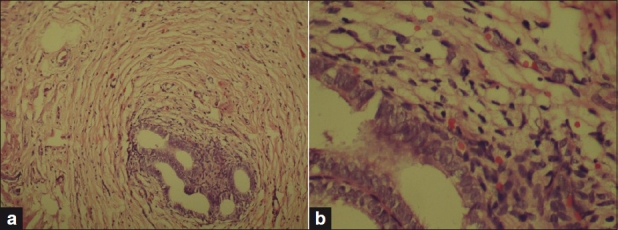
(a) Endometrial glands and stroma within bladder musculature (100× magnification), (b) same under 400× magnification (H and E staining)
Punch biopsy usually does not provide appropriate tissue for definite diagnosis. A positive punch biopsy definitively documents the presence of endometriosis, but a negative biopsy does not exclude it. Transurethral resection is usually diagnostic, but it is not recommended as a definite treatment, because any attempt at complete resection of the transmural involvement may result in bladder perforation.[15]
To determine the appropriate therapy for endometriosis, patient age, desire for reproduction, severity of symptoms, overall distribution of endometriosis and size of vesical lesion must be considered. Therapy for endometriosis includes bilateral oophorectomy, castration by drugs such as danazol or gonadotropin-releasing hormone agonists, as well as surgical resection of the lesion.[16]
Danazol is a derivative of 17α-ethinyl testosterone. It eliminates the mid-cycle surge of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), lowers basal levels of FSH and LH and suppresses ovarian steroidogenesis. As a result, estrogen and progesterone concentrations are decreased, thereby removing hormonal stimulation of endometriotic implants.[17] Benefits of danazol include pain relief and prevention of disease progression. Discontinuation of the drug frequently leads to recurrence of symptoms. For endometriosis of the urinary tract, treatment should be aimed primarily at elimination of the obstructive uropathy. Cases of diffuse endometriosis or large vesical lesions are more likely to need surgery for effective and definitive management.
When the bladder lesion is small and the symptoms are not debilitating, it is appropriate to use hormone manipulation as a first-time approach, especially in patients who have previously had children and desire some relief from symptoms before another pregnancy. Transurethral resection of vesical endometrioma followed by Gn-RH analogue is the preferred treatment, particularly in young patients where fertility should be preserved.
Apart from traditional surgery, laparoscopic partial cystectomy is a better choice if the surgeon is experienced enough, and it does not require ureteric transplantation. Nezhat et al. have performed the procedure laparoscopically with good surgical outcome.
If renal function is normal and there is minimal hydronephrosis with no functional obstruction, hormonal therapy may be prescribed. Rivlin et al. treated two of three patients with ureteric obstruction with injection Leuprolide for 6–9 months.[18] However, hormonal therapies cannot be recommend for obstructive uropathy because of lack of response of fibrotic tissue to hormone suppression.[19]
Ureterolysis may correct ureteral obstruction in patients with extrinsic disease. Choice between conservative and aggressive surgical approach depends more upon surgeon personal opinion rather on medical evidence. If laparoscopic ureterolysis is undertaken, the transperitoneal approach is preferable as it allows superior assessment of endometrial implants on the peritoneum overlying the ureter.[20] Our results with laparoscopic ureterolysis are consistent with previous reports that support the effectiveness of conservative laparoscopic strategy. Nezhat et al.[10] reported the resolution of ureteral obstruction in a series of 21 patients with severe ureteral endometriosis, 10 of which were operated with laparoscopic ureterolysis. Seracchioli et al.[21] successfully performed laparoscopic ureterolysis in 22 cases of 30 with ureteral endometriosis, whereas eight patients were treated either with open uretero-ureterostomy or with ureteroneocystostomy.
In case of intrinsic disease or cases in which ureterolysis fails or is unlikely to work, distal ureterectomy with reimplantation is reported to have excellent long-term results with regard to renal preservation.[22]
In view of the frequently prolonged diagnostic delay with related morbidity and erroneous treatments, we suggest a high index of suspicion of vesical endometriosis in all premenopausal women complaining of irritative urinary symptoms with negative urine cultures. Imaging and cystoscopy should be scheduled during or near a menstrual period to allow for the best chance of diagnosis. Open or laparoscopic partial cystectomy is a better alternative option in comparison with transurethral resection in vesical endometriosis. Ureteral endometriosis needs individualised treatment to maximise the preservation of renal function.
Footnotes
Source of Support: Nil,
Conflict of Interest: None.
