Abstract
The hyperpolarization-activated cation channels (Ih) play a distinct role in rhythmic activities in a variety of tissues, including neurons and cardiac cells. In the present study, we investigated whether Ca2+ can permeate through the hyperpolarization-activated pacemaker channels (HCN) expressed in HEK293 cells and Ih channels in dorsal root ganglion (DRG) neurons. Using combined measurements of whole-cell currents and fura-2 Ca2+ imaging, we found that there is a Ca2+ influx in proportion to Ih induced by hyperpolarization in HEK293 cells. The Ih channel blockers Cs+ and ZD7288 inhibit both HCN current and Ca2+ influx. Measurements of the fractional Ca2+ current showed that it constitutes 0.60 ± 0.02% of the net inward current through HCN4 at –120 mV. This fractional current is similar to that of the low Ca2+-permeable AMPA-R (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) channels in Purkinje neurons. In DRG neurons, activation of Ih for 30 s also resulted in a Ca2+ influx and an elevated action potential-induced secretion, as assayed by the increase in membrane capacitance. These results suggest a functional significance for Ih channels in modulating neuronal secretion by permitting Ca2+ influx at negative membrane potentials.
As the most important second messenger, Ca2+ controls many physiological events, such as neurotransmitter release and muscle contraction (1, 2). Whereas the classic voltage-dependent calcium channels provide an important pathway for Ca2+ entry into neurons (3, 4), many ligand-gated cation channels are also permeable to Ca2+, providing another pathway for Ca2+ influx (5–8). Fractional Ca2+ current, the percentage of current carried by Ca2+ in the total current through cation channels, has been determined for nicotinic acetylcholine receptors (nAChRs) (6), for glutamate receptors [N-methyl-d-aspartate receptors (NMDA-Rs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA-Rs)] (7, 9), for cyclic nucleotide-gated (CNG) channels (8), and for voltage-dependent Ca2+ channels (VDCC) (3). The Ca2+ influx through these channels may result in transmitter release or muscle contraction (e.g., CNG and AMPA-Rs, see refs. 8–10; VDCC and NMDA-Rs, see refs. 3 and 11; and nAChRs and P2X-R, see refs. 12 and 13).
The voltage-dependent hyperpolarization-activated cyclic nucleotide-gated (HCN) channels generate a hyperpolarization-activated cation inward current, named Ih (for hyperpolarization-activated current) in neurons and If (for funny current) in cardiac cells. Opened by hyperpolarization, these channels are thought to be permeable only to Na+ and K+ ions (14–17). Near the membrane resting potential, Ih channels conduct more Na+ into and less K+ out of the cell, generating a net inward current. In rhythmically pacing cells, this inward current contributes to the slow depolarization toward the threshold for firing (4).
Four pore-forming subunits (HCN1 to -4) of Ih channels have been identified in brain (16, 17). They resemble the voltage-gated potassium channel superfamily. HCN channel subunits contain six transmembrane domains (S1–S6), with a pore-forming P region between S5 and S6 (16). All four HCN subunits have an identical pore region, indicating that their ion selectivity should be similar. HCN messenger RNA is widely and nonuniformly expressed in central neurons (18), photoreceptors (19), dorsal root ganglion (DRG) neurons and cardiac myocytes (19, 20).
Recently, Ih channels in neurons have received increasing attention because the activation of Ih affects a variety of neural functions including synaptic plasticity. At the crayfish neuromuscular junction, activation of Ih channels by cAMP or by hyperpolarization induces synaptic facilitation (21). In hippocampal neurons, the presynaptic mossy fiber long-term potentiation (LTP) is also dependent on Ih activation (ref. 22, but see refs. 23 and 24). Biophysical studies have shown that the Ih channel is permeable only to monovalent cations in physiological solutions (25), and Ih-mediated membrane depolarization is not responsible for Ih modulation of synaptic plasticity. Thus, it is not surprising that possible mechanisms for any Ih-mediated events are assumed to be downstream from Ca2+ (21, 22). However, this interpretation may need to be revised, considering the possibility of Ca2+ influx through the Ih channel itself.
Using a combined whole-cell patch clamp recording and fluorescence Ca2+ imaging method, we show that Ca2+ permeates through Ih channels. Furthermore, activation of Ih channels causes a marked facilitation of action potential-induced secretion from DRG neurons, as revealed by membrane capacitance measurements. Thus, Ca2+ entry through Ih channels may provide a cellular basis for Ih-mediated events, such as presynaptic facilitation and LTP.
Materials and Methods
Heterologous Expression of HCN Channels. Human HCN4 subcloned into HindIII/XbaI sites in pcDNA1.1/Amp vector was generously provided by U. B. Kaupp (Forshungszentrum Julich, Germany). HEK293 cells were grown in DMEM, supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. When cells approached confluence, they were seeded into 35-mm dishes and subsequently transfected with the HCN plasmid by using a calcium phosphate method. HCN4 was cotransfected with the GFP to guide selection of cells expressing HCN channels. After 48–96 h, transfected cells with green fluorescence were selected for patch clamp experiments.
Cell Dissociation. Adrenal chromaffin cells from Wistar rats (SLACCAS Inc., Shanghai) were isolated and cultured as described (26, 27). Cells were used in the experiments after 2–6 days in culture.
DRG neurons were isolated as described with slight modification and used 4–16 h after preparation (28). We used small- to middle-sized (25–40 μm) neurons.
