Abstract
Aim:
As injection is not an ideal means for insulin delivery, various attempts have been made to administer insulin orally until now. The development of an oral dosage form of insulin would help diabetic patients and make the treatment more convenient. The aim of the present study is to evaluate the effectiveness of an oral insulin formulation containing polar and non-polar ingredients.
Materials and Methods:
New excipient for oral insulin administration in normal and diabetic rats was evaluated by measuring blood glucose concentrations in two groups (10 rats each) of normal and streptozotocin-induced diabetic rats. Oral insulin was administrated and blood glucose was measured by glucometer at 0, 1, 2, 3 and 4 h post-feeding. The data was compared by Student's t test.
Results:
Oral insulin formulation significantly (P<0.05) reduced blood glucose from 100 mg/dl to 33.73 mg/dl and 451.66 mg/dl to 200.83 mg/dl at 4 h in normal and diabetic rats, respectively.
Conclusion:
The novel excipient used could protect insulin from gastric and pancreatic enzymes and reduce blood glucose concentration in both healthy and diabetic rats suggesting that oral delivery of insulin is feasible in a near future.
KEY WORDS: Blood glucose, normal and diabetic rats, oral insulin
Introduction
Since the initial discovery of insulin by Banting and Best in 1922, an orally effective form of the drug has been an elusive goal for many investigators. Current dosage regimens of insulin in diabetes mellitus comprise of multiple subcutaneous injections per day.[1,2]
Considerable research effort has been devoted to the development of alternative modes of safe and effective insulin delivery. Protection of the insulin against degradation in the gastrointestinal tract is the major obstacle.[3] Such a formulation would have to bypass the enzymatic barrier of the intestinal tract and the physical barrier of the intestinal epithelium. Permeation enhancers, protease inhibitors enteric coatings and polymer microsphere formulations have been applied to develop oral insulin formulations with varying degrees of success.[4–8] Oral formulations are designed to resist insulin digestion in the gastro intestinal tract using enteric coatings, micro and nano-particulates, mucoadhesive excipients, hydrogel liposomes and permeation enhancers.[9,10]
Since our observation that insulin can cross the intestinal epithelium,[1] the formulation was designed to contain biologically active insulin and protect the protein from enzymatic degradation in the intestinal tract and facilitate systemic uptake of the molecule. The present study evaluates the effectiveness of new oral insulin formulation containing polar and non-polar ingredients.
Materials and Methods
Individual processing parameters in the preparation of insulin immobilized in a semi synthetic matrix were first refined, and two polar (26818) and non-polar (26819) formulations were subsequently investigated. The in vivo activity of oral immobilized insulin in both systems were evaluated by measuring blood glucose concentrations in normal and streptozotocin (60 mg/kg, i.p.) diabetic Wistar rats, after oral administration at the dose of 10 I.U./kg body weight. The animal care was provided under the supervision of a qualified veterinarian. This study was performed under control of Iranian Society for the Prevention of Cruelty to Animals. Adult Wistar rats, weighing 250-265 g were obtained from the animal center of the university of Jondishapour. The animals were kept under standard conditions and had access to a standard diet and clean drinking water.
The experiment was conducted to assess the oral activity of the various insulin formulations in two groups of 10 rats each in normal and streptozotocin-induced diabetic rats. The first was a simple oral bioactivity test in which fasted rats were weighed. Baseline blood samples (approximately 100 μl) were taken by tail vein and blood glucose was estimated with commercial glucometer (Cleverchek, Taiwan) before administration of the drug. Each rat was its own control and received vehicle (water) orally and blood glucose at zero time before active drug was used. Oral insulin samples in 1 ml of suspension buffer were fed to each rat through a stainless steel gastric tube. Repeat blood samples were taken at 1, 2, 3 and 4 h post-feeding. The blood glucose of each sample was estimated with the aid of a glucose sensor immediately. The data was analyzed and compared statistically by Student's t-test. P≤ 0.05 was considered as statistically significant.
Results
The polar system of oral insulin produced dose-dependent reduction in blood glucose in normal and diabetic rats. This formulation significantly reduced blood glucose from 100 mg/dl (before treatment) to 33.73 mg/dl 4 h after treatment in normal rats (P<0.05). This change was dependent to time of treatment [Figure 1]. Also, this treatment significantly decreased blood glucose from 451.66 mg/dl (before treatment) to 200.83mg/dl 4 h after treatment in diabetic rats [Figure 2](P<0.05).
