Abstract
Amyloid-β peptide (Aβ) is central to the pathogenesis of Alzheimer's disease, and the low-density lipoprotein receptor-related protein (LRP) has been shown to alter Aβ metabolism in vitro. Here, we show that overexpression of a functional LRP minireceptor in the brain of PDAPP mice results in age-dependent increase of soluble brain Aβ, with no changes in Aβ plaque burden. Importantly, soluble brain Aβ was found to be primarily in the form of monomers/dimers and to be highly correlated with deficits in spatial learning and memory. These results provide in vivo evidence that LRP may contribute to memory deficits typical of Alzheimer's disease by modulating the pool of small soluble forms of Aβ.
Alzheimer's disease (AD) is characterized by cognitive impairment and neuronal loss that have been primarily linked to the accumulation of extracellular neuritic plaques and intracellular neurofibrillary tangles in the brain (1). The major component of neuritic plaques is amyloid-β peptide (Aβ), which is derived from the cleavage of amyloid precursor protein (APP). Accumulation of fibrillar aggregates of Aβ in the brain parenchyma, caused by Aβ overproduction, impaired clearance, or both, is the basis for the amyloid cascade hypothesis long proposed to explain the etiology of AD (2).
The low-density lipoprotein (LDL) receptor-related protein (LRP) has been genetically linked to AD (3, 4) and has been shown to influence Aβ metabolism in vitro (5–12). LRP is an ≈600-kDa cell-surface endocytic receptor member of the LDL receptor family (13). LRP is highly expressed in the brain and is considered the major neuronal receptor for apolipoprotein E (apoE) and α2-macroglobulin (α2M), also implicated in the pathogenesis of AD by both biochemical and genetic evidence (14).
A putative role for LRP in AD is supported by in vitro studies showing that apoE and α2M can form stable complexes with Aβ and promote its clearance via cell-surface LRP (5–10). Furthermore, LRP appears to influence APP endocytic trafficking and cellular distribution such that processing to Aβ and its extracellular release are enhanced (11, 12). To assess the effect of LRP on Aβ deposition in vivo, we generated transgenic (TG) mice that overexpress a functional minireceptor of LRP in the brain. We bred LRP TG mice to PDAPP TG mice, an animal model that develops amyloid plaques similar to those seen in AD (15). Although brain Aβ plaque burden was not significantly altered by the overexpression of LRP, double TG mice showed an age-dependent increase of soluble brain Aβ levels when compared to littermate mice overexpressing APP alone. The Aβ levels in this soluble brain extracts, which we found to be mostly Aβ monomers and dimers, were highly correlated with deficits in spatial learning and memory. These data provide strong evidence that, in addition to Aβ plaques, small soluble forms of Aβ appear to impair neuronal function and contribute to memory deficits in vivo.
Materials and Methods
Animals and Tissue Preparation. mLRP2 TG mice were generated in a B6/C3H background and the MoPrP.Xho vector (16) was used for expression of the mLRP2 transgene. Double TG mice overexpressing APPV717F and mLRP2 transgenes were obtained by breeding homozygous (+/+) PDAPP mice with heterozygous (+/–) mLRP2 mice that were bred back into the C57BL/6 background for at least five generations. PDAPP mice were derived from a hybrid background representing combinations of C57BL/6, DBA, and Swiss–Webster strains (15). Mice were screened for the presence of mLRP2 by PCR. Tissues were obtained after transcardial perfusion with ice-cold PBS. The right hemisphere was immersion-fixed for 24 h in 4% paraformaldehyde and then cryoprotected in 30% sucrose in PBS for histological analysis. The left hemisphere was further dissected and brain regions were frozen for biochemical analysis. Cerebrospinal fluid was isolated from the cisterna magna compartment as described (17).
Western Blot. Brain regions were Dounce-homogenized in 10 vol of PBS containing 1 mM Mg2+, 0.5 mM Ca2+, 1% Triton X-100, 1 mM PMSF, and protease inhibitor mixture. Equal amounts of protein from tissue lysates were separated by SDS/PAGE, followed by Western blotting with anti-hemagglutinin (HA) or anti-LRP tail antibodies and detection by ECL or ECF (Amersham Biosciences).
Primary Neuronal Cultures. Mixed cortical and hippocampal neurons were obtained from 15- to 17-day-old embryos and maintained as described (18). Nearly pure neuronal cultures were plated onto 24-well plates coated with poly(D)-lysine and laminin, in Eagle's minimum essential medium (GIBCO) supplemented with 20 mM glucose, 5% FBS, and 5% horse serum. Nonneuronal cell growth was inhibited by cytosine arabinoside (3.3 μM) added 48 h later. After 10 days of plating, neurons were incubated with 0.5 nM 125I-receptor-associated protein (RAP) in serum-free media for 4 h at 37°C, washed, lysed with 1 N NaOH, and counted (19). Receptor-mediated internalization was calculated from duplicate experiments where 125I-RAP was incubated in the presence or absence of 0.5 μM unlabeled RAP. For the biotinylation experiments, cell-surface proteins were labeled with 0.5 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce) for 1 h on ice (20), and unbound biotin was quenched with glutathione. Biotinylated proteins were pulled down with streptavidinagarose beads (Pierce), followed by SDS/PAGE and Western blotting with anti-HA antibody.
