Abstract
Leptin is an adipose-derived hormone that regulates a wide variety of physiological processes, including feeding behavior, metabolic rate, sympathetic nerve activity, reproduction, and immune response. Circulating leptin levels are tightly regulated according to energy homeostasis in vivo. Although mechanisms for the regulation of leptin production in adipocytes are not well understood, G protein-coupled receptors may play an important role in this adipocyte function. Here we report that C2–C6 short-chain fatty acids, ligands of an orphan G protein-coupled receptor GPR41, stimulate leptin expression in both a mouse adipocyte cell line and mouse adipose tissue in primary culture. Acute oral administration of propionate increases circulating leptin levels in mice. The concentrations of short-chain fatty acids required to stimulate leptin production are within physiological ranges, suggesting the relevance of this pathway in vivo.
Leptin is a circulating hormone secreted primarily by white adipose tissue, an organ that functions not only in energy storage but also as an important site for the production of endocrine factors (1). Among the many known diffusible factors produced by the adipose tissue, leptin is of considerable importance, because of its intimate involvement in energy homeostasis and many other physiological processes. Failure of leptin signaling is associated with numerous defects in mice (1–3), rats (4), and humans (5, 6), including severe obesity, hyperphagia, infertility, and immunological defects. Exogenous leptin administration reduces food intake and body weight and increases energy expenditure in both wild-type and leptin-deficient ob/ob mice (7, 8). Administration of leptin to mice also blunts neuroendocrine response to fasting, accelerates sexual maturation, and reverses starvation-induced immune suppression (9–11). Circulating leptin levels are tightly regulated by energy balance. Leptin levels positively correlate with adiposity and are believed to serve as an index of body fat stores (12). Fasting dramatically decreases leptin levels, whereas refeeding restores them. Injection of insulin to fasted animals increases leptin production in adipose tissue, suggesting that postprandial insulin surges play a role in the regulation of leptin production (13). Several other factors have also been documented to regulate leptin gene expression in adipocytes, including peroxisome proliferator-activated receptor γ activators (14), catecholamines (15), glucocorticoids (16), cytokines (17), UDP-N-acetylglucosamine (18), adenosine (19), and endothelins (20).
Among the above regulators, catecholamines, adenosine, and endothelins all act by means of cognate G protein-coupled receptors (GPCRs) expressed in adipocytes. GPCRs are seven-transmembrane receptors that activate heterotrimeric G proteins during ligand binding. The human genome project has resulted in the discovery of many “orphan receptors” for which cognate ligands have yet to be identified. Because GPCRs play pivotal roles in a variety of physiological processes and have proven to be excellent therapeutic targets, great efforts have been put into ligand discovery for orphan GPCRs. GPR41 is one such orphan GPCR, cloned during a search for novel galanin receptor subtypes (21). Here we report that short-chain fatty acids (SCFAs) are specific agonists of GPR41, confirming an independent study by another group that was recently published (22). We further demonstrate that GPR41 is expressed in adipose tissue and SCFAs stimulate leptin production both in cultured adipocytes and in the whole animal. SCFAs are a major product of food digestion and the primary energy source in ruminants. In humans and other omnivorous mammals, SCFAs are also generated in large amounts primarily by fermentation of dietary fibers in the lower intestine, and they contribute significantly to caloric intake (23). Our findings suggest that SCFAs also function as signaling molecules, which may serve as one of the factors that regulate leptin production in accordance with dietary intake.
Materials and Methods
Chemical Compounds. All compounds tested for GPR41 agonist activity were purchased from Sigma. Medium-chain and long-chain fatty acids (≥C10) were dissolved in 100% ethanol to make 10 mM stock solutions. Other compounds were dissolved or diluted with H2O to make 100 mM stock solutions. Stock solutions were serially diluted with culture medium to obtain final concentrations in the assay.
Xenopus Melanophore Culture and Pigment Aggregation Assay. Culture of the Xenopus melanophore, DNA and transfection, and the pigment aggregation assay were performed exactly as described (24). The coding sequence of human GPR41 cDNA (GenBank accession no. AF024688) was cloned into the pCDNA3.1 expression vector.
cAMP Determination in CHO-K1 Cells. CHO-K1 cells were transfected with expression plasmids by using Lipofectamine 2000 (GIBCO/BRL). For the cAMP assay, cells were pretreated with 1 mM 3-isobutyl-1-methylxanthine for 20 min, then incubated with SCFAs for 10 min, and finally stimulated with 50 μM forskolin in the presence of SCFAs for 5 min. The cAMP assay of cell lysates was performed with a cAMP enzyme immunoassay system by following the manufacturer's instructions (Amersham Pharmacia). The coding sequence of mouse GPR41 cDNA (GenBank accession no. AC087143) was subcloned into the pCDNA3.1 expression vector.