Figure 1.
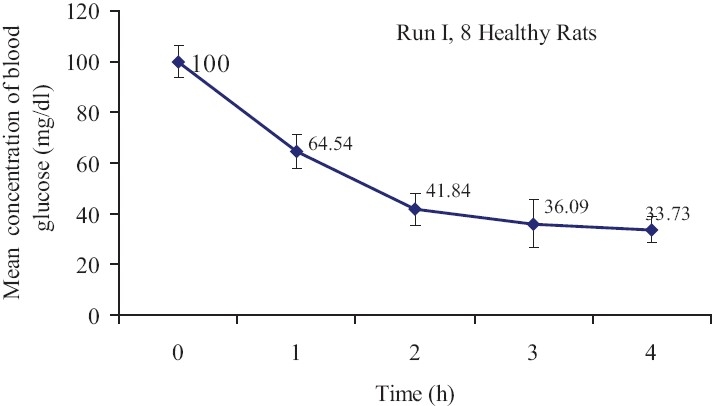
Mean blood glucose concentration (mean±SE) in healthy rats after oral insulin administration. The initial concentrations of blood glucose (mg/ dl) were considered as 100% and the weight of rats were between 250 and 265 g(n=10, P<0.05)
Figure 2.
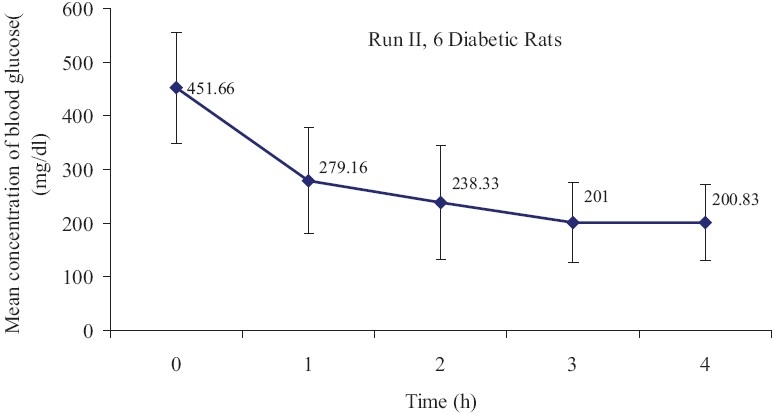
Mean concentration of blood glucose (mean±SE) in diabetic rats after oral insulin administration(n=10, P<0.05)
Discussion
Insulin is a sensitive protein that is digested in acidic pH of stomach. It needs to be changed to be more acid stable. Five of seven batches of the two immobilized insulin formulations fit the baseline criteria for approval of the new formulation. The formulation consists of an aqua sol containing a solid hydrophilic phase.
The present study indicates a significant reduction in blood glucose with the oral insulin and supports the usage of this novel system for better glycemic control. There is no doubt that the oral form of insulin eliminates or reduces the need to use injection as a mode of administering insulin. Investigation of the safety and tolerability of a single oral dose of modified human insulin is necessary.
While these results are certainly encouraging in animal model systems, there is still a long way to go before oral insulin is introduced for diabetic patients.
