Abstract
Objective:
To evaluate the effect of Ginkgo biloba extract (EGB) on the serum levels of cytokines in patients suffering from chronic, age-related neurological disorders (NDs).
Materials and Methods:
Patients received 9.6 mg of EGB twice daily for 8 weeks. Serum levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were measured by enzyme-linked immunosorbent assay (ELISA) before and after treatment.
Results:
The serum level of IL-6 was significantly higher in ND patients as compared to the healthy controls. After these patients underwent 4 and 8 weeks of EGB treatment, their IL-6 levels were shown a statistically significant (P<0.05) decline to near normal values. No significant changes were observed in serum levels of IL-1β and TNF-α after EGB treatment. We also observed an inverse relationship between ND and serum cholesterol levels.
Conclusions:
EGB may exert its beneficial effects in patients suffering from NDs through down-regulation and suppression of IL-6 secretion.
KEY WORDS: ELISA, interleukin-6, Ginkgo biloba extract, neurological disorder
Introduction
Neurological disorders (NDs) are functional abnormalities that occur in diverse body areas and often appear as age-related decrease in the functioning of the brain, spinal cord, muscles, or nerves in adults.[1] Both acute and chronic ND, including stroke and Alzheimer's disease (AD), are associated with inflammation. This neuroinflammation is characterized by activation of local glial cells and production of various pro-inflammatory mediators which lead to the abnormalities in neurons and astrocytes.[2,3] Thus, cytokine IL-1β has been implicated in the extensive inflammation and progressive neurodegeneration that occurs after ischemia.[4] Brain ischemia induces production of both TNF-α and IL-1β which may disrupt phosphatidylcholine homeostasis by increasing its hydrolysis and inhibiting the synthesis.[5] IL-6, a pleiotrophic cytokine, appears to participate in neurodegenerative processes including AD[6] and may be an early predictor of the one-year survival in patients following an ischemic stroke or transient ischemic attack.[7] In patients suffering from ND, the serum level, IL-6, rises markedly as a response of neural cells in almost every infectious and/or inflammatory condition. Il-6 has both pro- and anti-inflammatory effects. However, the exact role of IL-6 is difficult to predict because of its pleiotropic nature.[8]
Ginkgo biloba extract (EGB), containing 24% ginkgo flavone glycoside and 6% terpenlactones, is extracted from Ginkgo biloba leaves.[9] The extract show a significant activity on central nervous system (CNS) and promotes antioxidant, anti-inflammatory, and immunomodulatory responses. It has also been shown to increase the viability of neuronal cells under hydrogen peroxide-induced oxidative stress.[10] Nitrite accumulation during the reaction of NO with oxygen is also inhibited by EGB.[11] As it has negligible side effects, EGB is commonly used as a treatment for central neural system disorders, acute pancreatitis, myocardial disease, and intestinal ischemia/reperfusion injury.[12–15]
The aim of this study was to evaluate the effect of EGB on a number of pro-inflammatory cytokines important for the progression of ND. We hypothesized that the in vivo benefits of EGB may be associated with serum levels of IL-1β, IL-6, and TNF-α in patients suffering from NDs. We also examined the potential effects of EGB on body mass index, plasma glucose, triglycerides, and total cholesterol.
Materials and Methods
Patients and Clinical Samples
Patients who had experienced NDs such as myelopathy, malignant spinal cord tumor, or cervical spinal cord trauma with myelopathic complications were recruited from the Kaohsiung Armed Forces General Hospital in southern Taiwan between April and October 2009. Healthy blood donors with matching age and sex to the ND patients were used as the control group. All individuals agreed to participate in the study. Clinical and laboratory examination included age and body mass index, plasma glucose, cholesterol, and triglyceride levels. The study plan was accepted and supported by the ethics committee of Kaohsiung Armed Forces General Hospital.
Preparation of the EGB was based on a reported method.[9] For the preparation of EGB, Ginkgo biloba leaves were harvested while they were still green, then they were dried and subjected to extraction procedure. The extraction procedure begins with an acetone–water mixture, followed by the removal of lipophillic constituents and then the concentration of the active components in the extract. EGB used in this study was purchased from Sinphar Pharmaceutical Co., Ltd., Taiwan. The ND patients received 9.6 mg of EGB twice daily orally for 8 weeks. A 5 ml blood sample was withdrawn at 4-week interval. The study protocol ensured that blood was taken at the same time of the day to exclude diurnal variations in cytokines and the procedure was standardized based upon the last administration of EGB.