Aβ ELISA. For analysis of soluble Aβ levels, frontal cortex and hippocampus were Dounce-homogenized in 0.1 M carbonate/50 mM NaCl buffer (pH 11.5) containing 10 μg/ml leupeptin and 20 μg/ml aprotinin (21). Lysates were centrifuged at 14,000 rpm and 4°C for 20 min and the supernatants were used for measurement of soluble Aβ by sandwich ELISA for human Aβ40 (2G3 antibody) and human Aβ42 (21F12 antibody), both detected with biotin-3D6 antibody. Pellets were dissolved in 5 M guanidine (in 50 mM Tris·HCl, pH 8.0) for 4 h at RT and used for measurement of insoluble Aβ40 and Aβ42 as above. Cerebrospinal fluid Aβ levels were also measured by ELISA.
Histological Analysis. Tissue sectioning, staining, and quantitative analysis of Aβ plaques were performed as described (21). Briefly, right hemispheres were fixed, and 50-μm sections were cut in the coronal plane on a freezing sliding microtome. Brain sections were stained with a polyclonal antibody to HA epitope (Upstate Biotechnology, Lake Placid, NY) to detect mLRP2 or with a polyclonal antibody to full-length LRP to detect both endogenous LRP and mLRP2, followed by Alexa568 goat antirabbit IgG (Molecular Probes). Fluorescence was visualized by confocal laser scanning microscopy. A rabbit polyclonal pan-Aβ antibody (BioSource International, Camarillo, CA), followed by diaminobenzidine detection, was used to visualize Aβ load. Fibrillar plaques were stained with 0.125% thioflavine S (Sigma). The percent surface area of the hippocampus covered by Aβ plaques (Aβ load) or by thioflavine S-positive (fibrillar) plaques was determined in three consecutive sections, each 300 μm apart, by standard stereological procedure with the Stereo Investigator system (MicroBrightField, Williston, VT).
Gel Filtration and Aβ Western Blot. After neutralization of pH with equal volume of 2 M Tris (pH 6.8), carbonate-soluble extracts were separated by gel filtration with FPLC with a Superdex 75 HR10/30 column (Amersham Biosciences) in PBS containing 0.2% Triton X-100 to reduce Aβ stickiness to the column. Fifty fractions of 0.5 ml each were collected and analyzed for Aβ40 and Aβ42 by ELISA. Recovery was ≈80% for Aβ40 and 70% for Aβ42. Gel filtration chromatography standards from Bio-Rad were used to calibrate molecular sizes. Aβ40 and Aβ42 standards were separated by FPLC under the same conditions as samples. For SDS/PAGE, carbonate-soluble extracts and Aβ standards were subjected to immunoprecipitation with 6E10 anti-Aβ monoclonal antibody (Signet) and separated by 15% tricine/polyacrylamide gel. PDVF membranes were incubated in boiling PBS for 5 min before Western blotting with 6E10 followed by ECL.
Morris Water Navigation Task. Mice were trained in a round pool of opaque water to learn the location of an escape platform (22). Swimming paths were recorded by a computerized tracking system used to calculate the escape latency and distance traveled (path length) to reach the platform for each trial. Mice were trained under three different conditions conducted in the following order: (i) cued condition (visible platform and variable location); (ii) place condition (submerged platform and fixed location); and (iii) probe condition (platform removed). After completion of place trials, a “trials-to-criterion” protocol was used (23). This procedure included testing the mice for four consecutive weeks in which the submerged platform was moved to a new location each week. Trials-to-criterion data were analyzed with reference to the number of trials it took a mouse to achieve three consecutive trials during which average escape latency was <20 s. However, unlike the protocol previously described, all mice were administered 32 trials for each platform location (two blocks of four trials per day for 4 days) to assure that each animal had equal experience with a given platform location. In addition, path length and swimming speed data were collected and analyzed for each platform location.