The material used in the present study is similar to other studies; but the technical approach is different. For examples, Sonaje et al, reported oral delivery of aspart-insulin in rat by using a pH-responsive nanoparticle system composed of chitosan and poly (gamma-glutamic acid). They demonstrated that nanoparticle system allowed the absorption of aspart-insulin into the systemic circulation, while the drug carrier was mainly retained in the gastrointestinal tract. Also it has been reported that enteric-coated capsule of aspart-insulin nanoparticle enhanced the intestinal absorption of insulin and provided a prolonged reduction in blood glucose levels.[11,12] Absorption of oral insulin is feasible under fasting conditions in patients with type 2 diabetes.[13] The serum level of insulin increased after 2 h, resulting in a decrease in blood glucose levels. It has been demonstrated that nanoparticles-based microemulsions had a consistent and significant hypoglycemic effect over controls for up to 36 h without significant change in serum insulin levels.[14] In addition, application of poly (epsilon-caprolactone)/eudragit nanoparticles increased oral absorption of aspart-insulin in diabetic rats and the postprandial peak suppression was prolonged for more than 24 h as compared to regular insulin.[15] These results are similar to our observation. Sharma et al., also evaluated insulin loaded microemulsions prepared by using didodceyldimethylammonium bromide as the surfactant, propylene glycol as the co-surfactant, triacetin as the oil phase and insulin solution as the aqueous phase. This microemulsion enhanced 10-fold bioavailability of insulin compared to orally administered plain insulin solution in healthy rats.[16]
To conclude, this unique formulation of oral insulin decreased blood glucose in normal and diabetic rats.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kooshapur H, Chaideh M. Intestinal transport of Human Insulin in Rat. Med J Islam Acad Sci. 1999;12:5–11. [Google Scholar]
- 2.Wong TW. Design of oral insulin delivery systems. J Drug Target. 2010;18:79–92. doi: 10.3109/10611860903302815. [DOI] [PubMed] [Google Scholar]
- 3.Arbit E, Kidron M. Oral Insulin: The rationale for this approach and current developments. J Diabetes Sci Technol. 2009;3:562–7. doi: 10.1177/193229680900300322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touitou E, Rubinstein A. Targeted internal delivery of insulin to rats. Int J Pharm. 1986;3:95–9. [Google Scholar]
- 5.Morishita I, Morishita M, Takayama K, Machida Y, Nagai T. Enteral insulin delivery by microspheres in three different formulations using Eudragit l100 and S100. Int J Pharm. 1993;91:29–37. [Google Scholar]
- 6.Morishita M, Morishita I, Takayama K, Machida Y, Nagai T. Site-dependent effect of aprotinin, sodium caprate, Na2 EDTA and sodium glycocholate on the intestinal absorption of insulin. Biol Pharm Bull. 1993;16:68–72. doi: 10.1248/bpb.16.68. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto A, Taniguchi T, Rikyuu K, Tsuji T, Fujita T, Murakami M, et al. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res. 1994;11:1496–500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- 8.Mesiha M, Plakogiannis F, Vejosoth S. Enhanced oral absorption of insulin from desolvated fatty acid–sodium glycocholate emulsions. Int J Pharm. 1994;111:213–6. [Google Scholar]
- 9.Heinemann L, Jacques Y. Oral insulin and buccal insulin: A critical reappraisal. J Diabetes Sci Technol. 2009;3:568–84. doi: 10.1177/193229680900300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer H, Khedkar A, Verma M. Oral insulin -A review of current status. Diabetes Obes Metab. 2010;12:179–85. doi: 10.1111/j.1463-1326.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, et al. Enteric-coated capsules filled with freeze-driedchitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–94. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Sonaje K, Lin KJ, Wey SP, Lin CK, Yeh TH, Nguyen HN, et al. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: Oral delivery using pH- Responsive nanoparticles vs.subcutaneous injection. Biomaterials. 2010;31:6849–58. doi: 10.1016/j.biomaterials.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Kapitza C, Zijlstra E, Heinemann L, Castelli MC, Riley G, Heise T. Oral insulin: A comparison with subcutaneous regular human insulin in patients with type 2 diabetes. Diabetes Care. 2010;33:1288–90. doi: 10.2337/dc09-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf A, Rades T, Hook SM. Oral insulin delivery using nanoparticles based onmicroemulsions with different structure-types: Optimization and in vivoevaluation. Eur J Pharm Sci. 2009;37:53–61. doi: 10.1016/j.ejps.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Damgé C, Socha M, Ubrich N, Maincent P. Poly (epsilon- caprolactone)/eudragit nanoparticles for oral delivery of aspart-insulin in the treatment of diabetes. J Pharm Sci. 2010;99:879–89. doi: 10.1002/jps.21874. [DOI] [PubMed] [Google Scholar]
- 16.Sharma G, Wilson K, van der Walle CF, Sattar N, Petrie JR, Ravi Kumar MN. Microemulsions for oral delivery of insulin: Design, development and evaluation in streptozotocin induced diabetic rats. Eur J Pharm Biopharm. 2010;76:159–69. doi: 10.1016/j.ejpb.2010.07.002. [DOI] [PubMed] [Google Scholar]