Ob-Luc Cells and Luciferase Assay. Culture of Ob-Luc cells and luciferase assay were performed exactly as described (20).
Primary Adipose Tissue Culture and Leptin Immunoassay. Epididymal fat was taken from 7-week-old male Swiss mice (Taconic Farms), finely minced, suspended at 100 mg of tissue per ml in low-glucose DMEM, and then cultured at 37°C under 5% CO2/95% air (GIBCO/BRL). After 1–12 h, culture medium was replaced with fresh medium containing appropriate ligands. Conditioned medium was taken for leptin immunoassay (R & D Systems) 24 h later.
RT-PCR. Total RNA was extracted from mouse tissues by using RNA STAT-60 (Tel-Test, Friendswood, TX) and treated with RNase-free DNase (Roche). cDNA was synthesized with the ImProm-II system (Promega). A pair of primers amplified the entire coding region of mouse GPR41: 5′-TGACCATGGGGACAAGCTTC-TGACCATGGGGACAAGCTTC-3′ and 5′-ACTAGCTCGGACACTCCTTG-3′.
Retroviral Infection. Recombinant retroviral infection was performed with the RetroMax system (Imgenex, San Diego) by following the manufacturer's instructions. Briefly, 293-EBNA cells were cotransfected with the PCL-10A1 package plasmid and an empty pQCXIP expression vector or the vector containing mouse GPR41 cDNA. Undifferentiated Ob-Luc cells were infected with virus supernatant at 3-fold dilution, selected with 30 μg/ml puromycin for three passages, and differentiated as described (20).
RNA Interference. The retrovirus-mediated RNA interference study was performed with the pSuper RNA interference system (Oligoengine, Seattle) by following the manufacturer's instructions. Briefly, each pair of 64-nt oligonucleotides were synthesized, annealed, and ligated into pSuper.Retro vector. Within the 64-nt oligonucleotides, a 19-nt target sequence is included in both sense and antisense orientation, separated by a 9-nt spacer sequence. 293-EBNA cells were cotransfected with the PCL-10A1 package vector and pSuper.Retro vector containing inserts. Ob-Luc cells were either infected with individual virus supernatant or coinfected with all three virus supernatants containing sequences targeting different regions of GPR41 cDNA, selected with 10 μg/ml puromycin for three passages, and then differentiated (20). The target sequences in GPR41 cDNA are 5′-TGGAACCTGCTACCTGGAA-3′, 5′-CTCTTGTATCGACCCCCTG-3′, and 5′-ACCTGACCATTTCGGACCT-3′. The negative control sequence was based on the Drosophila rhodopsin cDNA: 5′-ACGGAAGTTGCTGACCGG-3′.
Isolation of Mouse Adipocytes and cAMP Quantification in Adipocyte Culture. Isolation of mouse adipocytes was performed as described with slight modifications (20). Mouse epididymal fat pads were dissected from 7-week-old male Swiss mice; the fat was then minced and digested. Dispersed adipose tissues were filtered through 500-μm nylon mesh (Small Parts, Miami) and centrifuged at 500 × g for 2 min. Fat cells were washed twice with Krebs–Ringer Hepes buffer and once with low-glucose DMEM. Packed fat cells (100 μl) were transferred into 1.5-ml tubes containing 400 μl of low-glucose DMEM with appropriate ligands and cultured at 37°C under 5% CO2/95% air for 15 min. Each reaction was then mixed with 500 μl of extraction buffer (160 μl of methanol/320 μl of chloroform/20 μl of 5 M HCl) and centrifuged at 11,000 × g for 5 min. The supernatant was then taken for cAMP immunoassay (R & D Systems).
Oral Administration of Propionate. Ten-week-old C57Bl6/J male mice (The Jackson Laboratory) were fed ad libitum and gavage-administered with water in the early light phase daily for 1 week. On day 8, these mice were gavage-administered with 200 μl of 2.5 M sodium propionate solution (pH 7.0) or an equimolar amount of sodium chloride solution instead of water. Animals were killed 7 h later. Epididymal fat pads were then dissected and weighed. Plasma propionate concentrations were determined by HPLC after chemical derivatization as described (25). Plasma leptin levels were measured by mouse leptin immunoassay (R & D Systems). For oral administration of hexanoate, mice were gavage-administered with 400 μl of 1.25 M sodium hexanoate solution (pH 7.0) or an equimolar amount of sodium chloride solution.
Statistical Analysis. Statistical analysis was performed with one-way ANOVA followed by a post hoc Dunnett's test.