The use and care of animals used in this study complied with the guidelines of the Animal Research Advisory Committee at the Shanghai Institutes of Biological Sciences.
Electrophysiology. Ionic currents were studied in the whole-cell configuration under voltage-clamp by using an EPC-9 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany). For switching external solutions, we used an RCP-2B perfusion system, which has a fast exchange time (100 ms) controlled electronically among seven channels (Inbio, Wuhan, China; ref. 28).
Solutions used for experiments are summarized in Tables 1 and 2. Pipette resistances were 2–5 MΩ for human embryonic kidney (HEK) cells, DRG neurons, and adrenal chromaffin cells.
Table 1.
Composition of internal solutions* (in mM)
| HEK cells | DRG neurons | Chromaffin cells | |
|---|---|---|---|
| NaCl | 10 | — | — |
| KCl | 145 | 150† | — |
| CsCl | — | — | 153 |
| MgCl2 | 1 | 1 | 1 |
| Hepes | 5 | 10 | 5 |
| ATP-Mg | — | 4 | — |
| pH | 7.2 | 7.2 | 7.2 |
For fluorescence calibration experiments, 1 mM fura-2 potassium salt was added to internal solutions. For calcium imaging experiments, 0.1 mM fura-2 salt was added to internal solutions.
For capacitance measurement of DRG neurons, 150 mM KCl was replaced with 153 mM CsCl to block K+ channels.
Table 2. Composition of external solutions (in mM).
| HEK cells | DRG neurons | Chromaffin cells | |
|---|---|---|---|
| NaCl | 138 | 150 | — |
| NMG | — | — | 138 |
| KCl | 5.6 | 5 | 5.6 |
| CaCl2 | 2.6 | 2.5 | 2.6 |
| MgCl2 | 1.2 | 1 | 1.2 |
| Hepes | 10 | 10 | 10 |
| Glucose | 10 | 10 | 10 |
| pH | 7.4 | 7.4 | 7.4 |
Fluorescence calibration experiments were performed in spherical adrenal chromaffin cells, 12–15 μm in diameter. High CsCl-containing intracellular solution (see Table 1) was used to measure voltage-gated Ca2+ currents.
The membrane capacitance measurements were carried out with a software lock-in amplifier of the pulse software controlling the EPC-9 amplifier (HEKA Electronics; ref. 28). Simulated action potential bursts for stimulation were constructed by computer from an action potential template, which was prerecorded from a DRG neuron under current clamp. This action potential (AP)-stimulation waveform was applied to the DRG neurons under whole-cell voltage-clamp.
DMEM and FBS were purchased from GIBCO. Fura-2 salt was from Molecular Probes. All other chemicals were from Sigma. All experiments were conducted at room temperature (22–24°C).
Fluorescence Measurements and Theory of Fractional Ca2+ Measurements. Intracellular calcium ([Ca2+]i) was measured by using a Ca2+ imaging system (TILL Photonics, Planegg, Germany). Fura-2 (0.1–1.0 mM) was loaded into the cell via a patch-pipette in the whole-cell configuration. The fluorescence was sampled at a frequency of 1 Hz (28).
Fractional Ca2+ current, Pf, is defined as the percentage of Ca2+ current in the total current passing through a cation channel (say, IHCN4 in this case). According to the original definition (6),
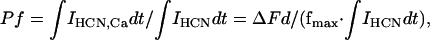 |
[1] |
where IHCN is the HCN4 current, and IHCN,Ca is the proposed fractional IHCN4 current carried by Ca2+. ΔFd is the change of Fd, which is the “modified Ca2+-sensitive fura-2 signal” immediately before (Fd′) and after (Fd″) the voltage-pulse induced Ca2+ influx (3), Fd = F340 –F380, ΔFd = Fd″ – Fd′, and fmax is a constant, which is determined by measuring Ca2+ influx through voltage-gated calcium channels in chromaffin cells under the condition that intracellular fura-2 is sufficiently high (>0.4 mM) (6). Under physiological conditions, all ions contributing to the current through Ca2+ channels are Ca2+ (3), or Pf = 100%. From Eq.1, we have fmax = ΔFd/(∫ICa dt), where ICa is the current through voltage-gated Ca2+ channels.
To record the time course of fura-2 dialysis, following ref. 6, we used the Ca2+-independent fluorescence signal F360, which can be calculated from F340 and F380. F360 = F340 + αF380, where α is the “isocoefficient” and can be determined by any experimental recording that shows rapid changes in Ca2+ concentration. In our setup, α = 0.35. Because F360 is Ca2+ independent, it can be used as an indicator of the intracellular fura-2 concentration [fura]i. After establishing the whole-cell recording configuration, fura-2 was dialyzed into the cell, and this was accompanied by a proportional F360 increase. Once F360 reached a steady-state level, we assumed that [fura]i was equal to the fura-2 concentration in the pipette (see Fig. 3 and ref. 6).
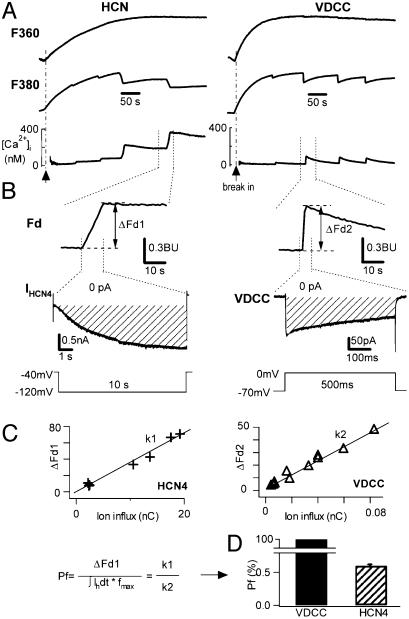
Fig. 3.