Enzyme-linked Immunosorbent Assay (ELISA)
Serum was kept frozen at –20°C until the measurement of IL-6, IL-1β, and TNF-α with commercial ELISA kits (Cayman Chemical Company) according to the manufacturer's instructions. The cytokines were measured in duplicate. Values were expressed as means ± S.E.M. Analysis of variance (ANOVA) was used to assess the statistical significance of the differences and P<0.05 was considered to be statistically significant.
Results
We recruited 79 patients affected by ND and 85 controls who did not have ND [Table 1]. Differences in cholesterol levels were statistically significant, but no significant differences were noted for body mass index, plasma glucose, or triglyceride levels. The initial serum level of IL-6 in ND patients was 18.25±5.25 pg/ml and was significantly higher than that of the control group (8.52±1.34 pg/ml). After four weeks of EGB administration, the mean level of serum IL-6 was decreased to 12.09±2.34 pg/ml and after 8 weeks, it was further decreased to 10.59±1.39 pg/ml [Table 2]. The decrease of IL-6 levels in patients of ND was statistically significant suggesting a role of this cytokine in prevention of ND [Figure 1]. No significant change was noticed in the serum levels of IL-1β and TNF-α before and after EGB treatment (data not shown).
Table 1.
Demographic details of study subjects
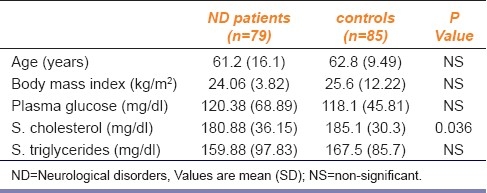
Table 2.
Effect of Ginkgo biloba extract on serum levels of interleukin-6 (IL-6)
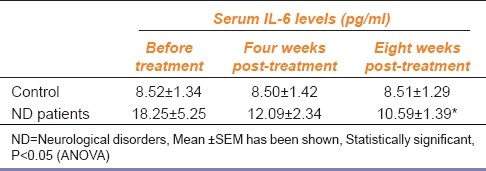
Figure 1.
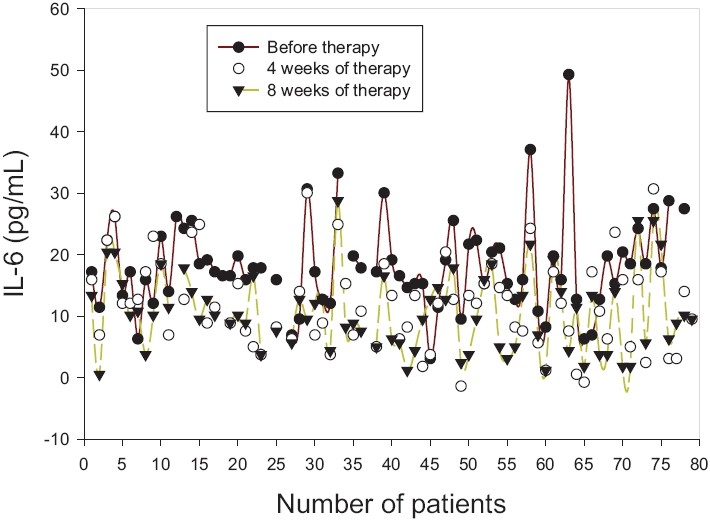
Profiles of the serum levels of IL-6 (pg/ml) in patients with neurological disorders (ND) during Ginkgo biloba extract therapy
Discussion
Inflammation has been reported in numerous NDs such as Parkinson's disease, stroke, and Alzheimer's disease (AD). In many of these disorders, pro-inflammatory cytokines like IL-1, IL-6, and TNF-α are produced by several cell types, including endothelial cells, microglia, neurons, platelets, leukocytes, and fibroblasts.[16] Clinical studies have reported increased levels of IL-6 during cerebral ischemia and during early neurological worsening after stroke.[17,18] The peripheral blood levels of IL-6 are particularly high in stroke patients and correlate with the infarct size.