Results
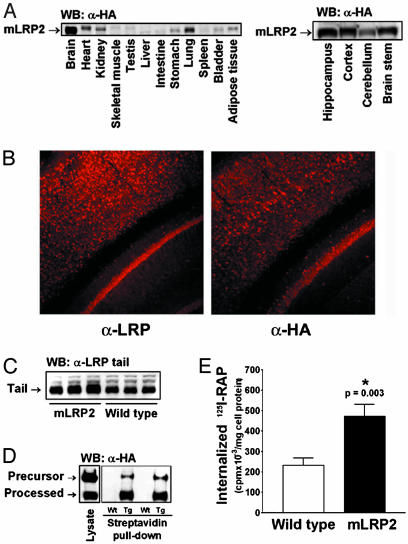
Characterization of mLRP2 TG Mice. Our TG mice overexpressed a minireceptor of LRP containing ligand-binding domain II of human LRP and the region representing the transmembrane subunit including the full cytoplasmic tail (mLRP2). Our previous in vitro studies have shown that mLRP2 folds and traffics similarly to endogenous full-length LRP and binds many of its physiological ligands (24). The transgene was tagged with a HA epitope near its amino terminus for distinction from endogenous LRP, and its expression was driven by the mouse prion protein (PrP) promoter (16), which resulted in expression of the transgene in CNS neurons (Fig. 1A), with a pattern of neuronal distribution similar to that of the endogenous LRP (Fig. 1B). Using an antibody directed against the cytoplasmic tail of LRP, we detected a 3.7-fold increase of LRP in the mLRP2 TG mouse brain compared to wild-type littermate control (Fig. 1C). Cell-surface biotinylation of mixed cortical and hippocampal neuronal cultures of mLRP2 mice followed by Western blotting with α-HA antibody confirmed that the overexpressed minireceptor was properly cleaved by furin before reaching the cell surface (Fig. 1D). Further studies with primary neuronal cultures from the mLRP2 mice also showed that mLRP2-overexpressing neurons internalized significantly more 125I-labeled RAP, a ligand for LRP (Fig. 1E). Together, these results confirmed that mLRP2 expressed in CNS neurons was properly processed and functional with respect to ligand binding and internalization.
Fig. 1.
Expression of PrP-mLRP2 transgene. (A) Expression of mLRP2 was highest in the brain, and, within the brain, mLRP2 was expressed in multiple regions. (B) mLRP2 staining pattern (Right) was similar to that of endogenous LRP (Left). (C) The overall LRP expression in the brain of mLRP2 TG mice was 3.7-fold higher than in wild-type mice as detected by an antibody to the tail region of LRP, which is identical between endogenous LRP and mLRP2. (D) mLRP2 was effectively processed to the cell surface as shown by cell-surface biotinylation. (E) mLRP2 overexpression increased receptor-mediated 125I-RAP internalization by primary neuronal cultures.
Overexpression of mLRP2 Increased Soluble Brain Aβ Levels in PDAPP TG Mice. We analyzed the impact of LRP overexpression on Aβ metabolism and amyloid plaque deposition in vivo by breeding mLRP2 TG mice with a well known mouse model of amyloid deposition, the PDAPP TG mouse (15). Double TG animals, PDAPP+/–/mLRP2+/– (hereafter referred to as PDAPP/LRP+), and littermate controls, PDAPP+/–/mLRP2–/– (PDAPP/LRP–), were aged for 9 (young), 16 (middle-aged), and 22 (old) months for behavioral studies, plaque analysis, and detection of Aβ levels in the brain. Littermate mice were used for each aging group, and no developmental or growth differences were observed between PDAPP/LRP+ and PDAPP/LRP– mice.
We observed an age-dependent increase in Aβ levels in the carbonate-soluble brain extracts from PDAPP/LRP+ mice when compared to PDAPP/LRP– mice (Fig. 2A). In old mice, soluble Aβ levels were ≈30% higher in the hippocampus and 60% higher in the frontal cortex. This increase was detected in both Aβ40 and Aβ42 forms, but it was greater for Aβ40 as the ratio Aβ40/Aβ42 was also elevated in PDAPP/LRP+ mice when compared to PDAPP/LRP– mice (hippocampus, 0.33 ± 0.03 vs. 0.23 ± 0.02, n = 17, P = 0.016; frontal cortex, 0.06 ± 0.03 vs. 0.03 ± 0.01, n = 14, P = 0.31; mean ± SEM, Student's t test). Insoluble Aβ levels were significantly increased in the frontal cortex but not in the hippocampus of old double TG mice (Fig. 2B). The overall differences in Aβ levels were not due to age-dependent differences in the expression of the transgene, because the amount of mLRP2 did not differ between young and old PDAPP/LRP+ mice by Western blotting of hippocampal lysates (data not shown). Furthermore, total APP expression in the hippocampus was not affected by mLRP2 transgene and did not change with age (data not shown).
Fig. 2.
Overexpression of mLRP2 in PDAPP mice increased soluble Aβ in an age-dependent manner. (A) Carbonate-soluble Aβ levels were increased in both hippocampus and cortex of aged PDAPP/LRP+ mice compared to littermate PDAPP/LRP– controls. (B) Insoluble Aβ level was also increased in the cortex but not in the hippocampus of aged PDAPP/LRP+ mice. Neither the percentage of hippocampal area covered by Aβ plaques (C) nor that of thioflavine S-positive plaques (D) differed between PDAPP/LRP+ and PDAPP/LRP– mice. *, Statistically significant differences by ANOVA.
