Abstract
Objectives:
The present study was undertaken to test the comparative efficacy of chlorpromazine and risperidone in patients of schizophrenia in a tertiary care hospital of Maharashtra.
Materials and Methods:
About 100 subjects of either sex between 15 and 75 years of age were randomly assigned either chlorpromazine or risperidone. Only those patients were included who met International Classification of Diseases 10 revision criteria by World Health Organization. To avoid bias, the test drugs were coded as A and B. The study coordinator was unaware of the prescribed drugs; however, the prescribing psychiatrist knew about the drug treatment.
Results:
Both chlorpromazine and risperidone significantly decreased the mean score of positive and general symptoms in patients of schizophrenia. Although chlorpromazine decreased the mean score of negative symptoms, it was not statistically significant. Risperidone reduced the mean score of negative symptoms to a significant extent. The cost (Rs. 3000-4000) of risperidone was more than the cost (Rs. 700-1000) of chlorpromazine per patient per annum. The dropouts were less (25%) in the risperidone group than in the chlorpromazine group (75%). The more purchase of risperidone than of chlorpromazine was observed in our study.
Conclusion:
The response rates for positive and general symptoms were found to be equal for both chlorpromazine and risperidone. However, risperidone was found to be more effective than chlorpromazine in treating negative symptoms. The dropout rate was less in the risperidone group than in the chlorpromazine group. The compliance was also better in the risperidone group, even though the cost of risperidone was more than that of chlorpromazine.
Keywords: Typical antipsychotic, atypical antipsychotic, schizophrenia
INTRODUCTION
Schizophrenia is a devastating mental disease affecting human population worldwide, with prevalence of about 1%. Patients suffering from schizophrenia may have predominant positive or negative symptoms or both.
There are number of drugs available for the treatment of schizophrenia; these drugs are classified into typical and atypical antipsychotics.
Many studies suggested that the typical antipsychotics have autonomic and extrapyramidal side effects such as dryness of mouth, constipation, cycloplegia, mydriasis, urinary retention, orthostatic hypotension, impotence, Parkinson's syndrome, akathisia, dystonias, and neuroleptic malignant syndrome, as well as many other side effects such as sedation, galactorrhea, and cholestatic jaundice that are less common with atypical antipsychotics.[1] However, the drugs are cheaper and were found to more effective in treating positive symptoms.[2,3] Among typical antipsychotics, chlorpromazine is frequently used as the first-line treatment for patients with schizophrenia.[4] However, about 5–25% of the patients show poor response to typical antipsychotics.[5,6]
Atypical antipsychotics take care of both positive and negative symptoms and have less side effects as compared with typical antipsychotics. These drugs are called ‘atypical’ because of having reduced or no risk for extrapyramidal side effects, reduced or no elevation in prolactin, antagonization of 5-HT2A, and minimal risk for tardive dyskinesia.[7] Currently, risperidone is being marketed for the treatment of schizophrenia, largely on the basis of claims of improved tolerability and effectiveness.[8] However, as compared with chlorpromazine, risperidone is an expensive drug.
Still there is a need for research in pharmacological intervention to treat symptoms of the disease having both positive and negative symptoms, and there are only a few studies that compare most commonly prescribed typical antipsychotics such as chlorpromazine and atypical antipsychotics such as risperidone.Therefore, it was found to be of interest to conduct a study to assess the efficacy of a typical antipsychotic chlorpromazine and an atypical antipsychotic risperidone on the positive and negative symptoms in patients of schizophrenia, as well as their cost-effectiveness and compliance.
MATERIALS AND METHODS
Study design
The present study was conducted in the psychiatry out patient department and psychiatry ward of Kasturba Hospital, Sewagram, Maharashtra, India, a rural medical college and hospital. It was a single-blind longitudinal prospective comparative study of the efficacy of a typical antipsychotic chlorpromazine and a newer or atypical antipsychotic risperidone on the positive and negative symptoms in patients of schizophrenia.