The expression of IL-6 is regulated mainly at the transcriptional level in neural cells. The plasma levels are strongly influenced by gene variants indicating a clinical relevance of IL-6 gene polymorphism in several diseases, including Alzheimer's disease and lacunar infarcts. EGB has been used clinically on the basis of its antioxidant activity and actions involving scavenging of free radicals, prevention of lipid peroxidation, and reduction in nitric oxide release. In vitro studies have shown that EGB significantly inhibits TNF-α-induced protein expression of vascular cellular adhesion molecule-1 and intracellular adhesion molecule-1 in human aortic endothelial cells (HAECs).[19] It has also been found that EGB treatment decreased the concentrations of PGE2, and TNF-α and the production of NO on LPS-induced RAW 264.7 cells.[20]
The anti-inflammatory effect of EGB may be linked to the down-regulation of transcriptional factor activity such as NF-κB or AP-1. In vivo experiments have shown that EGB could reduce CCl4-induced chronic liver injury and liver fibrosis in rats,[21] apparently by reducing oxidative stress that normally would result in lipid peroxidation in the hepatocytes. Experiments have also indicated that ginkgo-flavone glycosides are the main components of EGB and that the hydroxyl functional group may serve to capture oxygen-derived free radicals.[22]
The present study is first to show a significant decline in the IL-6 level in ND patients. Continuous treatment with EGB for eight weeks resulted in a significant decrease in the serum level of IL-6. Our findings are consistent with the results described before showing that EGB significantly reduces the expression of IL-6 at both the mRNA and protein levels in colon tissues of an experimental colitis rat model.[23] IL-6 is recognized for its role in the acute phase inflammatory response, which is characterized by production of a variety of hepatic proteins termed acute-phase proteins, such as C-reactive protein (CRP) and fibrinogen. Activation of the acute-phase response has been implicated in the pathogenesis of ischemic stroke. Elevated serum levels of IL-6 have been observed in patients with stroke. IL-6 also appears to be an important regulator of acute-phase responses in neurons and astrocytes.[24,25] Our study demonstrates that EGB has marked anti-inflammatory action in addition to its antioxidant properties. The mechanism underlying EGB effect on inflammation is not yet clear. It is reported that EGB could inhibit hydrogen peroxide-induced activation of NF-κB in vascular endothelial cells.[26] It can also inhibit the generation of reactive oxygen species induced by TNF-α and the activation of NF-κB in human aortic endothelial cells.[20] Several cis-acting response elements mediate activation of the IL-6 promoter including those for AP-1, nuclear factor IL-6, NF-κB, and the multiple response elements. Since the promoter regions of IL-6 contain a NF-κB binding motif, one possible mechanism for the decreased levels of IL-6 in response to EGB may involve regulation of NF-κB.[27]
Our study, thus, demonstrated that EGB may have beneficial effects in patients suffering from NDs. It is a natural antioxidant, has no adverse side-effects, and appears to be safe and inexpensive. It may therefore be useful as a herbal medicine for clinical treatment of NDs.
Acknowledgments
We thank all individuals who agreed to participate in this study. We thank the Kaohsiung Armed Forces General Hospital in Kaohsiung collecting samples and giving us certificate of Institutional Review Board approval. We thank the Asia-Pacific Biotech Developing, Inc. in Kaohsiung assisting the analysis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Watanabe CM, Wolffram S, Ader P, Rimbach G, Packer L, Maguire JJ, et al. The in vivo neuromodulatory effects of the herbal medicine ginkgo biloba. Proc Natl Acad Sci U S A. 2001;98:6577–80. doi: 10.1073/pnas.111126298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slegers H, Joniau M. Lipopolysaccharide-enhanced expression of interleukin-6 in dibutyryl cyclic AMP-differentiated rat C6 glioma. J Neurochem. 1996;66:466–73. doi: 10.1046/j.1471-4159.1996.66020466.x. [DOI] [PubMed] [Google Scholar]
- 3.Fiebich BL, Lieb K, Berger M, Bauer J. Stimulation of the sphingomyelin pathway induces interleukin-6 gene expression in human astrocytoma cells. J Neuroimmunol. 1995;63:207–11. doi: 10.1016/0165-5728(95)00145-x. [DOI] [PubMed] [Google Scholar]