Results
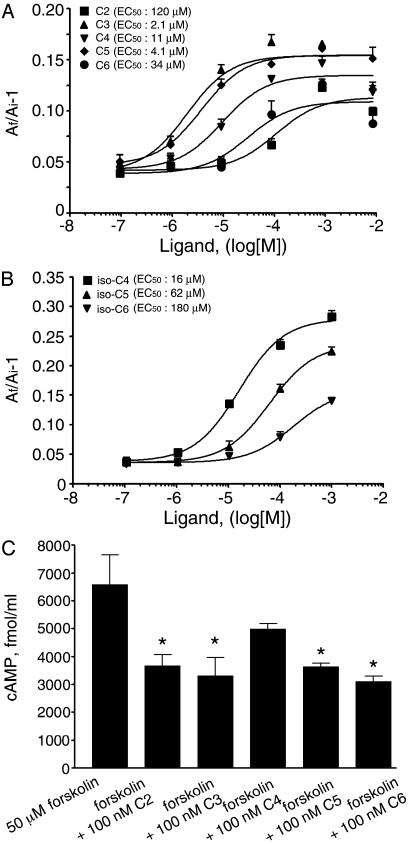
SCFAs Activate Human GPR41 Expressed in Xenopus Melanophores. In the course of ligand screening for orphan GPCRs by using the Xenopus melanophore assay system (26), we unexpectedly found that acetic acid, used as a solvent for certain peptide ligands, activates the human GPR41. This finding led us to recognize that other SCFAs and their various salts also activate GPR41. SCFAs (C2-C6) induce pigment aggregation in melanophores transfected with human GPR41 cDNA, with EC50 values of 120, 2.1, 11, 4.1, and 40 μM for C2, C3, C4, C5, and C6 straight-chain fatty acids, respectively (Fig. 1A). Interestingly, C3 and C5 are consistently more potent than other SCFAs in this assay, indicating a peculiar potency rank order. We also screened a group of molecules structurally or metabolically related to SCFAs for their abilities to activate GPR41 in melanophore cells (see Table 1, which is published as supporting information on the PNAS web site). Among the compounds we screened, only C4–C6 branched-chain fatty acids are active in addition to C2–C6 SCFAs (Fig. 1B). These results suggest that activation of GPR41 specifically requires simple short-chain monocarboxylates.
Fig. 1.
SCFAs activate GPR41. (A) Dose–response curves of C2–C6 straightchain fatty acids in stimulating pigment aggregation in Xenopus melanophore cells transfected with human GPR41 cDNA. (B) Dose–response curves of C4–C6 branched-chain fatty acids in stimulating pigment aggregation. (C) Reduction of cAMP levels in response to SCFA in CHO-K1 cells expressing GPR41. *, P < 0.01 compared with cells treated with forskolin only. Experiments were performed in quadruplicate in A and B and triplicate in C. Error bars represent SEM.
SCFAs Activate the Gi Pathway Through GPR41 Expressed in CHO-K1 Cells. Activation of the Gi pathway in melanophores induces pigment aggregation. The fact that SCFAs stimulate pigment aggregation suggests that the human GPR41 is a Gi-coupled receptor. We identified and cloned the mouse homolog of GPR41 from the GenBank database (accession no. AC087143). The mouse GPR41 shares 75% identity with the human receptor in both nucleic acid and amino acid sequences. To determine whether the mouse GPR41 couples to the Gi protein in mammalian cells, the effect of SCFAs on forskolin-induced cAMP production was examined in CHO-K1 cells transfected with the mouse GPR41 cDNA. As expected, C2–C6 SCFAs suppress forskolin-induced cAMP production in CHO-K1 cells (Fig. 1C). In contrast, CHO-K1 cells transfected with an unrelated GPCR, human endothelin-B receptor, do not respond to SCFAs (data not shown).
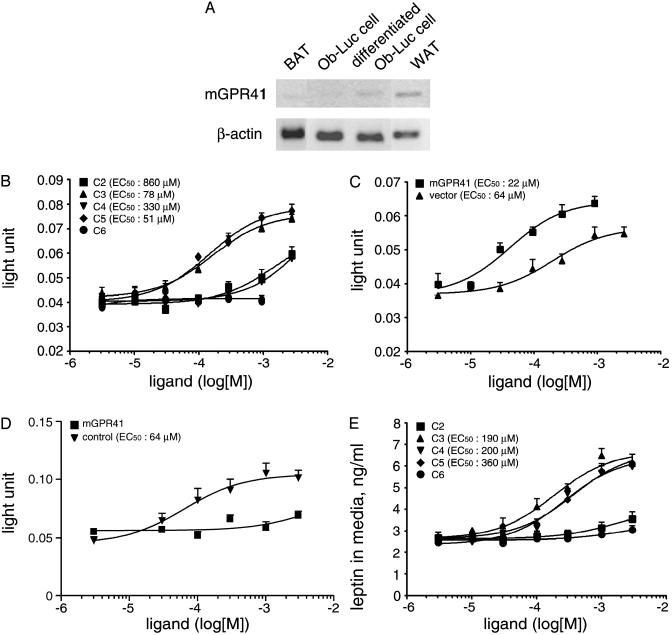
GPR41 Is Expressed in White Adipose Tissue and SCFAs Stimulate Leptin Production in Adipocytes Through GPR41. By using RT-PCR analysis, we observed that GPR41 mRNA is highly expressed in human adipose tissues (data not shown). RT-PCR analysis showed that GPR41 is expressed in mouse white adipose tissue and a mouse adipogenic cell line Ob-Luc cells following differentiation, but it is barely detectable in brown adipose tissue and undifferentiated Ob-Luc cells (Fig. 2A). Control reactions without reverse transcriptase do not produce detectable signals.