At an old age, LRP-dependent increase in total Aβ levels was statistically significant in the frontal cortex (28.11 ± 2.18 vs. 21.65 ± 1.78 pg/mg tissue; n = 14, P = 0.03, Student's t test) but not in the hippocampus (92.36 ± 3.47 vs. 85.27 ± 3.58 pg/mg tissue; n = 7, P = 0.16, Student's t test). Accordingly, no significant changes in Aβ plaque load or thioflavine S-positive (fibrillar) Aβ load (Fig. 2 C and D) were detected in the hippocampus of LRP overexpressing PDAPP mice at any age. Importantly, whereas the hippocampus was mostly covered by individual dense core compact Aβ plaques, the frontal cortex contained a great amount of diffuse Aβ staining between compact plaques that made the quantification of Aβ plaque load in this region technically difficult.
Recent in vitro studies have shown that small oligomeric Aβ species display higher neuronal toxicity than fibrillar forms of Aβ (25, 26). Therefore, we next analyzed carbonate-soluble brain extracts from old mice by gel filtration chromatography, with subsequent quantification of Aβ in the fractions by ELISA (Fig. 3A). A significant small fraction of carbonate-soluble Aβ was eluted at molecular size larger than 100 kDa (void volume), which could represent small portions of Aβ plaques that contaminated the soluble extract. Under native conditions, most of the Aβ present in carbonate-soluble extracts of both frontal cortex and hippocampus (frontal cortex shown) was detected in fractions that eluted at ≈9 kDa, suggesting that soluble extracts contained primarily Aβ dimers. However, when monomeric Aβ40 and Aβ42 standards were dissolved in carbonate buffer and run in the same conditions as brain extracts, all of the Aβ quantified by ELISA eluted in the same 9-kDa peak (data not shown). This finding indicated that we were unable to separate monomeric and dimeric Aβ forms present in soluble brain extracts by FPLC. Therefore, we subjected carbonate-soluble brain extracts to immunoprecipitation with an anti-Aβ antibody and separation by SDS/PAGE, followed by Western blotting. An immunoreactive band with apparent molecular mass of ≈9 kDa was detected, but the majority of Aβ was found as monomers (Fig. 3B). Even though potential LRP-mediated differences in the proportion between monomers and dimers in the soluble brain fraction could not be detected by gel filtration, differences in the total amount of Aβ40 and Aβ42 between PDAPP/LRP+ and PDAPP/LRP– mice present in the major 9-kDa peak (Fig. 3A) were similar in magnitude to differences measured in the unfractionated carbonate-soluble extracts from the same brain samples (Fig. 3B). These findings indicate that small soluble forms of Aβ were selectively increased by LRP overexpression in old PDAPP mice. Finally, we did not detect changes in cerebrospinal fluid Aβ levels between old PDAPP/LRP+ and PDAPP/LRP– mice (7.76 ± 0.41 and 7.54 ± 0.47 ng/ml, respectively; n = 10), suggesting that the LRP-mediated increase of soluble brain Aβ does not appear to be reflected in this pool. Plasma Aβ levels were also similar in old PDAPP/LRP+ and PDAPP/LRP– mice (37.17 ± 2.46 and 33.36 ± 5.71 pg/ml, respectively; n = 10).
Fig. 3.
Aβ monomers and dimers were increased in carbonate-soluble brain extracts of PDAPP/LRP+ mice. (A) By gel-filtration chromatography, most of the carbonate-soluble Aβ eluted at dimer size (≈9 kDa). Graphs shown are representative of three separate experiments in which extracts from five to six animals of each group were combined for analysis. (B) When extracts from frontal cortex of PDAPP/LRP– were separated by Tris-tricine gel, carbonate-soluble Aβ (lane 2) was detected mostly as monomers (arrow) as well as dimers (arrowhead). Aβ standards are shown in lane 1. (C) Soluble Aβ40 and Aβ42 differences between PDAPP/LRP+ and PDAPP/LRP– brain extracts before gel filtration were similar to the ones observed in the ≈9-kDa peaks shown in A.
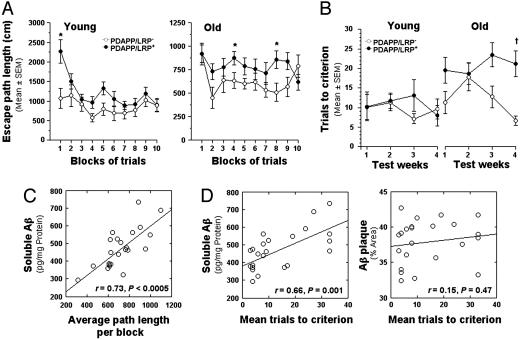
Overexpression of mLRP2 Enhanced Memory Impairment in PDAPP TG Mice. To evaluate whether the effect of LRP overexpression on Aβ levels had an impact on spatial learning and memory, we tested young and old PDAPP/LRP+ and PDAPP/LRP– mice by using both a traditional water-maze test (22) and a new watermaze test recently described (23). In the traditional water-maze navigation task, young (n = 9) and old (n = 12) groups performed similarly in the initial cued trials (visible platform). All groups showed progressive improvement over the blocks of trials, suggesting that learning had taken place. These results confirmed that PDAPP/LRP+ and PDAPP/LRP– mice did not differ in relation to nonassociative disturbances that could possibly affect performance on subsequent place trials. In contrast, the performance of PDAPP/LRP+ mice during place trials (hidden platform) in terms of path length was inferior to that of PDAPP/LRP– mice in both young and, more significantly, old mice (Fig. 4A). The impaired performance of PDAPP/LRP+ mice was confirmed by ANOVA, with a significant main effect of group [F(1, 18) = 5.30, P = 0.033] and a significant group by blocks of trials interaction [F(9, 162) = 2.19, P = 0.047 (Huynh–Feldt, corrected)] for young groups, and a significant main effect of group [F(1, 23) = 6.81, P = 0.016] for old groups. Although PDAPP/LRP+ mice were impaired in their ability to locate the platform during the place condition, there was little evidence of learning beyond the first block of trials for either PDAPP/LRP+ or PDAPP/LRP– mice from the young and old groups. The two groups of mice did not differ in terms of retention performance as measured by time in the target quadrant or platform crossing during probe trials (data not shown).