The patients of schizophrenia were diagnosed by a psychiatrist according to International Classification of Diseases-10 (ICD-10) criteria by World Health Organization.
Initially, 142 patients were included in the study, of which 42 patients dropped out of the study due to various reasons. The study was conducted in remaining 100 patients of schizophrenia, 50 each taking either risperidone or chlorpromazine who had fulfilled the inclusion criteria.
The written informed consent from patients or their guardians were taken before starting the study. The ethical approval was taken from the ethics committee of the institute.
To avoid bias, the test drugs were coded as A and B. The study coordinator was unaware of the prescribed drugs; however, the prescribing psychiatrist knew about the drug treatment.
The dose of chlorpromazine used in the study was 100–2000 mg/day and the dose of risperidone was 3–16 mg/day in divided dosage.
If the patients dropped out of the study due to reasons such as lost during follow-up, poor compliance to drug, pregnancy, generalized poor medical condition, change of drug in the view of better treatment of the patient, and change of diagnosis, more patients were added subsequently to the study. The study started in the month of November 2006, and the patients were assessed for 3 months. During each visit, patients who were taking drugs regularly and who were having good drug compliance were only included in the study and they were examined for positive, negative, and general symptoms of schizophrenia according to the Positive and Negative Syndrome Scale (PANSS) score. At the end of the study, the drug responses were analyzed statistically. The cost-effectiveness of these drugs was also studied by calculating the cost of drugs. An approximate sample size was calculated statistically to get significant results.
Inclusion criteria
The subjects who satisfied the following criteria were enrolled in the study:
New patients and follow-up patients who discontinued the drugs for more than 3 months of their own were included in the study;
Male or female patients between 15 and 75 years of age;
Subjects who met with ICD-10 criteria for schizophrenia: F20.0 Paranoid schizophrenia, F20.1 Hebephrenic schizophrenia, F20.2 Catatonic schizophrenia, F20.3 Undifferentiated schizophrenia, F20.4 Post schizophrenic depression, F20.5 Residual schizophrenia, F20.6 Simple schizophrenia, F20.8 Other schizophrenia, F20.9 Schizophrenia, unspecified;
Subjects or their guardians who gave the informed consent before starting the screening procedure;
Those subjects who were not danger to themselves (suicidal) or others and those who have family support available to be maintained as outpatients;
Only those female patients who were either (a) incapable of pregnancy because of hysterectomy or tubal ligation, (b) capable of pregnancy if they were ready to accept the method of contraception for at least 1 month before and throughout the entire study period;
It was ensured that during the study a responsible person must be available to accompany the patient to the investigational site at each visit in order to provide reliable information for the PANSS evaluation and accurate and reliable administration of the test drug to the patients;
They must also agree for hospitalization at any time during the study in case of need; and/or
They must be physically healthy as per the physical examination and medical history
Exclusion criteria
Those subjects who met any of the following criteria were excluded from participating in the study:
Subjects with a known or suspected history of substance dependence (including alcohol, but excluding nicotine and caffeine) according to the ICD-10 criteria during the 3 months before screening.