Fig. 2.
SCFAs stimulate leptin production in adipocytes. (A) RT-PCR analysis demonstrating expression of mouse GPR41 mRNA in white adipose tissue and Ob-Luc cells: from left to right, brown adipose tissue (BAT), undifferentiated Ob-Luc cells, differentiated Ob-Luc cells, white adipose tissue (WAT). (B) Dose–response curves of C2–C5 SCFAs in stimulating the leptin locus in Ob-Luc cells. Luciferase activity was presented as luminescent light units. (C) Dose–response curves of propionic acid in stimulating the leptin locus in Ob-Luc cells infected with control virus or virus containing mouse GPR41 cDNA. (D) Dose–response curves of propionic acid in stimulating the leptin locus in Ob-Luc cells infected with virus containing unrelated control sequence or virus supernatants containing siRNA sequences targeting three different regions of GPR41 cDNA. (E) Dose–response curves of SCFAs in increasing leptin produced from mouse adipose tissues in primary culture. Experiments were performed in quadruplicate in B and with n = 6 in C–E. Error bars represent SEM.
The observation that GPR41 is expressed in the white adipose tissue prompted us to study the effects of SCFAs on leptin expression by using the Ob-Luc cell line. Ob-Luc cells have one allele of leptin gene knocked in with a luciferase cassette, allowing leptin gene expression to be monitored by luciferase assay (20). SCFAs increase the luciferase activity in differentiated Ob-Luc cells, with EC50 values of 860, 78, 330, and 51 μM for C2, C3, C4, and C5 fatty acids, respectively (Fig. 2B).
To examine whether the stimulatory effects of SCFAs on the leptin locus are indeed mediated by GPR41, we first overexpressed the mouse GPR41 cDNA in Ob-Luc cells by retrovirus-mediated gene transfer. Overexpression of mouse GPR41 in infected cells was confirmed by Northern blot analysis (data not shown). The EC50 value of propionic acid in stimulating luciferase activity is 64 μM in Ob-Luc cells infected with control virus and 22 μM in cells infected with virus containing GPR41 cDNA (Fig. 2C).
We next performed retrovirus-mediated RNA interference study by infecting Ob-Luc cells with virus containing short interfering RNA (siRNA) sequences targeting different regions of the GPR41 mRNA. RT-PCR demonstrated a marked reduction of GPR41 mRNA levels in Ob-Luc cells after infection with the GPR41 siRNA viruses but not with the control virus containing a Drosophila rhodopsin siRNA sequence (data not shown). Although the response to propionic acid remained intact in Ob-Luc cells infected with control virus, coinfection with virus supernatants containing sequences targeting three different regions of GPR41 mRNA almost completely abolished the stimulatory effect of propionic acid (Fig. 2D). Infection with individual stocks of virus containing siRNA sequence no. 1 or no. 2 also significantly decreased the response of Ob-Luc cells to 1 mM propionic acid by 68% and 69%, respectively (data not shown). These observations indicated that the effect of propionic acid inOb-Ob-Luc cells is mediated by GPR41.
Furthermore, in primary cultures of mouse white adipose tissue, SCFAs increased the level of leptin secreted into culture medium with EC50 values of 190, 200, and 360 μM for C3, C4, and C5 fatty acids, respectively (Fig. 2E). C2 and C6 fatty acids were barely active at high concentrations in this assay.