Fig. 4.
Deficits in spatial learning and memory were increased in PDAPP/LRP+ mice and correlated with soluble Aβ levels measured in the hippocampus. (A) Performance of PDAPP/LRP+ mice during standard place trials in terms of path length was inferior to that of PDAPP/LRP– mice in both young and old groups. (B) The average number of trials to reach the acquisition criterion (three consecutive trials with an average escape latency of <20 s) in both young and old groups of mice as a function of 4 weeks of training. (C) In aged mice, performance deficits observed during standard place training were highly correlated with soluble Aβ levels in the hippocampus. (D) A significant correlation was also found between mean trials-to-criterion scores in old mice during week 4 and hippocampal soluble Aβ levels but not with Aβ plaque burden. In all figures, * indicates P < 0.05, and † indicates P < 0.005 (by ANOVA).
In the new water-maze protocol, designed to evaluate an episodic-like memory component embedded in a large test battery and used to study the effects of aging and plaque load on performance of PDAPP mice (23), animals were presented on a weekly basis with a new location of the escape platform (4 weeks, four locations). Performance was evaluated in terms of trials-to-criterion (number of trials with average escape latency <20 s for each platform location) (23). Although young mice from both groups performed similarly with regard to reaching the weekly acquisition criteria, performance of old PDAPP/LRP+ mice was significantly impaired in the trials-to-criterion task when compared to old PDAPP/LRP– mice (Fig. 4B; see Fig. 5, which is published as supporting information on the PNAS web site, for complete results). An ANOVA confirmed this impairment by revealing a significant main effect of group [F(1, 38) = 5.77, P = 0.021], a significant main effect of age [F(1, 38) = 12.05, P = 0.001], and, importantly, a significant group by age interaction [F(1, 38) = 4.43, P = 0.042]. Subsequent analysis yielded a significant main effect of group for old mice [F(1, 22) = 9.01, P = 0.007] but not for young mice. To further characterize the potential cognitive deficits in old PDAPP/LRP+ mice, we also evaluated average path lengths on trial 2 of the first day of training for each platform location. Performance on this trial likely invokes a relatively “pure” form of working memory, in that the mice must remember the response from the immediately preceding trial rather than the correct response from previous weeks. Again, a significant impairment in PDAPP/LRP+ mice at old age was detected {significant main effect of group [F(1,22) = 4.84, P = 0.039] by ANOVA}, whereas analysis of the young groups showed no differences in performance on trial 2 (data not shown).
Soluble Brain Aβ Was Highly Correlated with Memory Deficits in Aged PDAPP/LRP TG Mice. Because both soluble Aβ levels and behavioral deficits were increased in aged PDAPP/LRP+ mice, we evaluated a potential relationship between these parameters. In old mice, the amount of soluble Aβ in the hippocampus correlated highly with the average distance per block of trials computed for the standard place condition shown in Fig. 4A (r = 0.73, P < 0.0005) (Fig. 4C). The percentage of area in the hippocampus covered by Aβ plaques (Aβ load) also correlated with performance during place trials, although to a lesser extent (r = 0.45, P = 0.031). For the trials-to-criterion performance of the old groups during week 4, when differences in learning appeared to be the greatest (Fig. 4B), we found that the average trials-to-criterion scores correlated highly with soluble Aβ levels (r = 0.66, P = 0.001) but not with the extent of plaque deposition in the hippocampus (r = 0.15, P = 0.47) (Fig. 4C). No significant correlations were found between Aβ parameters and watermaze performance for the young mice (data not shown).
Discussion
LRP is a neuronal receptor for several ligands believed to be important in AD pathogenesis including apolipoprotein E (apoE) and APP (14, 27). Here, we showed that neuronal overexpression of a functional minireceptor of LRP (mLRP2) increased soluble levels of brain Aβ in PDAPP mice in an age-dependent manner. We also found that learning and memory deficits observed in aged mice were better correlated with soluble Aβ levels than with Aβ plaque burden.