Patients with cardiac conditions, such as sick sinus syndrome, complete Atrioventricular block, congestive heart failure, polymorphic ventricular tachycardia, clinically relevant hypocalcemia, hypokalemia, or hypomagnesemia; concomitant use of drugs that prolong the QTc interval [including Class I antiarrythmic (e.g., quinidine and procainamide) or Class III antiarrythmic (e.g., amiodarone and sotalol medications]; or the presence of congenital heart diseases;
Female subjects with pregnancy;
Subjects with a known or suspected history of seizure disorder, or neuroleptic malignant syndrome, encephalopathic syndrome, tardive dyskinesia, or insulin-dependent diabetes mellitus;
Presence of any significant or unstable cardiovascular, respiratory, renal, hepatic, hematological, endocrine, immunological, or other systemic disease;
History of any prior surgical procedures (i.e., gastric or bowel resection), which may interfere with the absorption and release of the drug;
Subjects who had received electroconvulsive therapy in the 1 month before the baseline visit;
Subjects who, despite washout, continued to use any prohibited concomitant medication, substance of abuse, or alcohol within 5 half-lives (up to a maximum of 5 days) before the baseline visit;
Subjects who have received a depot antipsychotic within two treatment cycles, 1 month before the baseline visit;
Subjects with known or suspected hypersensitivity or intole rance to risperidone or chlorpromazine; and/or
Those who refuse to give consent
Data analysis
At the end of the study, the mean and standard error of mean of the PANSS score in each group were analyzed. Two sample t tests for equal variances were applied. P<0.05 was considered statistically significant. Drugs were decoded only after data analysis.
RESULTS
The scoring for positive, negative, and general symptoms of 100 patients (50 each taking either chlorpromazine or risperidone) was done at an interval of 3 months over a period of 1 year (total five visits including first and last visits). The scores of 50 individuals taking either of the drug for positive, negative, and general symptoms were added separately for each visit, and the mean was taken by dividing the total by 50.
Compliance
This was studied by considering the factors such as drugs, purchase of drugs, efficacy of the drugs on symptoms, and dropouts of patients.
Age and sex of patients included in study groups
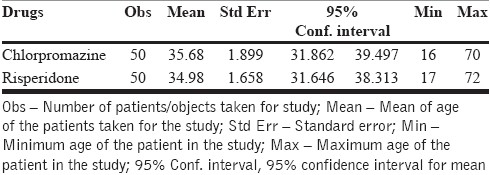
The age of the patients ranged from 16 to 72 years, with the mean age of approximately 35 years in both chlorpromazine and risperidone groups. For the chlorpromazine group, the mean age of 50 patients was 35.68 years, and for the risperidone group, the mean age was 34.98 years [Table 1].
Table 1.
Mean age of the patients in chlorpromazine and risperidone groups
In this study, the total number of males was 51 and the total number of females was 49. This indicates that the male and female ratio was almost equal as far as the distribution of schizophrenic patients is concern [Table 2].
Table 2.
Sex-wise distribution of patients taking chlorpromazine and risperidone

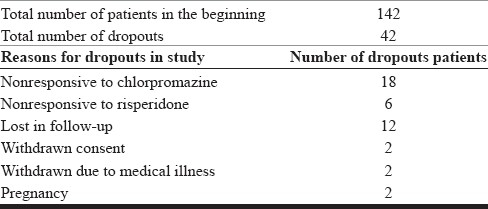
Dropouts
Of 142 patients enrolled in our study, 42 patients dropped out. The dropouts were due to nonresponse to drugs (chlorpromazine=18, risperidone=6, total 26), lost in the follow-up (12), and 2 patients each due to withdrawn consent and pregnancy [Table 3]. Among dropouts due to nonresponse to drugs, the chlorpromazine group composed of 75% and the risperidone group composed of 25% patients.
Table 3.
Number of patients and dropouts in the study
Comparative effect between chlorpromazine and risperidone on positive symptoms on each visit
In the fist visit, the mean scores for both chlorpromazine and risperidone were around 30 and 27, respectively, and these values were treated as a control because no drug was started yet.
In fifth visit, the mean score for chlorpromazine was 9 and for risperidone it was 7. The drop in the mean values for both the drugs was around 20 [Table 4, Figure 1].
Table 4.
Comparative effect between chlorpromazine and risperidone on positive symptoms on each visit
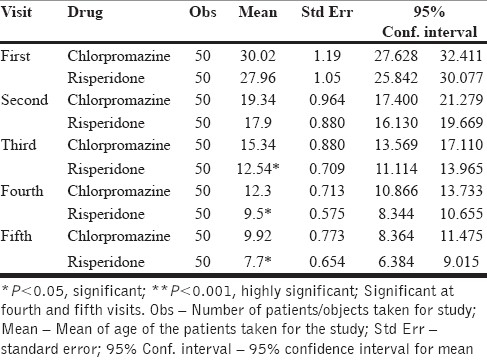
Figure 1.
