Abstract
Ischemic stroke following viper bite is rare. We report a case of posterior circulation ischemic infarction following viper bite in a previously healthy woman. Soon after being bitten by the snake on the left leg, she developed local redness, echymosis and one hour later became drowsy. On examination she had skew deviation of eyes and down gaze preference, generalized hypotonia. A CT scan of brain showed infarcts in cerebellar hemispheres and occipital lobes on both sides and that was confirmed on magnetic resonance imaging of brain. Her coagulation profile was deranged. Most common and serious central nervous system complication following snake bite is intracranial hemorrhage. Ischemic stroke commonly involves anterior circulation. Bilateral cerebellar and occipital infraction is not yet reported in literature. Exact cause for the development of infarction is not clear. The possible mechanisms of infarction in this scenario are discussed. Patient was treated with anti-snake venom and showed a good recovery. Early imaging and early treatment with anti-snake venom is important for a favorable outcome.
Keywords: Viper envenomation, ischemic stroke, posterior circulation
Introduction
Snake bites are common cause of morbidity and mortality in India. On an average, 200 000 fall prey to snake bite per year in India, and an estimated mortality may range from 20 000 to 50 000 cases. Viperidae species consisting of Russell's viper (Daboia russelli) and saw scaled viper (Echis carinatus) are the leading cause of fatal snake bite in India.[1–3] The common clinical characteristics of viper bite include local cellulites, renal failure, and systemic hemorrhagic manifestation. Neurological deficit following viper bite is not uncommon and is usually due to intracerebral or subarachnoid bleed. Ischemic infarction following viper envenomation has been described by only few authors.[3–7] In majority of the cases reported, ischemic infarction involved the anterior circulation. Korean viper bite resulting in brain stem infarction has also been reported.[8] We report a posterior circulation infarct involving bilateral cerebellum and occipital lobe following Russell's viper bite.
Case Report
A 40-year-old healthy woman was admitted with history of snake bite on her left foot. The incident occurred while she was working in the paddy field. The snake was identified as Russell's viper, as per the descriptions given by relatives. A few minutes after the bite, she noticed redness and ecchymoses over the left foot. One hour later, her sensorium deteriorated and she became very drowsy. There was no history of convulsion or any bleeding manifestations. She was transported to local hospital immediately and the blood pressure recorded at the hospital was normal. She was treated with three vials of polyvalent anti-snake venom (ASV) and referred to our hospital.
At the time of admission, her pulse rate was 84 beats per minute, blood pressure was 110/74 mmHg, and respiratory rate was 20 per minute. Local examination showed two deep bite marks with surrounding redness, edema, and cellulites over the dorsum of left foot. There was an ecchymotic patch over the left thigh. Neurological examination revealed semiconscious state with a Glasgow coma scale of 9/15. Her pupils were 2 mm bilaterally with normal light reflex. She had downward gaze preference and skew deviation of both the eyes. Examination of ocular fundus was normal. She had hypotonia of all the four limbs with bilateral brisk deep tendon reflexes and bilateral extensor plantar response. She had no focal neurological deficits and flexion withdrawal of all the four limbs was noticed to a painful stimulus. Detailed motor examination and coordination tests could not be done due to altered mentation. Examinations of all other systems were normal. Her hemoglobin was 8.8 gm/dl, total leukocyte count was 20 500/mm3 with 90% neutrophils, platelet count was 195 000/mm3, creatine kinase was 5850 units/l, blood urea was 24 mg/dl, serum creatinine was 1.0 mg/dl, and serum electrolytes and liver function tests including liver enzymes were normal. Coagulation profile revealed prothrombin time (PT) of 47 sec (control 15 sec), activated thromboplastin time of 46 sec (control 28.2 sec). Her fibrinogen degradation product and D-dimer were positive. Her urine examination showed microscopic hematuria without hemoglobinuria. Her renal functions remained normal throughout the hospital stay. Peripheral smear showed microcytic hypochromic picture with neutrophilic leukocytosis.
Her serum iron and ferritin levels were low with high iron binding capacity, suggesting pre-existing chronic iron deficiency anemia. Serum folate and vitamin B12 levels were normal. Electrocardiogram was normal. Brain computed tomography scan showed bilateral cerebellar and both occipital lobe hypo-intensities, suggesting infarction. Magnetic resonance imaging of brain showed hypo-intensities in both lobes of cerebellum, vermis, cerebellar tonsils, and both occipital lobes on T1, and hyper intensities on T2 weighted images [Figures 1 and 2].
Figure 1.