Several in vitro studies suggest two opposing effects of LRP in Aβ metabolism. Some LRP ligands appear to form stable complexes with Aβ and promote its clearance (5–10), thus decreasing extracellular Aβ levels. In contrast, LRP appears to influence APP endocytic trafficking and cellular distribution such that processing to Aβ and its extracellular release are enhanced (11, 12). In our present in vivo study, overexpression of mLRP2 increased soluble brain Aβ levels in an age-dependent manner, suggesting that a cumulative effect of LRP to increase Aβ production may be dominant over its potential role in Aβ clearance. Recently, an interaction between LRP and APP has been confirmed by both coimmunoprecipitation (28) and fluorescence resonance energy transfer analysis (29). This LRP–APP interaction has been demonstrated to occur at the cell surface (29), suggesting that LRP has the potential to influence APP processing by the endocytic pathway. The increase in Aβ40/Aβ42 ratio observed in both soluble and insoluble brain fractions of PDAPP/LRP+ mice is consistent with this hypothesis, because previous in vitro observations have shown that Aβ40 is mostly produced in the endocytic pathway (30, 31), and that overexpression of LRP appears to accelerate this route of APP processing (11, 12, 32).
In contrast with our results, a significant increase in amyloid deposition in human APP TG mice that were deficient in RAP, a chaperone for all members of the low-density lipoprotein (LDL) receptor family, was recently reported and linked to a role for LRP in Aβ clearance (33). However, because the levels of all LDL receptor family members are markedly reduced in the absence of RAP, this effect cannot be attributed specifically to LRP. It is also possible that a role for LRP in clearing extracellular Aβ was underestimated in our LRP-overexpressing model because the levels of certain ligands, such as apolipoprotein E (apoE), were unchanged by mLRP2 overexpression (data not shown).
When analyzed by gel filtration chromatography and SDS/PAGE, we found that carbonate-soluble brain fractions were almost exclusively composed of small Aβ forms (monomers and dimers). It has been recently shown that a heterogeneous population of Aβ derived from transfected cell lines contains an insulin-degrading enzyme-resistant form that can selectively inhibit long-term potentiation in hippocampal slices (26). Additionally, exogenous Aβ oligomers applied to primary neuronal cultures consistently results in Aβ-induced neurotoxicity (25). However, our results suggest that Aβ monomers and dimers can selectively disrupt learning and memory in vivo
Using a water-maze protocol designed to evaluate “episodic-like” memory, Chen et al. (23) found a negative correlation between trials-to-criterion for platform locations 4 and 5 and plaque burden in older PDAPP mice. Our analysis also showed a negative but insignificant correlation between trials-to-criterion for platform location 4 and plaque burden in old mice. However, a strong and significant correlation was found between trials-to-criterion and soluble Aβ levels in old mice, suggesting that soluble Aβ may further impair memory performance in PDAPP mice. Although we have not yet fully completed behavioral analysis of aged mLRP2 mice, our initial results from young LRP-overexpressing mice indicate that overexpression of mLRP2 transgene alone does not affect performance in place training (unpublished observations). These results support the hypothesis that the effect of mLRP2 overexpression on Aβ metabolism arising from overexpression of human APP likely underlies the increased behavioral deficits observed.
The amyloid cascade hypothesis posits that the conversion of the Aβ peptide from soluble to insoluble forms is the ultimate insult to susceptible brain regions such as the hippocampus and frontal cortex. However, reports showing that dementia is more highly correlated with soluble Aβ species than with plaque load challenge this hypothesis (34–37). Furthermore, mouse models of amyloid deposition display behavioral deficits in the absence of amyloid plaques (38, 39). Recent studies using oligomer-specific antibodies suggest that oligomers can be detected in the human AD brain but not in normal brain (40, 41). The specific Aβ oligomer staining appears to be physically distinct from that of fibrillar amyloid (40), suggesting the independent nature of these two forms of Aβ aggregates. The results presented here suggest that monomeric and dimeric forms of Aβ can significantly disrupt learning and memory in vivo. Consistent with this finding, passive immunization with an anti-Aβ monoclonal antibody has been shown to cause rapid efflux of Aβ into the plasma and to rapidly reverse memory impairment in PDAPP mice without altering brain Aβ plaque burden (42). Also, long-term deafferentiation dramatically reduces diffuse Aβ deposits but not Aβ plaques in deafferented terminal fields in the hippocampus of APP/PS1 double TG mice (43). A critical challenge to the soluble Aβ hypothesis of AD pathogenesis is to clearly identify and isolate from a physiologically relevant matrix (brain, cerebrospinal fluid, plasma) the exact species of Aβ that causes memory impairment (35).
In summary, our results support the hypothesis that small soluble forms of Aβ may result in neuronal dysfunction and memory deficits in vivo, and that LRP may play a role in the pathogenesis of late-onset AD via age-dependent effect on the steady-state levels of this Aβ pool. Our findings suggest that targeting soluble brain Aβ may be an effective therapeutic strategy for treating AD.