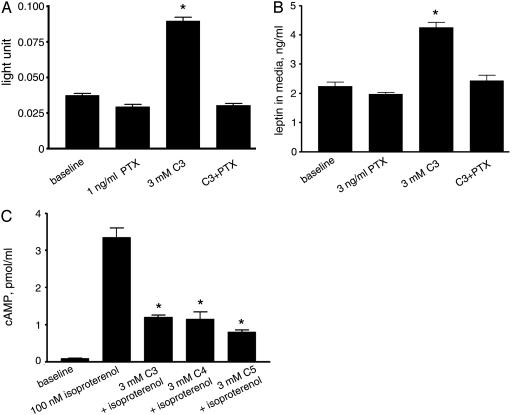
SCFAs Stimulate Leptin Production Through the Gi Pathway. Because we found that GPR41 activates the Gi pathway in both melanophores and CHO-K1 cells, we examined whether the stimulation of leptin production by SCFAs in adipocytes is inhibited by pertussis toxin treatment, which specifically inactivates the Gi pathway. Pertussis toxin pretreatment completely abolishes the stimulatory effect of propionic acid in Ob-Luc cells (Fig. 3A) and mouse adipose tissue in culture (Fig. 3B), suggesting that the effects of SCFAs in adipocytes are mediated by activated Gi proteins.
Fig. 3.
Stimulatory effect of propionate in adipocytes is mediated by the Gi pathway. (A) Stimulation of the leptin locus by propionic acid in Ob-Luc cells pretreated with or without pertussis toxin. *, P < 0.01 compared with baseline. (B) Response of native mouse adipose tissues to propionic acid after treatment with or without pertussis toxin. *, P < 0.01 compared with baseline. (C) Inhibition of isoproterenol-induced cAMP accumulation by SCFAs in adipocytes. *, P < 0.01 compared with cells treated with forskolin only. Experiments were performed in quadruplicate. Error bars represent SEM.
Adipocytes express high levels of β-adrenergic receptors, and activation of the Gs pathway by the β-adrenergic agonist isoproterenol increases intracellular cAMP levels (27). We examined whether SCFA-induced Gi activation in native adipocytes counteracts isoproterenol-induced elevation of cAMP. Although the basal level of cAMP in the adipocyte preparation is low, isoproterenol (100 μM) markedly increases cAMP levels, and coadministration of 3 mM C3, C4, or C5 SCFAs decreases the cAMP levels by ≈70% (Fig. 3C).
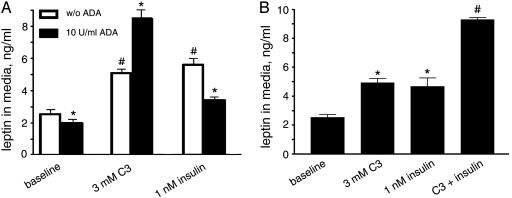
Effect of Propionic Acid Is Modulated by Adenosine and Insulin. Adipocytes produce adenosine, which acts as an autocrine factor through the Gi-coupled A1 receptor present in these cells (28). Addition of adenosine deaminase to the medium depletes adenosine released by adipose tissue in primary culture (29). The basal level of leptin production in primary cultures of native adipose tissue is reduced in the presence of 10 units per ml adenosine deaminase, in accordance with a previous report showing that adenosine is an activator of leptin production (19). However, rather than being diminished, the response of adipose tissue to propionic acid is further enhanced by adenosine deaminase treatment, implying that the Gi pathway is desensitized by the constant presence of autocrine adenosine, whereas depletion of adenosine resensitizes this pathway on which propionate/GPR41 works (Fig. 4A).
Fig. 4.
Stimulatory effect of propionic acid on leptin production is modulated by adenosine and insulin. (A) Leptin levels in conditioned medium from mouse adipose tissue cultures treated with propionic acid or insulin in the presence or absence of adenosine deaminase. *, P < 0.05 compared to corresponding experiments without adenosine deaminase treatment. #, P < 0.01 compared to corresponding experiments without treatment of propionic acid or insulin. (B) Leptin levels in conditioned medium from mouse adipose tissue cultures in the presence of propionic acid, insulin, or both together. *, P < 0.01 compared with baseline. #, P < 0.01 compared with cultures treated with propionic acid or insulin alone. Experiments were performed in quadruplicate. Error bars represent SEM.
Insulin signaling and G protein signaling have been reported to affect each other, suggesting crosstalks between these pathways (30). Moreover, at the whole-animal level, insulin sensitivity is augmented in guanine nucleotide-binding regulatory protein-deficient mice and reduced in Gi-deficient animals (31, 32). We observed that adenosine deaminase treatment reduces the stimulatory effect of insulin on leptin production in the adipose tissue in primary culture (Fig. 4A). Furthermore, propionic acid and insulin display more than additive effects in stimulating leptin production when coadministered (Fig. 4B). Taken together, these findings suggest that adenosine and SCFAs use the same Gi pathway in adipocytes, and insulin communicates with this pathway in stimulating leptin production.