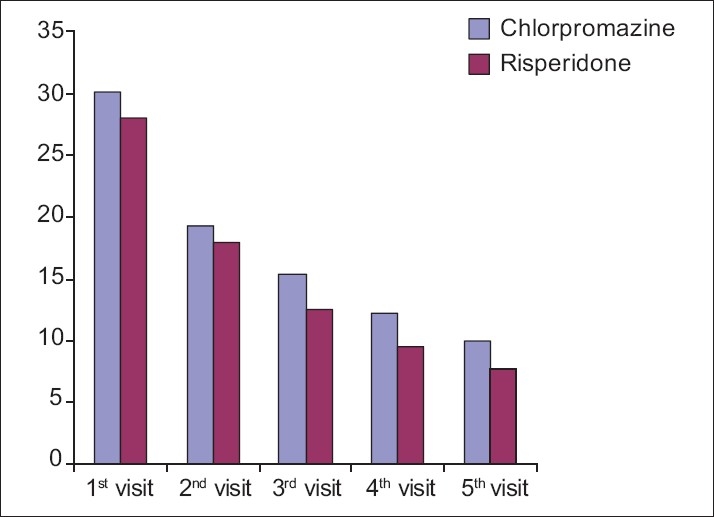
Graph showing the comparative effect of chlorpromazine and risperidone on positive symptoms as per PANSS
On positive symptoms, chlorpromazine and risperidone were found to be equally efficacious in patients of schizophrenia.
Comparative effect between chlorpromazine and risperidone on negative symptoms on each visit
The risperidone showed a gradual decrease in the mean score of negative symptoms from the first visit (27) to the final visit (7). The reduction in incidence was 74% as compared to the first visit, which was highly statistically significant (P=0.000). Chlorpromazine also reduced the mean score of negative symptoms from the first visit to the final visit to the extent of 30% only, which was not statistically significant [Table 5, Figure 2]. Hence, risperidone was found to have a better effect on treating negative symptoms as compared with chlorpromazine.
Table 5.
Comparative effect between chlorpromazine and risperidone on negative symptoms on each visit
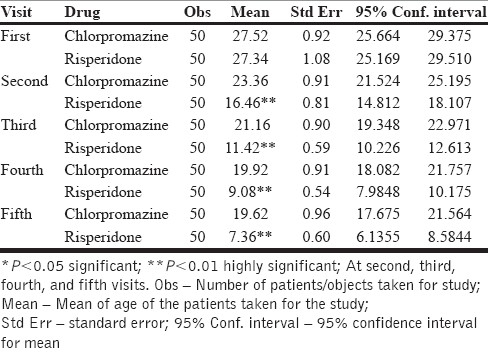
Figure 2.
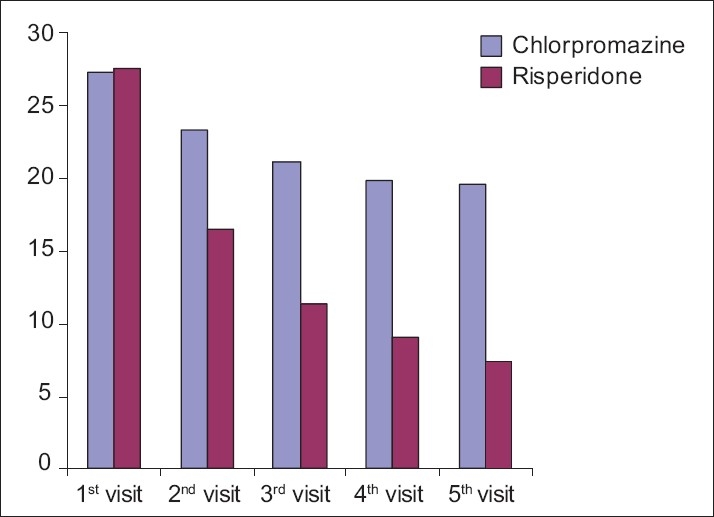
Graph showing the comparative effect of chlorpromazine and risperidone on negative symptoms according to PANSS
Comparative effect between chlorpromazine and risperidone in each visit on general symptoms
The mean score observed for general symptoms in patients of schizophrenia in the first visit was 61 and 56 for chlorpromazine and risperidone groups, respectively.