Protocols to measure Ca2+ influx through HCN4 channels. (A) Ca2+ signals in response to depolarizing pulses (B Bottom Right) in a chromaffin cell (voltage-gated Ca2+ current, VDCC, Right) and to hyperpolarizing pulses (B Bottom Left) in a HEK293 cell expressing HCN4 (HCN, Left). Ca2+ signals during fura-2 loading (1 mM in the pipette) F360 (top traces, indicating fura-2 entry into the cell), F380 (middle traces, indicating Ca2+ influx), and [Ca2+]i (bottom traces) are shown. Dashed lines are baselines. (B)Ca2+ current (Right) and IHCN4 (Left) with the corresponding Fd (top traces). Fd changes induced by the voltage protocols (Bottom) are shown as ΔFd1 and ΔFd2 for HCN4 and VDCC, respectively. Dashed lines in current traces indicate zero. The shaded areas indicate the total ion influx charge through the channels. (C) Determination of the fractional Ca2+ current through HCN4 channels. ΔFd1 for HCN4 and ΔFd2 for VDCC are plotted against the ion influx. (Inset) The equation used to calculate Pf (see Materials and Methods). (D) Compared with VDCC (Pf = 100%) in chromaffin cells (3), the Pf of HCN4 is 0.60 ± 0.02% (n = 7).
According to Eq. 1, by measuring the fura-2 signal evoked after activation of IHCN4 in HEK293 cells expressing HCN channels, the Pf of HCN channels can be determined.
Intracellular free Ca2+ concentration, [Ca2+]i, was measured according to Grynkiewicz et al. (29): [Ca2+]i = Keff·(R – R0)/(R1 – R), where R0 = 0.1, R1 = 3.4, and Keff = 1938 nM, as determined by standard calibrations (29, 30).
Data were analyzed with igor pro-3.12 software (WaveMetrics, Lake Oswego, OR). Unless otherwise stated, the data were presented as mean ± SD. Statistical significance was tested with Student's t test. P < 0.05 was considered statistically significant.
Results
To explore Ca2+ permeation through Ih channels, we first needed a simple expression system with the least contamination by other ion channels. We chose human embryonic kidney (HEK293) cells in which the expression of endogenous voltage-gated ion channels, including Ih channels, is either undetectable or very small compared with that in excitable cells.
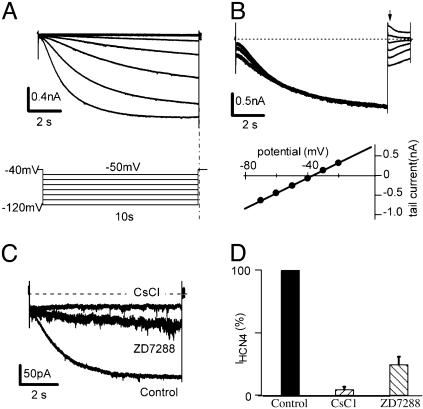
Fig. 1 provides a typical example of the hyperpolarization-activated current in a HEK cell expressing HCN 4. Fig. 1 A shows the currents induced by HCN4 channels (IHCN4) in response to 10-s hyperpolarizing pulses from –50 to –120 mV in 10-mV increments (holding potential, –40 mV). To determine the reversal potential, a prehyperpolarizing pulse to –120 mV was applied to fully activate IHCN4, and the membrane was then clamped back to test pulses ranging from –70 to –20 mV for 1.5 s. The reversal potential was determined by plotting the amplitudes of tail currents (Fig. 1B Upper) against the test potentials. Averaging over six cells, the reversal potential of HCN4 was –37 mV (Fig. 1B Lower). At –120 mV, the time constant of activation was 2.4 ± 0.3s(n = 12). IHCN4 was blocked by Cs+ and a selective Ih channel blocker, ZD7288 (21) (Fig. 1C). The currents were elicited by a hyperpolarizing pulse to –120 mV for 10 s from a holding potential of –40 mV. Application of 2 mM CsCl to the external solution blocked IHCN4 whereas 30 μM ZD7288 inhibited the current. On average, 2 mM Cs+ blocked 95 ± 3% (n = 4) of the IHCN4 whereas ZD7288 (30 μM) blocked 75 ± 3% (Fig. 1D, n = 6). The currents generated by HCN channel activation are largely insensitive to Ba2+ (which is widely used for blocking a variety of K+ currents; data not shown). The results are consistent with the typical properties of HCN4 expressed in HEK cells (31).
Fig. 1.
Properties of HCN channels expressed in HEK293 cells. (A) HCN4 currents (IHCN4, Upper) and voltage protocol (Lower). Cells expressing HCN4 were clamped from –40 mV to various voltages (–120 to –50 mV in 10-mV increments) for 10 s. (B) Determination of reversal potential of HCN4. An 8-s prepulse to –120 mV was applied to fully activate IHCN4. Test pulses of 1.5 s ranging from –70 to –20 mV in 10-mV increments were then applied to deactivate IHCN4. (Upper) A typical series of IHCN4 where the dashed line indicates zero current. The arrow indicates the start of the test pulses. By plotting the tail currents against the test potentials, the reversal potential of –37 mV was determined (Lower, n = 6). (C) Inhibition of IHCN4 by extracellular application of Cs+ (2 mM) or ZD7288 (30 μM). IHCN4 were evoked from –40 mV to –120 mV for 10 s and inhibited by Cs+ or by ZD7288. (D) Statistics of IHCN4 blockade by CsCl (2 mM) or ZD7288 (30 μM). Cesium blocked 95 ± 3% (n = 4) and ZD7288 blocked 75 ± 6% (n = 6) of IHCN4.
