Abstract
The war against cancer has seen a proliferation in armamentarium over the last decades. A new antineoplastic agent, trastuzumab, was synthesized in 1991 and gained United States Food and Drug Administration approval in 1998 for treatment of metastatic breast cancer. Cardiotoxicity manifesting as dilated cardiomyopathy is a rarely reported adverse effect of trastuzumab. We hereby report a case of dilated cardiomyopathy, which occurred following trastuzumab chemotherapy in a 32–year-old female. The patient responded to discontinuation of trastuzumab and standard medical treatment. Extensive search of Indian literature revealed no reported case of dilated cardiomyopathy occurring due to trastuzumab.
KEY WORDS: Breast cancer, chemotherapy, dilated cardiomyopathy, trastuzumab
Introduction
Breast cancer is one of the most common cancers occurring among females in India. Reports state that about 75000 new cases of breast cancer occur in India, every year.[1] Current treatment for breast cancer is based on a multimodality approach of surgery, radiotherapy and chemotherapy including biological therapy, hormone therapy and immunotherapy.
In 25–30% patients of metastatic breast cancer, the gene Human Epidermal growth factor Receptor 2 (HER-2) located on chromosome 17q is amplified and over expressed, which makes the cancer cells grow and divide more rapidly.[2] HER-2 overexpression is associated with a more aggressive tumor phenotype, and women with HER-2-positive disease have faster relapse times, resistance to hormonal therapy and poor overall prognosis.[3,4] Trastuzumab is a recombinant monoclonal antibody against the defective HE-2 protein and constitutes a form of passive immunotherapy. It has a proven efficacy as monotherapy and in combination with other chemotherapeutic agents in HER-2-overexpressing metastatic breast cancer and early breast cancer.[5,6]
Clinical experience with this drug however is not much. The risk of cardiotoxicity with trastuzumab has been reported with monotherapy and in combination therapy.[7] In India, cardiotoxicity with trastuzumab has not been reported till date, to the best of our knowledge. We came across a patient who developed dilated cardiomyopathy after treatment with trastuzumab. We did the causality, severity and preventability assessment of the adverse drug reaction as per the Naranjo scale, adapted Hartwig scale and the modified Schummock and Thornton scales, respectively.
Case Report
A 32-year-old female was referred to department of Pulmonary Medicine, Sanjay Gandhi Post Graduate Institute of Medical Sciences with chief complaint of increasing dyspnoea for two weeks. Dyspnoea was of Modified Medical Research Council grade IV. She had no past history of respiratory complaints. Her general examination revealed absent right breast with healed surgical scar at that site and bilateral pedal edema.
Her treatment history as per perusal of records revealed that she had noticed a right breast lump about 5 × 4 cm size in the outer lower quadrant 1 and 1/2 year back. A fine needle aspiration cytology (FNAC) of the lump had revealed ductal carcinoma in situ. She had undergone a detailed workup that ruled out any cardio-pulmonary disease. She was given four cycles of CEF neoadjuvant chemotherapy (cyclophosphamide, epirubicin, 5-fluorouracil) after which she underwent modified radical mastectomy of right breast. Histopathology of the excised breast, nipple and axillary lymph nodes had confirmed the findings of FNAC and also revealed metastasis to axillary lymph nodes. Immunohistochemistry had revealed tumor negative for ER/PR and positive for BRCA-1 and 3+ve for HER-2 neu. A diagnosis of metastatic carcinoma right breast stage pT2N1M0 was made. She was administered paclitaxel weekly for 12 cycles. She tolerated both phases of chemotherapy. This was followed by radiotherapy. A post radiotherapy cardiac evaluation was done with 2-D echocardiogram which revealed a normal left ventricle size and normal left ventricle ejection fraction of 60%.
She was subsequently started on three weekly regimen of trastuzumab, one month after completing radiotherapy. Trastuzumab was administered 8 mg/kg over 90 min at first cycle followed by 4 mg/kg over 30 min in next cycles. She tolerated the initial infusion without reaction. She started having dyspnoea on exertion progressing to dyspnoea on rest along with pedal edema after the 6th 3 weekly injection. Her 7th dose was cancelled and she was referred to our center.
Chest X-ray PA view revealed cardiomegaly and straightening of left heart border. 2-D echocardiogram was done which revealed dilated left ventricle, global hypokinesia and left ventricular ejection fraction of 22%. On this basis, a diagnosis of dilated cardiomyopathy was made. To have objective assessment of the extent of cardio pulmonary involvement, we did a CT of thorax which revealed cardiomegaly and dilated heart chambers [Figure 1].