In subsequent visits up to the fifth visit, both chlorpromazine and risperidone showed a gradual decline in the mean score of the symptoms to the extent of 20 for chlorpromazine and 14 for risperidone, which were highly significant (P=0.0001).
Both chlorpromazine and risperidone were noticed to be equi-efficacious in treating the general symptoms in patients of schizophrenia [Table 6, Figure 3].
Table 6.
Comparative effect between chlorpromazine and risperidone on general symptoms on each visit
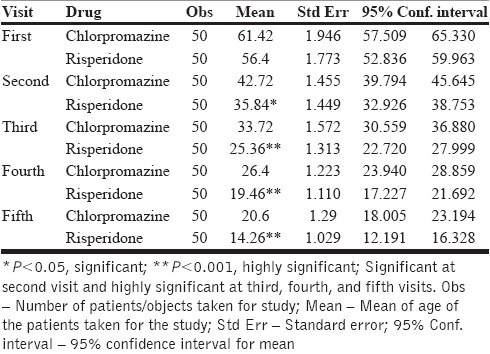
Figure 3.
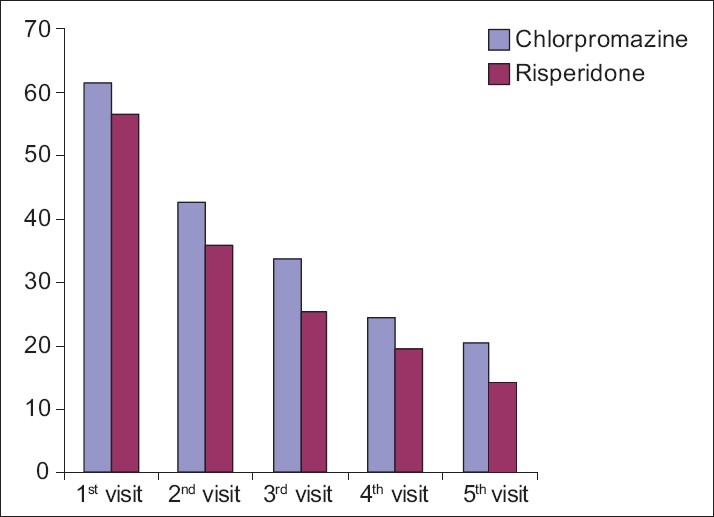
Graph showing the comparative effect of chlorpromazine and risperidone on general symptoms according to PANSS
Cost-effectiveness
The cost of risperidone (6 mg/day) treatment for 1 year was Rs. 3000-4000, whereas the cost of chlorpromazine treatment (400 mg/day) was Rs. 700-1000, which means that the cost of risperidone was more than the cost of chlorpromazine per patient per annum.
Compliance
Better compliance was noticed with risperidone.
DISCUSSION
It was found that the dropout of patients was as high as 30%. Initially, 142 patients were taken; of those 142, 100 patients came for regular follow-up and responded well to the drugs.
Among dropouts due to nonresponse to drugs, the chlorpromazine group was composed of 75% and the risperidone group composed of 25% patients.
It was found that the mean PANSS score for positive symptoms decreased for chlorpromazine from 30.02 to 9.92 while the mean PANSS score for positive symptoms decreased for risperidone from 27.96 to 7.7 in 1 year. This indicates that both drugs are good in treating positive symptoms of schizophrenia, such as delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness, and hostility. Reduction in the positive symptoms was equal, that is, approximately 70% for both the drugs as compared with the first visit.