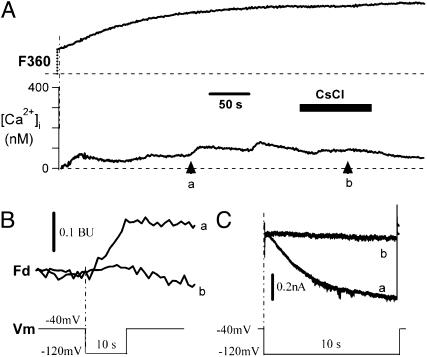
Fig. 2 presents an example of the fluorescence signals (F360 and [Ca2+]i, Fig. 2 A and B) and the HCN4 currents expressed in a HEK293 cell in response to a step to –120 mV from a holding potential of –40 mV (Fig. 2C). Surprisingly, there was [Ca2+]i rise during activation of HCN4 channels, implying existence of Ca2+ influx through the cation channel. When the HCN4 current (Fig. 2C, trace a) was blocked by Cs+ (Fig. 2C, trace b), the simultaneous increase in [Ca2+]i (Fig. 2A, arrow a) was also eliminated in the presence of Cs+ (Fig. 2A, arrow b). Fd changes in the absence and presence of Cs+ are illustrated in Fig. 2B.
Fig. 2.
Cs+ blockade of Ca2+ influx through HCN4 channels in HEK293 cells. (A) Ca2+ signals in response to hyperpolarizing pulses in the absence and presence of 2 mM CsCl. Two of the pulse-induced Ca2+ signals are analyzed below. Similar results were observed in all three cells tested. The dashed lines are baselines. (B) Fd signals corresponding to arrows a and b in A. (C) IHCN4 (trace a) was blocked by 2 mM Cs+ (trace b). Dashed line represents zero current. (Lower) The voltage protocol is shown.
Fig. 3 shows the protocol used to determine Ca2+ permeation through HCN4 channels expressed in HEK293 cells. A high concentration of fura-2 (1 mM), the calcium-sensitive fluorescent probe, was included in the patch-pipette and loaded into the cell in a whole-cell patch configuration. Entry of fura-2 into the cell was monitored by the Ca2+-insensitive signal, F360 (Fig. 3A, top traces). On binding to Ca2+, the fluorescence signal at F380 (Fig. 3A, middle traces) and the modified Ca2+-sensitive Fd (Fig. 3B, top traces, see Materials and Methods) were used to detect the net Ca2+ influx through voltage-gated calcium channels (Fig. 3A, right bottom trace) and HCN4 channels (Fig. 3A, left bottom trace). When intracellular fura-2 concentration [Ca2+]i is higher than 0.4 mM, the buffering capacity of fura-2 out-competes the endogenous Ca2+ buffers so that all inflowing Ca2+ is bound by fura-2 and reported by the Ca2+-sensitive Fd signals (3, 6).
Fig. 3 B and C illustrates how the fractional Ca2+ current (Pf) of the HCN4 channel was obtained by using Eq. 1 (see Materials and Methods). In Fig. 3B Right, we show that, in a rat adrenal chromaffin cell (RACC), a depolarizing step to 0 mV from a holding potential of –70 mV activated a voltage-dependent Ca2+ current (VDCC) and simultaneously induced an increase in Fd (ΔFd2, Fig. 3B, top trace). The shaded region marks the area over which the time integral of the ion flux through the calcium channels was calculated. In Left, we show that, in a HEK293 cell, a hyperpolarizing step to –120 mV from a holding potential of –40 mV activated the HCN4 current (middle trace), and simultaneously induced an increase in Fd (ΔFd1, top trace). The shaded region defines the time integral of ion flux through HCN4 channels. Fig. 3C shows the relationship between total ion influx and the corresponding increase in Fd (ΔFd) obtained with different durations of stimulation. Data were best fitted by a linear equation, indicating a correlation between the increased ΔFd and the increased ion flux for both VDCC and HCN4. The ratio, defined as ΔFd over ion influx (Fig. 3C Inset), is ≈200 times larger for voltage-gated Ca2+ channels (k1) than for HCN4 channels (k2). Using Eq. 1, we determined Pf to be 0.60 ± 0.02% of total IHCN4 (n = 9, Fig. 3D).
We have thus far shown that (i) activation of HCN4 can simultaneously induce an increase in Fd that indicates net Ca2+ influx and (ii) when HCN4 is blocked by Cs+, the concomitant increase in Fd is also blocked. These results strongly suggest that the hyperpolarization-induced Ca2+ influx is likely caused by a fractional Ca2+ current through open HCN channels.