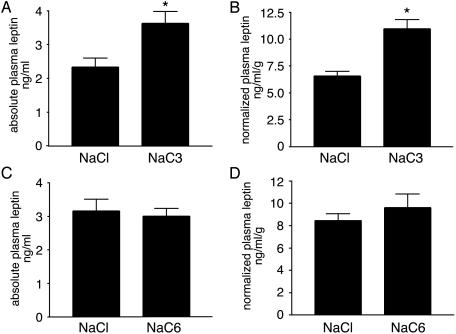
Oral Administration of Propionate Increases Circulating Leptin Levels in Vivo. We examined whether SCFAs are able to affect leptin levels in the whole animal. In mice, oral administration of 500 μmol of propionate in bolus by gavage increased plasma propionic acid level from ≈25 to ≈250 μM 7 h after gavage. Plasma leptin levels are elevated by ≈80% after propionate administration (Fig. 5A). We observed similar elevations when leptin levels were normalized to body adiposity by using the gonadal fat weight as an index (Fig. 5B). In contrast, oral administration of 500 μmol of sodium hexanoate had no effect on plasma leptin levels (Fig. 5 C and D), showing the specificity of the effect of propionate.
Fig. 5.
Acute oral administration of propionate in mice increases circulating leptin levels. (A) Plasma leptin levels in mice administered with sodium propionate or control sodium chloride solutions (n = 9 per group). *, P < 0.05 compared with control group. (B) Plasma leptin levels normalized to epididymal fat mass in mice administered with sodium propionate or sodium chloride solutions. *, P < 0.01 compared with control group. (C) Plasma leptin levels in mice administered with neutral sodium hexanoate or control sodium chloride solutions (n = 6 per group). (D) Plasma leptin levels normalized to epididymal fat mass in mice administered with sodium hexanoate or sodium chloride solutions. Error bars represent SEM.
Discussion
In addition to the production through endogenous metabolic pathways, a large amount of SCFAs are generated from fermentation of dietary fibers in the lower gastrointestinal tract. The concentration of total SCFAs in humans is ≈50–100 μM in peripheral blood and ≈300–450 μM in portal blood (33). Indeed, SCFAs are a prominent source of dietary energy in mammals. They constitute ≈70% of the energy supply in ruminants and ≈5–10% in nonruminants (23). In the present study, we identified a molecular mechanism for monitoring extracellular SCFA levels. We demonstrated that SCFAs are activators of GPR41 and that they stimulate leptin production in both cultured adipocytes and whole animals. GPR41 is expressed in the white adipose tissue, and overexpression of GPR41 increased the potency of propionic acid in stimulating leptin production in adipocytes. In addition, suppression of GPR41 expression by RNA interference inhibited the stimulatory activity of propionic acid, confirming the direct involvement of GPR41 in the stimulatory effect of SCFAs on leptin production. The EC50 values of SCFAs in stimulating leptin production are close to the concentrations of total SCFAs in the circulation, suggesting that our findings may be relevant in vivo. The EC50 values of SCFAs in stimulating pigment aggregation in Xenopus melanophores transfected with GPR41, however, are significantly lower than the EC50 of SCFAs in stimulating leptin production in adipocytes. We speculate that this discrepancy is caused by differences in the expression levels of GPR41, the time course of assays, and intracellular signaling machineries in these distinct cellular systems. Further studies are needed to address this point.
The finding that SCFAs activate GPR41 and stimulate leptin production in adipocytes establishes a role for this group of molecules in cellular signaling and suggests their potential therapeutic applications. GPR43 is another orphan GPCR that shares 38% identity with GPR41 in amino acid sequence (21). In an assay that examines elevation of intracellular calcium concentration, we detected weak activities of SCFAs in CHO-K1 cells transfected with human GPR43 cDNA, with acetic acid being significantly more potent than other SCFAs (data not shown). This observation suggests that SCFAs can activate the Gq pathway through GPR43. Importantly, we also detected expression of GPR43 in mouse adipose tissue by RT-PCR analysis (data not shown). Our data indicate that SCFA-induced leptin production is mediated by Gi activation and that acetic acid is less potent than C3–C5 SCFAs in stimulating leptin production. Although these findings argue against a role for GPR43, further studies are needed to address the questions of whether GPR43 can couple to Gi proteins in vivo and whether this receptor is involved in the up-regulation of leptin production by SCFAs in adipocytes.