In the present comparative study, the negative symptoms in patients taking risperidone and chlorpromazine, risperidone was found to be superior to chlorpromazine in controlling the negative symptoms such as blunted affect, emotional withdrawal, poor rapport, apathetic, social withdrawal, difficulty in abstract thinking, lack of spontaneity, and flow of conversation and stereotyped thinking. The reduction in the negative symptoms was 30% for chlorpromazine and 74% for risperidone as compared with the first visit.
In our study the general symptoms of schizophrenia were reduced significantly by both drugs as evident for a decrease in the mean PANSS score for chlorpromazine slides from 61.42 to 20.6 and for risperidone from 56.4 to 14.26. Both the drugs are equally effective in treating general symptoms such as somatic concern, anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, and active social avoidance.
Risperidone is a relatively expensive antipsychotic. Treatment for 1 yearcosts approximately Rs. 3000-4000 for risperidone (6 mg/day) and Rs. 700-1000 (400 mg/day) for chlorpromazine.
Although risperidone is an expensive drug, dropouts were less in this group; this indicates a better effect of risperidone as compared with chlorpromazine. In this study, the cost of the drug did not influence the treatment by the drugs.
The dropouts in the risperidone group were less in comparison to those in the chlorpromazine group. This indicates compliance is better with the risperidone group compared with the chlorpromazine group.
In our study the age of the patient varied from 16 to 72 years. This suggests that the disease is prevalent in all age groups.
The distribution of the disease in our study has been observed almost equal in males and females. This is an agreement with the previous study by Castle et al.,[9] as they have also shown the equal ratio in distribution of schizophrenia.
Our study is in agreement with the study by Rabinowitz et al.,[10] who compared the effects of haloperidol (typical antipsychotic) and risperidone (atypical antipsychotic) on positive and negative symptoms in patients of schizophrenia by using PANSS and observed that patients receiving risperidone (4 mg) improved significantly more than those treated with haloperidol (10 mg). Although in our study, the effects were compared between chlorpromazine (100–1000 mg) and risperidone (3–16 mg) drugs.
However, in another similar comparative study between haloperidol and risperidone, Schooler et al.[11] did not notice any significant difference in the improvement of positive and negative symptoms in patients of schizophrenia.
Another study was conducted by Sanger et al.;[12] for the reduction of positive and negative symptoms of schizophrenia by using atypical antipsychotic (risperidone) and typical antipsychotic (haloperidol). Our study is also in agreement with the above study for negative symptoms, where a higher reduction in symptoms was reported for risperidone in comparison to chlorpromazine. However, the reduction in positive symptoms was found to be equal, i.e., approximately 70% for both the drugs.
Lieberman et al.[13] had also shown the higher reduction in symptoms for atypical antipsychotic (clozapine reduction in symptoms 71%) than typical antipsychotic (chlorpromazine reduction in symptoms 64%).
Kennedy et al.[14] in their study on cost-effectiveness and compliance reported that the conventional antipsychotic drugs such as haloperidol and chlorpromazine are frequently used as the first-line treatment in schizophrenia even after their poor response. This is because of the cost of the drugs, which also increases the compliance.
This is in contrast with our study where we have shown that the dropouts were less in the risperidone group than in the chlorpromazine group even though the cost of risperidone was high; this might be contributing to the better response of risperidone.
All these above studies clearly suggest the superiority of atypical antipsychotic drugs over the typical one, in terms of not only better efficacy in treating both positive and negative symptoms of schizophrenia but also decrease in side effects. Nevertheless, the role of typical antipsychotics cannot be ignored for the management on positive symptoms of schizophrenia and cost-effectivity when compared with the newer atypical group of antipsychotics.