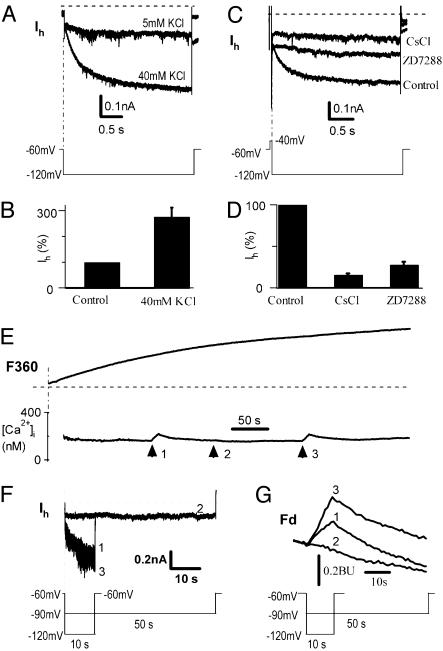
To extend our observations on calcium permeation through Ih channels to normal cells, we performed similar experiments in DRG neurons where Ih channels (32) and HCN messenger RNA have been detected (19). In whole-cell recording of small- to medium-sized (25–40 μM) C-type DRG neurons, we recorded typical Ih currents (Fig. 4). The Ih was enhanced by elevating external potassium concentration, [K+]o, from 5 mM to 40 mM (Fig. 4A), a well-known feature of Ih (15). Averaging over six neurons, Ih increased 277 ± 34% (n = 6, P < 0.01) in response to an 8-fold increase in [K+]o (Fig. 4B). The hyperpolarizing currents were blocked by Cs+ (2 mM) and ZD7288 (30 μM) (Fig. 4C). The average inhibition was 84 ± 3% (n = 11, P < 0.01) by Cs+ and 75 ± 5% (n = 5, P < 0.01) by ZD7288 (Fig. 4D). The activation time constant of Ih in DRG neurons was 0.51 ± 0.05 s (n = 15), which is faster than HCN4 channels (2.35 s, Fig. 1). The activation time constant of 0.51 s is consistent with HCN1 and HCN2, which are the major components of Ih in rat DRG neurons and have time constants of 0.03–0.2 s (19).
Fig. 4.
Ca2+ influx through Ih channels in DRG neurons. (A) Enhancement of Ih by increasing [K+]o from 5 to 40 mM in a neuron. The pulse protocol is shown below. (B) Normalized Ih is increased by 277 ± 34% in 40 mM vs. 5 mM KCl (P < 0.001, n = 6). (C) Inhibition of hyperpolarization-induced Ih by extracellular Cs+ (2 mM) or ZD7288 (30 μM). (D) Ih was blocked significantly by 2 mM CsCl (84 ± 3%; P < 0.01, n = 11) or 30 μM ZD7288 (75 ± 5%, P < 0.01, n = 5). (E) Ca2+ signals in response to hyperpolarizing pulses of –120 mV for 10 s (arrows 1 and 3) or –90 mV for 50 s (arrow 2) in a DRG neuron (n = 15). (F) Ih at –120 mV for 10 s (traces 1 and 3) and at –90 mV for 50 s (trace 2). Protocols are shown in the text. (G) Fd signals corresponding to arrows 1, 2, and 3 in E and F. Similar to HCN4 in HEK293 cells, the fractional Ca2+ current of Ih was 0.5 ± 0.1% in DRG neurons (n = 3).
Similar to reconstituted HCN channels, activation of Ih channels by hyperpolarization to –120 mV induces Ca2+ influx into DRG cells. Fig. 4E shows an example of Ih activation and the associated fluorescence changes recorded in a DRG neuron. In response to a hyperpolarizing step to –120 mV for 10 s from a holding potential of –60 mV, Ih was activated (Fig. 4F, traces 1 and 3), and accompanied by increases in [Ca2+]i (Fig. 4E, arrows 1 and 3, and Fig. 4G). When a weaker hyperpolarizing pulse to –90 mV was applied for 50 s, Ih was not activated (Fig. 4F, trace 2, and Fig. 4G), and no change in [Ca2+]i was observed (Fig. 4E, arrow 2).