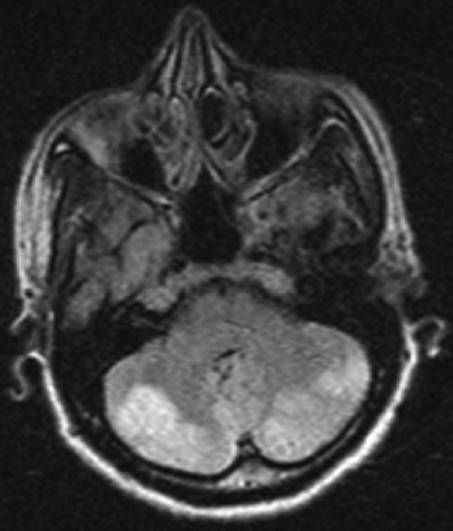
MRI brain (STIR axial) showing bilateral cerebellar infarct
Figure 2.
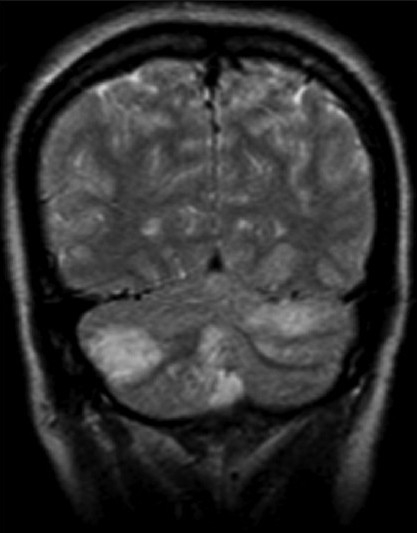
MRI brain (T2W) coronal images showing bilateral cerebellar and occipital infarct
She was immediately treated with equine polyvalent ASV (Bharat Serum and Vaccines Ltd. ASV – ASIA, India, Thane). After an initial test dose, continuous infusion of ASV was administered for four days. A total of 24 vials of ASV were administered during the stay. Her coagulation parameters were monitored regularly and they normalized by third day. She was also treated with broad spectrum antibiotics, anti-edema measures, and plasma transfusion. An injection of adsorbed tetanus toxoid was given. Her 2D and color Doppler echocardiography was normal. Carotid and vertebral Doppler did not show atherosclerotic stenosis or luminal irregularities. Magnetic resonance angiography did not reveal any abnormality. tests for lupus anticoagulant and anticardiolipin antibodies were negative. Her lipid profile, protein C, S and antithrombin III levels were normal. Over a period of three weeks, she showed gradual improvement in her sensorium and her muscle power recovered to almost normal. At the time of discharge, she had a significant ataxia of gait, for which she required assistance.
Discussion
The manifestations following viper bites depend upon the severity of envenomation. In cases of minimal envenomation, local swelling, ecchymoses, and blisters will be noticed. In case of severe envenomation, local signs will be profound with tissue necrosis and systemic manifestations. Systemic complications are primarily related to bleeding due to depletion of fibrinogen and clotting factors. This is manifested by hemorrhage in different parts of the body including gums, nose, central nervous system, gastrointestinal and urinary tract bleeding. Hemorrhagins and hemolysins present in snake venom destroy the walls of blood vessel and cause coagulation defect. Myoglobinuria from muscle destruction can cause renal failure. Cardiotoxins present in venom may lead to arrhythmias, decreased cardiac contractility, and hypotension. The neurological feature of viper bite includes drowsiness, confusion, fainting, dizziness, blurred vision, loss of muscle coordination, and convulsions.[8,9]
Cerebral complications, particularly ischemic infarction after viper bite is rare. Ischemic stroke has been reported by few authors[3–6] reported ischemic infarction in middle cerebral artery territory following viper bite. Brainstem stroke had been reported following Korean viper bite[8] and envenomation from Bathrops lanceolatus that is found only in Matinique[10] To the best of our knowledge, bilateral cerebellar and occipital infraction following viper bite is not yet reported.