Figure 1.
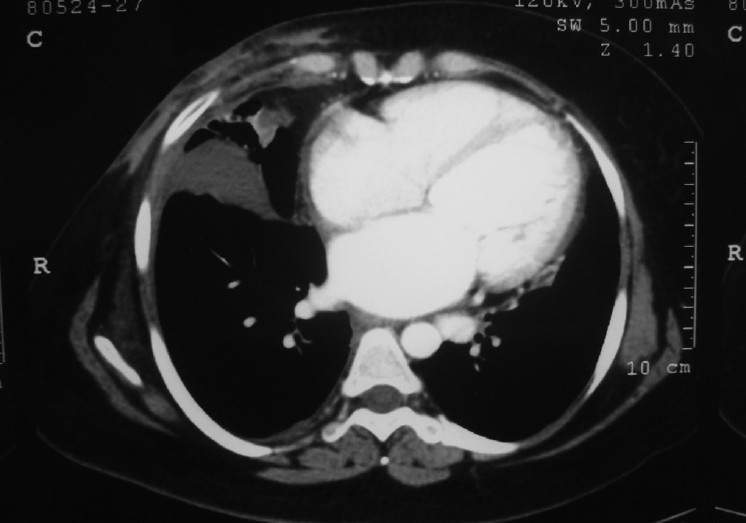
CECT of thorax showing an enlarged heart with dilated cardiac chambers
We started her on Tab carvedilol 6.25 mg OD, Tab frusemide –spironolactone (20/50 mg) OD and Tab enalapril 2.5 mg OD. Her pedal edema resolved after seven days and breathlessness on exertion after 14 days. She was subsequently discharged on the above medications. On follow-up visit after 12 weeks, she had none of the previous symptoms and a 2-D echocardiogram revealed a normal LV size and a left ventricular ejection fraction of 52%. She refused further chemotherapy and a repeat contrast enhanced computed tomography (CECT) of thorax. Assessment of causality by Naranjo's algorithm[8] revealed a score of 6 which suggests a ‘probable’ adverse drug reaction. Adapted Hartwig scale assessment of severity level[9] revealed a score of 4 in a scale of 1 to 7. Application of Schumock and Thornton preventability criteria[10] revealed the reaction as ‘not preventable’. Written informed consent was taken from the patient for collection of her details and images.
Discussion
Cardiac metabolism, biochemical properties, structure and ultimately, function are affected by antineoplastic agents. Newer antineoplastic agents have improved the survival of patients with cancer, so they survive long enough for cardiovascular adverse effects to become apparent.
Cardiotoxicity is defined by the National Cancer Institute as the ‘toxicity that affects the heart’. This definition includes a direct effect of drug on the heart as well as, an indirect effect due to enhancement of hemodynamic flow alterations or due to thrombotic events.[11]
Drug-associated cardiotoxicity is defined as one or more of the following: 1) cardiomyopathy in terms of a reduction in left ventricular ejection fraction , either global or more severe in the septum; 2) symptoms associated with heart failure (HF); 3) signs associated with HF, such as S3 gallop, tachycardia, or both; 4) reduction in left ventricular ejection fraction from baseline in the range of less than or equal to 5% to less than 55% with accompanying signs or symptoms of HF, or a reduction in left ventricular ejection fraction in the range of equal to or greater than 10% to less than 55%, without accompanying signs or symptoms.[12]
Many antineoplastic agents are cardiotoxic. Anthracyclines (doxorubicin, daunorubicin, epirubicin and mitoxantrone), alkylating agents (cyclophosphamide, ifosfamide), antimicrotubule molecules (paclitaxel, docetaxel and Vinca alkaloids) and antimetabolites (capecitabine, cytarabine, 5-flourouracil) cause cardiotoxicity.