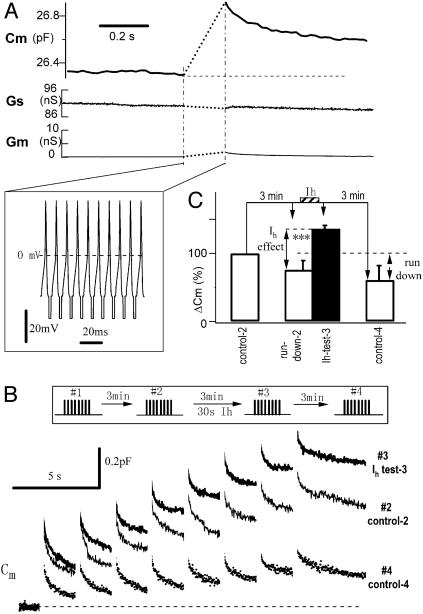
To investigate the physiological relevance of the Ca2+ influx through Ih channels, we used Cm (membrane capacitance) to measure AP-induced secretion before and after activation of Ih channels in DRG neurons (28). We recorded a typical AP and used it as a template to build a burst of 10 APs at 100 Hz (Fig. 5A Inset) and applied the AP burst to the voltage-clamped cell. The computer-constructed AP burst induced a capacitance increase of 0.57 pF (Fig. 5A), which corresponds to the exocytosis of 1,140 vesicles (vesicle diameter 140 nm, corresponding to 0.5 fF/vesicle; ref. 28). The stimulation-induced changes in Gs (whole-cell series conductance) and Gm (membrane conductance) were negligible, indicating that the lock-in assay of Cm is accurate (33). The neuron was stimulated four times with eight AP bursts separated by 2-s intervals (i.e., 80 APs each time; Fig. 5B Inset). Three minutes after the first set of stimuli (control-1, data not shown), the second stimulation (Fig. 5B, control-2) showed a 16% rundown. To test the effect of Ih on AP-induced Cm, immediately before applying the third AP series, the neuron was hyperpolarized to –120 mV for 30 s. The third stimulation evoked a Cm response (Fig. 5B, Ih test-3), which was 43% larger than control-2. Finally, 3 min later, the fourth stimulation evoked a response (Fig. 5B, control-4), which was 59% smaller than control-2. Similar results were observed in all cells tested. On average, activation of Ih for 30 s increased AP-induced secretion to 136 ± 5% of control (P < 0.001, n = 10). This is an underestimate because of the 24 ± 14% rundown (comparing control-1 and -2) during the 3-min whole-cell recording. If the rundown effect is compensated, the total facilitation of AP-induced secretion would be 179 ± 19% (P < 0.001, n = 10, paired t test). Thus, consistent with the findings from the crayfish neuromuscular junction (21), activation of Ih channels enhances AP-induced secretion from DRG neurons. Activation of Ih was responsible for the facilitation because 2 mM Cs+ blocked both Ih currents and AP-induced Cm facilitation (n = 5, data not shown). Ca2+ influx through Ih channels is most likely the mechanism underlying the facilitation of AP-induced secretion in DRG neurons because combined measurements of fura-2 fluorescence Ca2+ imaging and patch-clamp Cm recording revealed that activation of Ih increased the basal Ca2+, and the subsequent AP-induced [Ca2+]i and Cm (Fig. 6, which is published as supporting information on the PNAS web site, and data not shown). These experiments strongly suggest that Ca2+ inflow through Ih channels is responsible for the Ih-induced facilitation in DRG neurons.
Fig. 5.
Ih modulation of AP-induced exocytosis in DRG neurons. (A) An AP burst (Inset) induced a Cm rise in a DRG neuron. (B) Ih facilitates AP-induced secretion. Cm traces were recorded as #1 (control-1, data not shown), #2 (control-2, after 3 min rundown), #3 (test-3, after activation of Ih), and #4 (control-4, without Ih activation) at 3-min intervals. Cm traces #2, #3, and #4 are overlapped for comparison. (C) Statistical analysis over 10 experiments. Note that the total Cm change induced by an AP train depends not only on exocytosis, but also on endocytosis after each Cm jump. To reduce this effect, secretion induced by an AP train was indicated by ΔCm, which was the sum of Cm jumps immediately after each AP burst. Three minutes after the third stimulation, the AP-induced secretion was decreased to 61 ± 21% of the control-2 level (P < 0.01, n = 10).
Discussion
We have shown that HCN channels are permeable to Ca2+. Brief activation of Ih in DRG neurons nearly doubles the action potential-induced secretion from DRG neurons. Thus, the fractional Ca2+ influx through the slow Ih channels may affect Ca2+-dependent synaptic transmission.
Permeability of HCN Channels to Ca2+. Ih channels are widely expressed in peripheral and central neurons as well as in cardiac myocytes. The distinct properties of Ih are believed to be associated with a variety of physiological events (25, 34). The discovery of calcium permeation through Ih channels could provide a novel mechanism coupling membrane hyperpolarization with Ih-mediated events.
The fractional Ca2+ current of the HCN4 channel (0.6%) is small compared with other Ca2+-permeable channels such as the nicotinic acetylcholine receptor (2.5%) (6), the NMDA-R (8–10%) (7, 9), the non-NMDA-R (0.5–5%) (7, 9), cyclic nucleotide-gated channels (>10%) (35), and L-type Ca2+ channels (100%) (3). However, Ih channels have slow kinetics and are activated during the long interval between two action potentials or bursts of action potentials. Under certain experimental conditions, the accumulated fractional Ca2+ current through Ih channels could be sufficient to modulate Ca2+-dependent cellular functions, such as neurotransmitter release from DRG neurons (Fig. 6).
Ih channels are permeable to both Na+ and K+ ions. Evidence from myocytes has shown that ion permeation although Ih channels does not fully obey the Goldman–Hodgkin–Katz (GHK) equation (15). The independent ion permeation through a multiple-cation channel, an assumption for using the GHK equation, apparently does not work for Ih channels. Our discovery of Ca2+ permeation through Ih channels provides an additional piece of evidence for the idea that precautions must be taken when applying the GHK equation to channels that are permeable to multiple ions.
Role of Ca2+ Influx Through Ih Channels in Neurons. Two general mechanisms have been proposed for synaptic facilitation: enhanced presynaptic calcium rise (Ca2+-dependent) and direct modulation of the release process (Ca2+-independent). In chromaffin cells and at many synapses, accumulation of presynaptic Ca2+ by higher frequencies of action potentials can facilitate synaptic transmission (26, 36–38).
In hippocampal mossy fibers, activation of cAMP/PKA mediates a form of presynaptic LTP, which is interpreted as a Ca-independent process, although the molecular mechanism remains unresolved. One report postulates that Ih is responsible for cAMP-dependent LTP because the Ih antagonist ZD7288 blocks the LTP (22) whereas two other reports argue that the block could be due to side-effects of ZD7288 so Ih is not involved in LTP (23, 24).