Leptin is a potent anorexigenic hormone that suppresses food intake through receptors expressed in the central nervous system (34). We found that acute administration of sodium propionate almost doubled the plasma concentration of leptin in mice. This led us to examine the effect of propionate administration on food intake as well. However, we did not observe any significant difference in food intake between propionate-treated and control groups after acute gavage. It may be that the observed 2-fold increase of leptin level in the peripheral blood is not enough to acutely induce significant effects on food intake, especially in the context of gavage-induced stress (35, 36). However, several independent studies have demonstrated that sodium propionate supplemented diets or i.v. propionate infusions reduce food intake in chicken and sheep through yet unidentified mechanisms (37, 38). Indeed, our preliminary experiments have shown a reproducible reduction of food intake in mice fed chow containing 3% sodium propionate in diet as compared to controls fed equimolar sodium chloride (unpublished data). Further investigation is required to examine whether the anorexic effect of chronic propionate treatment is in part mediated by the leptin pathway.
In this study, we focused on a function of GPR41 in adipocytes and found that adipocytes can sense SCFA levels and positively regulate leptin production in response to elevated SCFA concentrations. Future studies will further define the physiological roles of SCFAs as a group of heretofore overlooked signaling molecules in various tissues.
Supplementary Material
Acknowledgments
We thank H. Kurosu, S. L. McKnight, M. R. Lerner, J. L. Chen, and M. S. Brown for discussions and S. D. Haferkamp, C. H. Michnoff, and J. Ou for technical help. J. T. Willie helped edit the manuscript. M.Y. is an Investigator of Howard Hughes Medical Institute. This work was supported in part by research funds from the Perot Family Foundation and Exploratory Research for Advanced Technology program of the Japan Science and Technology Agency.
Abbreviations: GPCR, G protein-coupled receptor; SCFA, short-chain fatty acid; siRNA, short interfering RNA.
References
- 1.Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L. & Friedman, J. M. (1994) Nature 372, 425–432. [DOI] [PubMed] [Google Scholar]
- 2.Chen, H., Charlat, O., Tartaglia, L. A., Woolf, E. A., Weng, X., Ellis, S. J., Lakey, N. D., Culpepper, J., Moore, K. J., Breitbart, R. E., et al. (1996) Cell 84, 491–495. [DOI] [PubMed] [Google Scholar]
- 3.Lee, G. H., Proenca, R., Montez, J. M., Carroll, K. M., Darvishzadeh, J. G., Lee, J. I. & Friedman, J. M. (1996) Nature 379, 632–635. [DOI] [PubMed] [Google Scholar]
- 4.Phillips, M. S., Liu, Q., Hammond, H. A., Dugan, V., Hey, P. J., Caskey, C. J. & Hess, J. F. (1996) Nat. Genet. 13, 18–19. [DOI] [PubMed] [Google Scholar]
- 5.Montague, C. T., Farooqi, I. S., Whitehead, J. P., Soos, M. A., Rau, H., Wareham, N. J., Sewter, C. P., Digby, J. E., Mohammed, S. N., Hurst, J. A., et al. (1997) Nature 387, 903–908. [DOI] [PubMed] [Google Scholar]
- 6.Clement, K., Vaisse, C., Lahlou, N., Cabrol, S., Pelloux, V., Cassuto, D., Gourmelen, M., Dina, C., Chambaz, J., Lacorte, J. M., et al. (1998) Nature 392, 398–401. [DOI] [PubMed] [Google Scholar]
- 7.Halaas, J. L., Gajiwala, K. S., Maffei, M., Cohen, S. L., Chait, B. T., Rabinowitz, D., Lallone, R. L., Burley, S. K. & Friedman, J. M. (1995) Science 269, 543–546. [DOI] [PubMed] [Google Scholar]
- 8.Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. (1995) Science 269, 546–549. [DOI] [PubMed] [Google Scholar]
- 9.Ahima, R. S., Prabakaran, D., Mantzoros, C., Qu, D., Lowell, B., Maratos-Flier, E. & Flier, J. S. (1996) Nature 382, 250–252. [DOI] [PubMed] [Google Scholar]
- 10.Chehab, F. F., Lim, M. E. & Lu, R. (1996) Nat. Genet. 12, 318–320. [DOI] [PubMed] [Google Scholar]
- 11.Lord, G. M., Matarese, G., Howard, J. K., Baker, R. J., Bloom, S. R. & Lechler, R. I. (1998) Nature 394, 897–901. [DOI] [PubMed] [Google Scholar]