REFERENCES
- 1.Westney OL, Amundsen CL, McGuire EJ. Bladder endometriosis: Conservative management. J Urol. 2000;163:1814–7. doi: 10.1016/s0022-5347(05)67550-7. [DOI] [PubMed] [Google Scholar]
- 2.Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1–6. doi: 10.1016/0090-4295(88)90560-2. [DOI] [PubMed] [Google Scholar]
- 3.Ridley JH. The validity of Sampson's theory of endometriosis. Am J Obstet Gynecol. 1961;82:777–82. doi: 10.1016/s0002-9378(16)36141-5. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Spada F, Squifflet J, Nisolle M. Bladder endometriosis must be considered as bladder adenomyosis. Fertil Steril. 2000;74:1175–81. doi: 10.1016/s0015-0282(00)01584-3. [DOI] [PubMed] [Google Scholar]
- 5.Batler RA, Kim SC, Nadler RB. Bladder endometriosis: Pertinent clinical images. Urology. 2001;57:798–9. doi: 10.1016/s0090-4295(00)01114-6. [DOI] [PubMed] [Google Scholar]
- 6.Patel A, Thorpe P, Ramsay JW, Shepherd JH, Kirby RS, Hendry WF. Endometriosis of the ureter. Br J Urol. 1992;69:495–8. doi: 10.1111/j.1464-410x.1992.tb15595.x. [DOI] [PubMed] [Google Scholar]
- 7.Stillwell TJ, Kramer SA, Lee RA. Endometriosis of the ureter. Urology. 1986;28:81–5. doi: 10.1016/0090-4295(86)90092-0. [DOI] [PubMed] [Google Scholar]
- 8.Fagan C. Endometriosis: Clinical and roentgenographic manifestations. Radiol Clin North Am. 1974;12:109–25. [Google Scholar]
- 9.Yates-Bell AJ, Molland EA, Pryor JP. Endometriosis of ureter. Br J Urol. 1974;44:58–67. doi: 10.1111/j.1464-410x.1972.tb12113.x. [DOI] [PubMed] [Google Scholar]
- 10.Nezhat C, Nezhat F, Nezhat CH, Nasserbakht F, Rosati M, Seidman DS. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996;66:920–4. [PubMed] [Google Scholar]
- 11.Schneider V, Smith MJ, Frable WJ. Urinary cytology in endometriosis of the bladder. Acta Cytol. 1980;24:30–3. [PubMed] [Google Scholar]
- 12.Savoca G, Trombetta C, Troiano L, Guaschino S, Raber M, Belgrano E. Ecographic. MRI and CT features in a case of bladder endometriosis. Arch Ital Urol Androl. 1996;68:193–96. [PubMed] [Google Scholar]
- 13.Fedel L, Bianchi S, Raffaelli R, Portuese A. Preoperative assessment of bladder ndometriosis. Hum Reprod. 1997;12:2519–22. doi: 10.1093/humrep/12.11.2519. [DOI] [PubMed] [Google Scholar]
- 14.Chapron C, Dubuisson JB, Jacob S, Fauconnieer A, Da Costa VM. Laparoscopy and bladder endometriosis. Gynecol Obstet Fertil. 2000;28:232–7. [PubMed] [Google Scholar]
- 15.Aldridge KW, Burns JR, Singh B. Vesical endometriosis: A review and two case reports. J Urol. 1985;134:539–41. doi: 10.1016/s0022-5347(17)47284-3. [DOI] [PubMed] [Google Scholar]
- 16.Nezhat CR, Nezhat FR. Laparoscopic segmental bladder resection for endometriosis. Obstet Gynecol. 1993;81:882–4. [PubMed] [Google Scholar]
- 17.Barbieri RL, Evans S, Kistner RW. Danazol in the treatment of endometriosis: Analysis of 100 cases with a 4-year follow-up. Fertil Steril. 1982;37:737–46. doi: 10.1016/s0015-0282(16)46331-4. [DOI] [PubMed] [Google Scholar]
- 18.Rivlin ME, Miller JD, Krueger RP, Patel RB, Bower JD. Leuprolide acetate in the management of ureteral obstruction caused by endometriosis. Obstet Gynecol. 1990;75:532–6. [PubMed] [Google Scholar]
- 19.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of the endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–69. [Google Scholar]
- 20.Watanabe Y, Ozawa H, Uematsu K, Kawasaki K, Nishi H, Kobashi Y. Hydronephrosis due to ureteral endometriosis treated by transperitoneal laparoscopic ureterolysis. Int J Urol. 2004;11:560–2. doi: 10.1111/j.1442-2042.2004.00828.x. [DOI] [PubMed] [Google Scholar]
- 21.Seracchioli R, Mabrouk M, Manuzzi L, Guerrini M, Villa G, Montanari G, et al. Importance of retroperitoneal ureteric evaluation in cases of deep infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15:435–9. doi: 10.1016/j.jmig.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Antonelli A, Simeone C, Frego E, Minini G, Bianchi U, Cunico SC. Surgical treatment of ureteral obstruction from endometriosis: Our experience with thirteen case. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:407–12. doi: 10.1007/s00192-004-1171-7. [DOI] [PubMed] [Google Scholar]