Supplementary Material
Acknowledgments
We thank Dr. David Borchelt (John Hopkins University School of Medicine, Baltimore) for providing the MoPrP.Xho vector and Drs. Eugene Johnson, Jonathan Gitlin, and Alan Schwartz for critical reading of the manuscript. This work was supported by National Institutes of Health Postdoctoral Fellowship NS41872 (to C.V.Z.) and National Institutes of Heath Grants P50 AG05681 (to G.B.) and AG11355 (to D.F.W.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Aβ, amyloid-β peptide; AD, Alzheimer's disease; APP, amyloid precursor protein; HA, hemagglutinin; LRP, low-density lipoprotein receptor-related protein; RAP, receptor-associated protein; TG, transgenic.
References
- 1.Hardy, J. & Selkoe, D. J. (2002) Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- 2.Hardy, J. A. & Higgins, G. A. (1992) Science 256, 184–185. [DOI] [PubMed] [Google Scholar]
- 3.Lendon, C. L., Talbot, C. J., Craddock, N. J., Han, S. W., Wragg, M., Morris, J. C. & Goate, A. M. (1997) Neurosci. Lett. 222, 187–190. [DOI] [PubMed] [Google Scholar]
- 4.Wavrant-DeVrieze, F., Lambert, J. C., Stas, L., Crook, R., Cottel, D., Pasquier, F., Frigard, B., Lambrechts, M., Thiry, E., Amouyel, P., et al. (1999) Hum. Genet. 104, 432–434. [DOI] [PubMed] [Google Scholar]
- 5.Narita, M., Holtzman, D. M., Schwartz, A. L. & Bu, G. (1997) J. Neurochem. 69, 1904–1911. [DOI] [PubMed] [Google Scholar]
- 6.Du, Y., Bales, K. R., Dodel, R. C., Liu, X., Glinn, M. A., Horn, J. W., Little, S. P. & Paul, S. M. (1998) J. Neurochem. 70, 1182–1188. [DOI] [PubMed] [Google Scholar]
- 7.Qiu, Z., Strickland, D. K., Hyman, B. T. & Rebeck, G. W. (1999) J. Neurochem. 73, 1393–1398. [DOI] [PubMed] [Google Scholar]
- 8.Van Uden, E., Sagara, Y., Van Uden, J., Orlando, R., Mallory, M., Rockenstein, E. & Masliah, E. (2000) J. Biol. Chem. 275, 30525–30530. [DOI] [PubMed] [Google Scholar]
- 9.Kang, D. E., Pietrzik, C. U., Baum, L., Chevallier, N., Merriam, D. E., Kounnas, M. Z., Wagner, S. L., Troncoso, J. C., Kawas, C. H., Katzman, R. & Koo, E. H. (2000) J. Clin. Invest. 106, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata, M., Yamada, S., Kumar, S. R., Calero, M., Bading, J., Frangione, B., Holtzman, D. M., Miller, C. A., Strickland, D. K., Ghiso, J. & Zlokovic, B. V. (2000) J. Clin. Invest. 106, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulery, P. G., Beers, J., Mikhailenko, I., Tanzi, R. E., Rebeck, G. W., Hyman, B. T. & Strickland, D. K. (2000) J. Biol. Chem. 275, 7410–7415. [DOI] [PubMed] [Google Scholar]
- 12.Pietrzik, C. U., Busse, T., Merriam, D. E., Weggen, S. & Koo, E. H. (2002) EMBO J. 21, 5691–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herz, J., Hamann, U., Rogne, S., Myklebost, O., Gausepohl, H. & Stanley, K. K. (1988) EMBO J. 7, 4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Uden, E., Kang, D. E., Koo, E. H. & Masliah, E. (2000) Microsc. Res. Tech. 50, 268–272. [DOI] [PubMed] [Google Scholar]
- 15.Games, D., Adams, D., Alessandrini, R., Barbour, R., Borthelette, P., Blackwell, C., Carr, T., Clemens, J., Donaldson, T., Gillespie, F., et al. (1995) Nature 373, 523–527. [DOI] [PubMed] [Google Scholar]
- 16.Borchelt, D. R., Davis, J., Fischer, M., Lee, M. K., Slunt, H. H., Ratovitsky, T., Regard, J., Copeland, N. G., Jenkins, N. A., Sisodia, S. S., et al. (1996) Genet. Anal. 13, 159–163. [DOI] [PubMed] [Google Scholar]
- 17.DeMattos, R. B., Bales, K. R., Parsadanian, M., O'Dell, M. A., Foss, E. M., Paul, S. M. & Holtzman, D. M. (2002) J. Neurochem. 81, 229–236. [DOI] [PubMed] [Google Scholar]
- 18.Rose, K., Goldberg, M. P. & Choi, D. W. (1993) in Methods in Toxicology: In Vitro Biological Methods, eds. Tyson, C. A. & Frazier, J. M. (Academic, San Diego), pp. 46–60.