- 12.Frederich, R. C., Hamann, A., Anderson, S., Lollmann, B., Lowell, B. B. & Flier, J. S. (1995) Nat. Med. 1, 1311–1314. [DOI] [PubMed] [Google Scholar]
- 13.Saladin, R., De Vos, P., Guerre-Millo, M., Leturque, A., Girard, J., Staels, B. & Auwerx, J. (1995) Nature 377, 527–529. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, B., Graziano, M. P., Doebber, T. W., Leibowitz, M. D., White-Carrington, S., Szalkowski, D. M., Hey, P. J., Wu, M., Cullinan, C. A., Bailey, P., et al. (1996) J. Biol. Chem. 271, 9455–9459. [DOI] [PubMed] [Google Scholar]
- 15.Mantzoros, C. S., Qu, D., Frederich, R. C., Susulic, V. S., Lowell, B. B., Maratos-Flier, E. & Flier, J. S. (1996) Diabetes 45, 909–914. [DOI] [PubMed] [Google Scholar]
- 16.Slieker, L. J., Sloop, K. W., Surface, P. L., Kriauciunas, A., LaQuier, F., Manetta, J., Bue-Valleskey, J. & Stephens, T. W. (1996) J. Biol. Chem. 271, 5301–5304. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld, C., Zhao, C., Fuller, J., Pollack, A., Moser, A., Friedman, J. & Feingold, K. R. (1996) J. Clin. Invest. 97, 2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, J., Liu, R., Hawkins, M., Barzilai, N. & Rossetti, L. (1998) Nature 393, 684–688. [DOI] [PubMed] [Google Scholar]
- 19.Rice, A. M., Fain, J. N. & Rivkees, S. A. (2000) Endocrinology 141, 1442–1445. [DOI] [PubMed] [Google Scholar]
- 20.Xiong, Y., Tanaka, H., Richardson, J. A., Williams, S. C., Slaughter, C. A., Nakamura, M., Chen, J. L. & Yanagisawa, M. (2001) J. Biol. Chem. 276, 28471–28477. [DOI] [PubMed] [Google Scholar]
- 21.Sawzdargo, M., George, S. R., Nguyen, T., Xu, S., Kolakowski, L. F. & O'Dowd, B. F. (1997) Biochem. Biophys. Res. Commun. 239, 543–547. [DOI] [PubMed] [Google Scholar]
- 22.Brown, A. J., Goldsworthy, S. M., Barnes, A. A., Eilert, M., Tcheang, L., Daniels, D., Muir, A. I., Wigglesworth, M. J., Kinghorn, I., Fraser, N. J., et al. (2003) J. Biol. Chem. 278, 11312–11319. [DOI] [PubMed] [Google Scholar]
- 23.Bergman, E. N. (1990) Physiol. Rev. 70, 567–590. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka, H., Yoshida, T., Miyamoto, N., Motoike, T., Kurosu, H., Shibata, K., Yamanaka, A., Williams, S. C., Richardson, J. A., Tsujino, N., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 6251–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa, H. & Yamamoto, M. (1990) J. Chromatogr. 523, 235–246. [DOI] [PubMed] [Google Scholar]
- 26.Lerner, M. R. (1994) Trends Neurosci. 17, 142–146. [DOI] [PubMed] [Google Scholar]
- 27.Granneman, J. G. (1995) Cell. Signalling 7, 9–15. [DOI] [PubMed] [Google Scholar]
- 28.Olah, M. E. & Stiles, G. L. (1995) Annu. Rev. Pharmacol. Toxicol. 35, 581–606. [DOI] [PubMed] [Google Scholar]
- 29.Green, A. (1987) J. Biol. Chem. 262, 15702–15707. [PubMed] [Google Scholar]
- 30.Katada, T., Kurosu, H., Okada, T., Suzuki, T., Tsujimoto, N., Takasuga, S., Kontani, K., Hazeki, O. & Ui, M. (1999) Chem. Phys. Lipids 98, 79–86. [DOI] [PubMed] [Google Scholar]
- 31.Yu, S., Castle, A., Chen, M., Lee, R., Takeda, K. & Weinstein, L. S. (2001) J. Biol. Chem. 276, 19994–19998. [DOI] [PubMed] [Google Scholar]
- 32.Moxham, C. M. & Malbon, C. C. (1996) Nature 379, 840–844. [DOI] [PubMed] [Google Scholar]
- 33.Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. (1987) Gut 28, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen, P., Zhao, C., Cai, X., Montez, J. M., Rohani, S. C., Feinstein, P., Mombaerts, P. & Friedman, J. M. (2001) J. Clin. Invest. 108, 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey, J. J., Kemnitz, J. W., Colman, R. J., Cunningham, D. & Swick, A. G. (1998) J. Clin. Endocrinol. Metab. 83, 3230–3235. [DOI] [PubMed] [Google Scholar]
- 36.Kastin, A. J. & Pan, W. (2000) Regul. Pept. 92, 37–43. [DOI] [PubMed] [Google Scholar]
- 37.Pinchasov, Y. & Elmaliah, S. (1995) Ann. Nutr. Metab. 39, 107–116. [DOI] [PubMed] [Google Scholar]
- 38.Farningham, D. A. & Whyte, C. C. (1993) Br. J. Nutr. 70, 37–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.