Viper snake venom is a complex toxin with rich components affecting hemostatic mechanisms. Most of the viper venom exhibit both anticoagulant and coagulant effects. In large doses, venom can cause massive intravascular coagulation, leading to occlusion of small and even large vessels resulting in cerebral infarction. Toxic vasculitis caused by certain viper species may result in thrombosis.[9] Bashir and Jinkins suggested direct action of the venom on vascular endothelial cells. Hemorrhagins, the complement-mediated toxic components of Viperidae snake venom result in severe vascular spasm, endothelial damage, and increased permeability, all of which may contribute to vascular occlusion.[10] Hypercoagulation due to procoagulants in venom, such as arginine, esterase, and hydrolase, and hyperviscosity caused by hypovolemia and hypoperfusion secondary to hypotension may also contribute to vessel occlusion.[3,9] Our patient was relatively young and had no premorbid illness. Carotid and vertebral Doppler did not show evidence of atherosclerosis. Echocardiography was negative for cardiogenic emboli, and autoimmune disease workup was negative. The infarction typically involved the arterial territory of the posterior cerebral circulation. The pathogenesis of cerebral infarction is not clear. Hypotension as the cause of cerebral infarction was not considered, as the infarction did not involve the typical watershed zone and her blood pressure recordings were normal. Occurrence of cerebral infarction has been reported without any coagulation and platelet abnormalities.[6] Prolonged PT and activated partial thromboplastin with positive fibrin degradation product and D-dimer suggested disseminated intravascular coagulation, which might be the cause of infarction in our patient. In most of the cases, infarction is multifactorial. Two factors which are mainly responsible are toxin-mediated coagulation abnormality and damage to vascular endothelium. Altered sensorium following an hour after the bite is probably related to direct arterial endothelial injuries caused by the venom itself. The diffuse cerebral disturbances may be caused by toxic encephalopathy due toxins in the venom. Occasionally, cerebral infarction may be unrelated to bite and could be due to underlying medical illness. Early administration of ASV reduces the thrombotic complications. In a study by Thomas et al.,[10] thrombotic complications were not noticed if the ASV was administered within six hours. Despite early administration of ASV, our patient devolved significant neurological problems. In conclusion, we reported a case of posterior circulation ischemic stroke following viper envenomation. Even though the occurrence of ischemic stroke is well known, exact pathogenesis remains unclear. Early imaging with work up for other possible causes of infarction is emphasized.
Footnotes
Source of Support: Nil,
Conflict of Interest: Nil.
References
- 1.David AW. New Delhi: World Health Organisation, Regional Office for South-East Asia region; 2005. Guidelines for the clinical management of snake-bites in South-East Asia region; pp. 1–77. [Google Scholar]
- 2.Brunda G, Sashidhar RB. Epidemiological profile of snake-bite cases Andhra Pradesh using immunoanalytical approach. Indian J Med Res. 2007;125:661–8. [PubMed] [Google Scholar]
- 3.Narang SK, Paleti S, Azeez Asad MA, Samina T. Acute ischemic infarct in the middle cerebral artery territory following a Russell's viper bite. Neurol India. 2009;57:479–80. doi: 10.4103/0028-3886.55594. [DOI] [PubMed] [Google Scholar]
- 4.Panicker JN, Madhusudanan S. Cerebral Infarction in a Young Male Following Viper Envenomation. J Assoc Phys Ind. 2000;48:744–5. [PubMed] [Google Scholar]
- 5.Murthy JM, Kishore LT, Shanti Naidu K. Cerebral infarction after envenomation by viper. J Comput Assist Tomogr. 1997;21:35–7. doi: 10.1097/00004728-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bashir R, Jinkins J. Cerebral infarction in a young female following snake bite. Stroke. 1985;16:328–30. doi: 10.1161/01.str.16.2.328. [DOI] [PubMed] [Google Scholar]
- 7.Kurian J, Thomas M, Krishna Das KV. Stroke following snake bite. J Assoc Phys Ind. 1989;37:38. [Google Scholar]
- 8.Byung-chul Lee, Sung-Hee Hwang, Jae-Chun Bae, Seok-Bum Kwon. Brainstem infarction following Korean viper bite. Neurology. 2001;56:1244–5. doi: 10.1212/wnl.56.9.1244. [DOI] [PubMed] [Google Scholar]
- 9.Boviatsis EJ, Kouyialis AT, Papatheodorou G, Gavra M, Korfias S, Sakas ES. Multiple hemorrhagic brain infarcts after viper envenomation. Am J Trop Med Hyg. 2003;68:253–7. [PubMed] [Google Scholar]
- 10.Thomas L, Tyburn B, Bucher B, Pecout F, Ketterle J, Rieux D, et al. Prevention of thrombosis in human patients with Bothrops lanceolatus envenoming in Martinique : f0 ailure of anticoagulant and efficacy of a monospecific antivenom. Am J Trop Med Hyg. 1995;52:419–26. doi: 10.4269/ajtmh.1995.52.419. [DOI] [PubMed] [Google Scholar]