- 4.Akuzawa S, Kazui T, Shi E, Yamashita K, Bashar AH, Terada H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J Vasc Surg. 2008;48:694–700. doi: 10.1016/j.jvs.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Adibhatla RM, Hatcher JF. Cytidine 5’-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30:15–23. doi: 10.1007/s11064-004-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: A field in its infancy. J Alzheimers Dis. 2010;19:355–61. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 7.Shenhar-Tsarfaty S, Ben Assayag E, Bova I, Shopin L, Fried M, Berliner S, et al. Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int J Stroke. 2010;5:16–20. doi: 10.1111/j.1747-4949.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1:795–805. [PMC free article] [PubMed] [Google Scholar]
- 9.De Feudis FV, Drieu K. Ginkgo biloba Extract (EGb 761) and CNS functions: Basic studies and clinical applications. Curr Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- 10.Oyama Y, Chikahisa L, Ueha T, Kanemaru K, Noda K. Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 1996;712:349–52. doi: 10.1016/0006-8993(95)01440-3. [DOI] [PubMed] [Google Scholar]
- 11.Kobuchi H, Droy-Lefaix MT, Christen Y, Packer L. Ginkgo biloba extract (EGb-761): Inhibitory effect on nitric oxide production in the macrophage cell line RAW 264.7. Biochem Pharmacol. 1997;53:897–903. doi: 10.1016/s0006-2952(96)00873-8. [DOI] [PubMed] [Google Scholar]
- 12.Pehlivan M, Dalbeler Y, Hazinedaroglu S, Arikan Y, Erkek AB, Gunal O, et al. An assessment of the effect of Ginkgo biloba egb 761 on ischemia reperfusion injury of intestine. Hepatogastroenterology. 2002;49:201–4. [PubMed] [Google Scholar]
- 13.Maitra I, Marcocci L, Droy-Lefaix MT, Packer L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem Pharmacol. 1995;49:1649–55. doi: 10.1016/0006-2952(95)00089-i. [DOI] [PubMed] [Google Scholar]
- 14.Kusmic C, Basta G, Lazzerini G, Vesentini N, Barsacchi R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: A link between vitamin E preservation and prostaglandin biosynthesis. J Cardiovasc Pharm. 2004;44:356–62. doi: 10.1097/01.fjc.0000137164.99487.42. [DOI] [PubMed] [Google Scholar]
- 15.Zeybek N, Gorgulu S, Yagci G, Serdar M, Simsek A, Kaymakcioglu N, et al. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. J Surg Res. 2003;115:286–93. doi: 10.1016/s0022-4804(03)00190-2. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–45. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Berti R, Williams AJ, Moffett JR, Hale SL, Velarde LC, Elliott PJ, et al. Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cereb Blood Flow Metab. 2002;22:1068–79. doi: 10.1097/00004647-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 19.Chen JW, Chen YH, Lin FY, Chen YL, Lin SJ. Ginkgo biloba extract inhibits tumor necrosis factor-alpha-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:1559–66. doi: 10.1161/01.ATV.0000089012.73180.63. [DOI] [PubMed] [Google Scholar]
- 20.Ilieva I, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, et al. The effects of Ginkgo biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2004;79:181–7. doi: 10.1016/j.exer.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 21.He SX, Luo JY, Wang YP, Wang YL, Fu H, Xu JL, et al. Effects of extract from Ginkgo biloba on carbon tetrachloride-induced liver injury in rats. World J Gastroenterol. 2006;12:3924–8. doi: 10.3748/wjg.v12.i24.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res. 2001;15:449–51. doi: 10.1002/ptr.814. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006:92642. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda Y, Matsumoto M, Hori O, Kuwabara K, Ogawa S, Yan S, et al. Hypoxia/reoxygenation-mediated induction of astrocyte interleukin-6: A paracrine mechanism potentially enhancing neuron survival. J Exp Med. 1994;180:2297–308. doi: 10.1084/jem.180.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Tanaka K, Nogawa S, Nagata E, Ito D, Dembo T, et al. Temporal profile and cellular localization of interleukin-6 protein after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1256–62. doi: 10.1097/00004647-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Wei Z, Peng Q, Lau BH, Shah V. Ginkgo biloba inhibits hydrogen peroxide-induced activation of nuclear factor-κB in vascular endothelial cells. Gen Pharmacol. 1999;33:369–75. doi: 10.1016/s0306-3623(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 27.Grassl C, Luckow B, Schlondorff D, Dendorfer U. Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol. 1999;10:1466–77. doi: 10.1681/ASN.V1071466. [DOI] [PubMed] [Google Scholar]