- 19.Bu, G., Morton, P. A. & Schwartz, A. L. (1992) J. Biol. Chem. 267, 15595–15602. [PubMed] [Google Scholar]
- 20.Ko, K. W., McLeod, R. S., Avramoglu, R. K., Nimpf, J., FitzGerald, D. J., Vukmirica, J. & Yao, Z. (1998) J. Biol. Chem. 273, 27779–27785. [DOI] [PubMed] [Google Scholar]
- 21.DeMattos, R. B., O'dell, M. A., Parsadanian, M., Taylor, J. W., Harmony, J. A., Bales, K. R., Paul, S. M., Aronow, B. J. & Holtzman, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 10843–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho, N., Liauw, J. A., Blaeser, F., Wei, F., Hanissian, S., Muglia, L. M., Wozniak, D. F., Nardi, A., Arvin, K. L., Holtzman, D. M., et al. (2000) J. Neurosci. 20, 6459–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, G., Chen, K. S., Knox, J., Inglis, J., Bernard, A., Martin, S. J., Justice, A., McConlogue, L., Games, D., Freedman, S. B., et al. (2000) Nature 408, 975–979. [DOI] [PubMed] [Google Scholar]
- 24.Obermoeller-McCormick, L. M., Li, Y., Osaka, H., FitzGerald, D. J., Schwartz, A. L. & Bu, G. (2001) J. Cell Sci. 114, 899–908. [DOI] [PubMed] [Google Scholar]
- 25.Dahlgren, K. N., Manelli, A. M., Stine, W. B., Jr., Baker, L. K., Krafft, G. A. & LaDu, M. J. (2002) J. Biol. Chem. 277, 32046–32053. [DOI] [PubMed] [Google Scholar]
- 26.Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J. & Selkoe, D. J. (2002) Nature 416, 535–539. [DOI] [PubMed] [Google Scholar]
- 27.Kounnas, M. Z., Moir, R. D., Rebeck, G. W., Bush, A. I., Argraves, W. S., Tanzi, R. E., Hyman, B. T. & Strickland, D. K. (1995) Cell 82, 331–340. [DOI] [PubMed] [Google Scholar]
- 28.Rebeck, G. W., Moir, R. D., Mui, S., Strickland, D. K., Tanzi, R. E. & Hyman, B. T. (2001) Brain Res. Mol. Brain Res. 87, 238–245. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita, A., Whelan, C. M., Smith, C. J., Mikhailenko, I., Rebeck, G. W., Strickland, D. K. & Hyman, B. T. (2001) J. Neurosci. 21, 8354–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo, E. H. & Squazzo, S. L. (1994) J. Biol. Chem. 269, 17386–17389. [PubMed] [Google Scholar]
- 31.Selkoe, D. J. (1998) Trends Cell Biol. 8, 447–453. [DOI] [PubMed] [Google Scholar]
- 32.Goto, J. J. & Tanzi, R. E. (2002) J. Mol. Neurosci. 19, 37–41. [DOI] [PubMed] [Google Scholar]
- 33.Van Uden, E., Mallory, M., Veinbergs, I., Alford, M., Rockenstein, E. & Masliah, E. (2002) J. Neurosci. 22, 9298–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean, C. A., Cherny, R. A., Fraser, F. W., Fuller, S. J., Smith, M. J., Beyreuther, K., Bush, A. I. & Masters, C. L. (1999) Ann. Neurol. 46, 860–866. [DOI] [PubMed] [Google Scholar]
- 35.Klein, W. L., Krafft, G. A. & Finch, C. E. (2001) Trends Neurosci. 24, 219–224. [DOI] [PubMed] [Google Scholar]
- 36.Selkoe, D. J. (2002) Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- 37.Lue, L. F., Kuo, Y. M., Roher, A. E., Brachova, L., Shen, Y., Sue, L., Beach, T., Kurth, J. H., Rydel, R. E. & Rogers, J. (1999) Am. J. Pathol. 155, 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mucke, L., Masliah, E., Yu, G. Q., Mallory, M., Rockenstein E. M., Tatsuno, G., Hu, K., Kholodenko, D., Johnson-Wood, K. & McConlogue, L. (2000) J. Neurosci. 20, 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koistinaho, M., Kettunen, M. I., Goldsteins, G., Keinanen, R., Salminen, A., Ort, M., Bures, J., Liu, D., Kauppinen, R. A., Higgins, L. S., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 14675–14680.11724968 [Google Scholar]
- 40.Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W. & Glabe, C. G. (2003) Science 300, 486–489. [DOI] [PubMed] [Google Scholar]
- 41.Gong, Y., Chang, L., Viola, K. L., Lacor, P. N., Lambert, M. P., Finch, C. E., Krafft, G. A. & Klein, W. L. (2003) Proc. Natl. Acad. Sci. USA 100, 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodart, J. C., Bales, K. R., Gannon, K. S., Greene, S. J., DeMattos, R. B., Mathis, C., DeLong, C. A., Wu, S., Wu, X., Holtzman, D. M., et al. (2002) Nat. Neurosci. 5, 452–457. [DOI] [PubMed] [Google Scholar]
- 43.Van Groen, T., Liu, L., Ikonen, S. & Kadish, I. (2003) Neuroscience 119, 1185–1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.