Other evidence for a Ca2+-independent mechanism has come from studies of 5-HT action at the crayfish neuromuscular junction (21). These studies showed that enhanced release induced by 5-HT is not due to PKA activation; the 5-HT-induced depolarization is dramatically inhibited by both Cs+ and ZD7288, in agreement with the pharmacological profile of Ih; and the activation of Ih by hyperpolarization is sufficient to enhance synaptic transmission. Thus, these workers suggested that the downstream event is probably due to direct interaction between Ih channels and vesicles, i.e., by means of a Ca2+-independent mechanism. In contrast to the study on mossy fiber LTP, which depended on the use of ZD7288, the hyperpolarization-mediated facilitation in crayfish was shown to be mediated by Ih channels (figure 8 of ref. 21) in experiments that did not require the use of this compound. Thus, the hyperpolarization-induced facilitation in crayfish observed by Beaumont and Zucker (21) was probably due to Ca influx through Ih channels.
Our results show that Ca2+ ions can pass through Ih channels and significantly elevate cytosolic Ca2+ levels. In DRG neurons, the amplitude of Ih is ≈20% of the 1 nA voltage-gated Ca2+ current (Fig. 4) so the Ca2+ inflow during a 60-s activation of Ih would be equivalent to the Ca2+ influx induced by a 72-ms (20%·0.6%·60 s) depolarization through voltage-gated Ca2+ channels (or 24 action potentials of 3 ms duration). The Ih-mediated Ca2+ increase may facilitate subsequent stimulus-induced secretion in two ways. First, like the facilitation induced by a high action-potential frequency (26, 36), the elevated basal Ca2+ assists the cell in secretion. Second, in contrast to voltage-gated Ca2+ channels or Ca2+ stores that both trigger secretion on stimulation (38), Ih channels could be a more effective pathway for increasing the readily releasable vesicle pool (RRP), because the slow influx of Ca2+ through the Ih channel is probably not sufficient to trigger secretion but ensures the increase of the RRP (39). Thus, Ca2+ influx through Ih channels provides a mechanism for Ih-mediated synaptic plasticity. Interestingly, the fractional Ca2+ current of Ih is close to that of the low Ca2+-permeable AMPA-R channels in Purkinje neurons (40). This result implies that low Ca2+ permeation cation channels may play important roles in synaptic transmission.
We note that, although we favor the hypothesis that Ca2+ influx through Ih channels is responsible for the facilitation of AP-induced secretion found in this work (as well as the synaptic facilitation induced by hyperpolarization at the crayfish neuromuscular junction; ref. 21), we cannot exclude other possibilities, such as a direct link between Ih channels and some factor(s) downstream from Ca2+ (21–24). In addition, although the 30-s hyperpolarization is sufficient to induce AP-induced facilitation of cell secretion, it is not clear whether such hyperpolarization occurs in DRG neurons in vivo. However, in pacemaker neurons (such as thalamic neurons) or cardiac cells, where Ih is cyclically activated, Ca2+ influx through Ih channels may modulate Ca2+-dependent synaptic transmission or heartbeat.
Mechanism of Ca2+ Permeation Through Ih Channels. Ca2+ must pass through the pore region of Ih channels because blockade of Ih also eliminates the Ca2+ influx. The four HCN channels (HCN1 to -4) share the same pore region (16), implying that the Ca2+ permeation of HCN1 to -4 channels (including Ih in DRG neurons, Figs. 4 and 5) are probably similar (i.e., Pf = 0.6%). However, we do not yet know the mechanism by which calcium permeates Ih channels. The molecular mechanism of Ca2+ permeation through Ih channels might be distinct from that of glutamate channels. In NMDA and AMPA channels, the fractional Ca2+ current increases 5-fold when external [Ca2+] is increased from 2 to 10 mM (9). The critical Q/R site for Ca2+ permeation through glutamate channels is thus not saturated in bathing solutions containing 2–10 mM Ca2+. In contrast, the Pf of HCN channels is unchanged when external [Ca2+] is changed from 2 to 20 mM (X.Y. and Z.Z., unpublished observations). This finding implies that Ca2+ may have saturated at some unknown Ca2+ binding site(s) in the Ih channel pore. Future work is needed to determine the critical site(s) in Ih channel proteins that are responsible for Ca2+ permeation.
Supplementary Material
Acknowledgments
We thank Drs. I. Bruce, M. M. Poo, and H. P. Cheng for suggestions on the manuscript. This work was supported by grants from the Major State Basic Research Program of China (Grant G2000077800), the National Natural Science Foundation of China (Grant 30330210), the Chinese Academy of Sciences Instrument Program, and the Science and Technology Commission of Shanghai Municipality.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NMDA-R, N-methyl-d-aspartate receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; VDCC, voltage-dependent Ca2+ channel; HCN, hyperpolarization-activated cyclic nucleotide-gated; DRG, dorsal root ganglion; Cm, membrane capacitance; LTP, long-term potentiation; HEK, human embryonic kidney; AP, action potential.
References
- 1.Katz, B. (1969) The Release of Neural Transmitter Substances (Thomas, Springfield, IL).
- 2.Bers, D. M. (2002) Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- 3.Zhou, Z. & Bers, D. M. (2000) J. Physiol. (London) 523, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hille, B. (2001) Ionic Channels in Excitable Membranes (Sinauer, Sunderland, MA), 3rd Ed.