Trastuzumab alters mitochondrial integrity, leading to adenosine triphosphate depletion and contractile dysfunction in cardiac muscle cells. Contractile dysfunction presents as ventricular dilatation, thinned ventricle walls, and reductions in pumping efficiency, leading to dilated cardiomyopathy. Recently, evidence of focal vacuolar changes, pleomorphic mitochondria, myocardial cell hypertrophy, and mild interstitial fibrosis of endomyocardial biopsy specimens have been found on electron microscopy. These ultramicroscopic features are consistent with reversible pattern of cardiac injury.[6] Clinically, it may manifest as asymptomatic decrease in LVEF or [GAP] symptomatic congestive heart failure. Trastuzumab-induced cardiotoxicity is not dose related, does not occur in all patients, and is expressed in a broad spectrum of severity, and is reversible once it is discontinued.[6] The risk factors include age greater than 50 years, borderline left ventricular ejection fraction before trastuzumab treatment, history of cardiovascular disease, cardiovascular risk factors such as diabetes, dyslipidemia or elevated body mass index (>30), sequence in which chemotherapy is administered, prior treatment with anthracyclines (cumulative doses greater than 300 mg/m2) and possibly, a genetic background and immune status.[13]
Cardiotoxicity was not reported in the preclinical and early clinical trials.[14] Early multicenter phase III trials which confirmed the clinical efficacy of trastuzumab for metastatic breast cancer, identified instances of cardiac dysfunction. As phase III trials progressed, data on cardiotoxicity began to emerge.
Four large trials evaluated efficacy of adjuvant trastuzumab in combination chemotherapy: NSABP B-31, NCCTG N9831, HERA and BCIRG 006. A meta-analysis of these trials showed that trastuzumab reduced the three-year risk of recurrence of breast cancer by about one half.[15]
A meta-analysis of patients treated with trastuzumab monotherapy revealed a 4% risk of cardiotoxicity. The risk of cardiac dysfunction is greater among patients receiving trastuzumab monotherapy as second-line therapy (7%) compared with as first-line therapy (0.8%). This difference is attributed to incidence of prior exposure to anthracyclines in the 2 populations (94 and 51%, respectively).[16]
There has been no follow up of more than two to three years on cardiotoxicity of trastuzumab among women enrolled in all trials. The incidence of cardiotoxicity among population of women treated outside of clinical trial is still unknown.[17]
Therapeutic decisions in the treatment of patients with HER-2 positive metastatic breast cancer should be based on ethical patient selection and clinical situation. Prior to therapy, the patient should be carefully evaluated for cardiovascular risk factors and cardiac disease. Cardiac function should be established at baseline and monitored regularly during treatment by measurement of left ventricular ejection fraction. The benefits and risks of trastuzumab chemotherapy must be explained to the patient.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Chopra R. The Indian scene. J Clin Oncol. 2001;19:106–11. [PubMed] [Google Scholar]
- 2.Srinivasan S, Parsa V, Liu CY, Fontana JA. Trastuzumab-induced hepatotoxicity. Ann Pharmacother. 2008;42:1497–501. doi: 10.1345/aph.1L217. [DOI] [PubMed] [Google Scholar]
- 3.Jackisch C. HER-2-positive metastatic breast cancer: Optimizing trastuzumab-based therapy. Oncologist. 2006;11:34–41. doi: 10.1634/theoncologist.11-90001-34. [DOI] [PubMed] [Google Scholar]
- 4.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association betweenHER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–53. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 5.Untch M, Ditsch N, Hermelink K. Immunotherapy: New options in breast cancer treatment. Expert Rev Anticancer Ther. 2003;3:403–8. doi: 10.1586/14737140.3.3.403. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta PP, Northfelt DW, Gentile F, Zamorano JL, Khandheria BK. Trastuzumab-induced cardiotoxicity: Heart failure at the crossroads. Mayo Clin Proc. 2008;83:197–203. doi: 10.4065/83.2.197. [DOI] [PubMed] [Google Scholar]
- 7.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 8.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 9.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 10.Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 11.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: The need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the Trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 13.Brana I, Tabernero J. Cardiotoxicity. Ann Oncol. 2010;21:173–9. doi: 10.1093/annonc/mdq295. [DOI] [PubMed] [Google Scholar]
- 14.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant Trastuzumab: A milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11:4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 16.Sparano JA. Cardiac toxicity of Trastuzumab (Herceptin): Implications for the design of adjuvant trials. Semin Oncol. 2001;28:20–7. doi: 10.1016/s0093-7754(01)90189-7. [DOI] [PubMed] [Google Scholar]
- 17.McArthur HL, Chia S. Cardiotoxicity of trastuzumab in clinical practice. N Engl J Med. 2007;357:94–5. doi: 10.1056/NEJMc070065. [DOI] [PubMed] [Google Scholar]