The dropout rate is as high as 30% in patients of schizophrenia. The commonest reason for dropout in our study is poor response to the drug. The dropout rate is 75% for chlorpromazine and 25% for risperidone from the total dropouts because of nonresponsiveness to the drugs. The dropouts from the risperidone group were less in comparison to those from the chlorpromazine group. This indicates compliance is better with the risperidone group than with the chlorpromazine group. Schizophrenia is widely distributed in all age groups, and there was equal distribution of the disease in males and females. Both chlorpromazine and risperidone can effectively treat positive symptoms of schizophrenia.
Risperidone is superior to chlorpromazine in terms of treating negative symptoms of schizophrenia. Both drugs take good care of general symptoms of schizophrenia.
As chlorpromazine is relatively cheaper than risperidone, it should be preferred in patients with predominant positive symptoms and problem of poor affordability due to economical conditions, which is common in patients of schizophrenia due to regression of work performance, leading to hampering of economic conditions.
Risperidone should be preferred in patients with both positive and negative symptoms or patients with predominate negative symptoms.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Kay SR, Oplar LA, Fiezbein A. Significance of Positive and negative syndrome in chronic Schizophrenia. Br J Psychiatry. 1986;149:439–448. doi: 10.1192/bjp.149.4.439. [DOI] [PubMed] [Google Scholar]
- 2.Joy CB, Adams CE, Lawrie SM. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2006;4:CD003082. doi: 10.1002/14651858.CD003082.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Thornley B, Rathbone J, Adams CE, Awad G. Chlorpromazine versus placebo for schizophrenia. Cochrane Database Syst Rev. 2003;2:CD000284. doi: 10.1002/14651858.CD000284. [DOI] [PubMed] [Google Scholar]
- 4.Kane JM. Treatment programme and long term outcome in chronicschizophrenia. Acta Psychiatr Scand Suppl. 1990;358:151–7. doi: 10.1111/j.1600-0447.1990.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis JM, Casper R. Antipsychotic drugs: Clinical pharmacology and therapeutic use. Drugs. 1997;14:260–82. doi: 10.2165/00003495-197714040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Christison GW, Kirch DG, Wyatt RJ. When symptoms persist: Choosing among alternative somatic symptoms for schizophrenia. Schizophr Bull. 1991;17:217–45. doi: 10.1093/schbul/17.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Borison RL, Pathiraja AP, Diamond BI, Meibach RC. Risperidone: Clinical safety and efficacy in schizophrenia. Psychopharmacol Bull. 1992;28:213–8. [PubMed] [Google Scholar]
- 8.Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Risperidone versus other atypical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev. 2000;3:CD002306. doi: 10.1002/14651858.CD002306. [DOI] [PubMed] [Google Scholar]
- 9.Castle D, Wesseley S, Der G, Murray RM. The incidence of operationally defined schizophrenia in Camberwell 1965–84. Br J Psychiatry. 1991;159:790–4. doi: 10.1192/bjp.159.6.790. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz J, Davidson M. Risperidone versus Haloperidol in long-term hospitalized chronic patients in a double blind randomized trial: A post hoc analysis. Schizophr Res. 2001;50:89–93. doi: 10.1016/s0920-9964(00)00163-8. [DOI] [PubMed] [Google Scholar]
- 11.Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, et al. Risperidone and haloperidol in first-episode psychosis: A long-term randomized trial. Am J Psychiatry. 2005;162:947–53. doi: 10.1176/appi.ajp.162.5.947. [DOI] [PubMed] [Google Scholar]
- 12.Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley C, Jr, Tollefson GD. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry. 1999;156:79–87. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, et al. Atypical and conventional antipsychotic drugs in treatment of first-episode schizophrenia: A 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28:995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy E, Song F, Hunter R, Clarke A, Gilbody S. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev. 2000;2:CD000440. doi: 10.1002/14651858.CD000440. [DOI] [PubMed] [Google Scholar]