- 5.Mayer, M. L. & Westbrook, G. L. (1987) J. Physiol. (London) 394, 501–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, Z. & Neher, E. (1993a) Pflügers Arch. 425, 511–517. [DOI] [PubMed] [Google Scholar]
- 7.Schneggenburger, R., Zhou, Z., Konnerth, A. & Neher, E. (1993) Neuron 11, 133–143. [DOI] [PubMed] [Google Scholar]
- 8.Dzeja, C., Hagen, V., Kaupp, U. B. & Frings, S. (1999) EMBO J. 18, 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. (1995) J. Physiol. (London) 485, 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn, J. T., Grunwald, M. E. & Yau, K, W. (1996) Annu. Rev. Physiol. 58, 395–426. [DOI] [PubMed] [Google Scholar]
- 11.Koh, D. S., Geiger, J. R., Jonas, P. & Sakmann, B. (1995) J. Physiol. (London) 485, 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollard, P., Seward, E. P. & Nowycky, M. C. (1995) Proc. Natl. Acad. Sci. USA 92, 3065–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers, M. & Dani, J. A. (1995) Biophys. J. 68, 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiFrancesco, D. (1993) Annu. Rev. Physiol. 55, 455–472. [DOI] [PubMed] [Google Scholar]
- 15.Yu, H. G., Chang, F. & Cohen, I. S. (1995) J. Physiol. (London) 485, 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro, B., Liu, D. T., Yao, H., Bartsch, D., Kandel, E. R., Siegelbaum, S. A. & Tibbs, G. R. (1998) Cell 93, 717–729. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig, A., Zong, X., Jeglitsch, M., Hofmann, F. & Biel, M. (1998) Nature 393, 587–591. [DOI] [PubMed] [Google Scholar]
- 18.Santoro, B., Chen, S., Luthi, A., Pavlidis, P., Shumyatsky, G. P., Tibbs, G. R. & Siegelbaum, S. A. (2000) J. Neurosci. 20, 5264–5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moosmang, S., Stieber, J., Zong, X., Biel, M., Hofmann, F. & Ludwig, A. (2001) Eur. J. Biochem. 268, 1646–1652. [DOI] [PubMed] [Google Scholar]
- 20.Shi, W., Wymore, R., Yu, H. G., Wu, J. Y., Wymore, R., Pan, Z., Robinson, R. B., Dixon, J. E., McKinnon, D. & Cohen, I. S. (1999) Circulation Res. 85, e1–e6. [DOI] [PubMed] [Google Scholar]
- 21.Beaumont, V. & Zucker, R. S. (2000) Nat. Neurosci. 3, 133–141. [DOI] [PubMed] [Google Scholar]
- 22.Mellor, J., Nicoll, R. A. & Schmitz, D. (2002) Science 295, 143–147. [DOI] [PubMed] [Google Scholar]
- 23.Chevaleyre, V. & Castillo, P. E. (2002) Proc. Natl. Acad. Sci. USA 99, 9538–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamiya, H., Umeda, K., Ozawa, S. & Manabe, T. (2002) J. Neurosci. 22, 10524–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegelbaum, S. (2000) Nat. Neurosci. 3, 101–102. [DOI] [PubMed] [Google Scholar]
- 26.Zhou, Z. & Misler, S. (1995) J. Biol. Chem. 270, 3498–3505. [PubMed] [Google Scholar]
- 27.Wu, J. J., He, L. L., Zhou, Z. & Chi, Z. W. (2002) Biochemistry 41, 2844–2849. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, C. & Zhou, Z. (2002) Nat. Neurosci. 5, 425–430. [DOI] [PubMed] [Google Scholar]
- 29.Grynkiewicz, G., Poenie, M. & Tsien, R. Y. (1985) J. Biol. Chem. 260, 3440–3450. [PubMed] [Google Scholar]
- 30.Zhou, Z. & Neher, E. (1993b) J. Physiol. (London) 469, 245–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig, A., Zong, X., Stieber, J., Hullin, R., Hofmann, F. & Biel, M. (1999) EMBO J. 18, 2323–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birch, B. D., Kocsis, J. D., Di Gregorio, F., Bhisitkul, R. B. & Waxman, S. G. (1991) J. Neurophysiol. 66, 719–728. [DOI] [PubMed] [Google Scholar]
- 33.Gillis, K. D. (1995) in Single Channel Recording, eds. Sakmann, B. & Neher E. (Plenum, New York), 2nd Ed., pp. 155–198.
- 34.Gauss, R., Seifert, R. & Kaupp UB. (1998) Nature 393, 583–587. [DOI] [PubMed] [Google Scholar]
- 35.Frings, S., Seifert, R., Godde, M. & Kaupp UB. (1995) Neuron 15, 169–179. [DOI] [PubMed] [Google Scholar]
- 36.Zucker, R. S. (1973) J. Physiol. (London) 229, 787–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elhamdani, A., Zhou, Z. & Artalejo, C. R. (1998) J. Neurosci. 18, 6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Augustine, G. J. & Neher, E. (1992) J. Physiol. (London) 450, 247–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Ruden, L. & Neher, E. (1993) Science 262, 1061–1065. [DOI] [PubMed] [Google Scholar]
- 40.Tempia, F., Kano, M., Schneggenburger, R., Schirra, C., Garaschuk, O., Plant, T. & Konnerth A. (1996) J. Neurosci. 16, 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.