Abstract
Objectives:
This study was designed to investigate the gastroprotective effect of L-citrulline against gastric ischemia-reperfusion injury.
Materials and Methods:
Sodium pentobarbital-anesthetized rats underwent occlusion of the celiac artery for 30 min, followed by 60 min of reperfusion. Sixty minutes before ischemia, L-citrulline at doses of 300, 600, 900 mg/kg was administered intragastrically. Based on this animal model of gastric ischemia-reperfusion injury, the gastroprotective effect of L-citrulline was assessed by determining and comparing the ulcerative index and the estimation of myeloperoxidase (MPO) activity and malondialdehyde (MDA) level in the gastric mucosal tissues. Moreover, the expression of inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and endothelial nitric oxide synthase (eNOS) protein was also determined.
Results:
Intragastric administration of L-citrulline (600 and 900 mg/kg) 60 min before ischemia significantly ameliorated the gastric mucosal damage and inhibited the increase in MPO and MDA contents. Also, the increase in expression of iNOS protein was also prevented by L-citrulline. The expression of nNOS and eNOS was not affected significantly by I/R or L-citrulline.
Conclusion:
The results suggest that L-citrulline, administered exogenously, exhibits gastric protection by inhibition of neutrophil infiltration in rats, which may be related in prevention of the increase in iNOS activity.
KEY WORDS: Gastric damage, ischemia-reperfusion, l-citrulline, myeloperoxidase, nitric oxide synthase
Introduction
Gastric ulcer is a common gastrointestinal disease. A growing body of evidence has demonstrated that neutrophils are implicated in the development of inflammation and injury in a variety of tissues including the gastric mucosa. Neutrophils, once activated, release oxygen-derived free radicals such as superoxide anion, hydrogen peroxide, hypochlorous acid, as well as enzymes such as myeloperoxidase (MPO) and proteases.[1] Neutrophils can also migrate into the surrounding tissues resulting in further disruption of the gastric mucosa[2] and their infiltration has been observed in the gastric mucosa in patients suffering from severe gastritis and chronic peptic ulcers.[3]
On the other hand, it is well recognized that nitric oxide (NO) is a molecule that plays an important role in the regulation of multiple physiological functions in the gastrointestinal tract.[4] It is produced in vivo through the conversion of L-arginine to L-citrulline and NO by nitric oxide synthase (NOS). The NOS enzyme family is composed of three major isoforms, endothelial NOS, neuronal NOS (nNOS), and inducible NOS (iNOS); the former two are expressed under normal conditions and referred to as constitutive NOS (cNOS), whereas iNOS is usually not expressed but induced by certain cytokines such as tumor necrosis factor-α.[5] NO plays a biphasic role in the ulcerogenic response of the gastric mucosa depending on the NOS isozyme; a protective effect by cNOS/NO, and a proulcerogenic effect by iNOS/NO.[6] Related reports have shown that a drastic increase in iNOS expression in the gastric mucosa occurs with the development of gastric mucosa lesions in rats with gastric ischemia-reperfusion (I/R).[7] Once the expression of iNOS is upregulated, the concentration of NO will increase, resulting in the production of reactive oxygen species and ultimately oxidative stress, which play proulcerogenic effect on I/R-induced gastric injury.
L-citrulline, which is a non-essential amino acid in mammals, is closely related to de novo biosynthesis of L-arginine. In the kidney, vascular endothelium and other tissues, L-citrulline can be readily converted to L-arginine, thus raising its plasma and tissue levels.[8] Evidence suggests that L-arginine can reduce heart and pulmonary diseases in rats and reverses the progression of atherosclerosis in rabbits.[9–11] Moreover, it has been demonstrated that L-arginine can elicit a gastroprotective effect by preventing the increase of inducible NOS (iNOS) activity in the gastric mucosa of rats with water immersion restraint stress.[12] As an indirect precursor of L-arginine, L-citrulline has a cardioprotective effect against ischemia/reperfusion injury in rats and an inhibitory effect on polymorphonuclear neutrophils (PMNs) burst in humans.[10,13] Because of its extensive presence and close relation to NO production in vivo, it has been attracting an increasing attention. In this study, we have attempted to determine the protective effect of L-citrulline on acute hemorrhagic gastric mucosal injury caused by I/R in rats.
Materials and Methods
Experimental Animal
Adult male Sprague-Dawley rats, weighing 200-240 g, were provided by the Experimental Animal Centre of Xuzhou Medical College. The animals, eight to ten per group, were deprived of food for 24 h before experiments but had free access to water. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drug Preparation and Treatment
L-citrulline (L-Cit) (Sigma Chemical, St. Louis, MO, USA) was dissolved in distilled water. It was prepared freshly each time and given at different doses (300, 600, and 900 mg/kg) by gavage 60 min before the experiment. N-(3-(aminomethyl)-benzyl) acetamidine (1400W) (a selective inhibitor of iNOS, Sigma Chemical, St. Louis, MO, USA) was dissolved in saline and given s.c. 30 min before ischemia. Control group received the vehicle in a comparable volume (10 ml/kg body weight) also by the same route. In the drug control group, the rats were treated with L-citrulline (900 mg/kg) without I/R. The animals were randomly divided into eight groups: control, drug control, sham, ischemia-reperfusion group, and L-citrulline (300, 600, and 900 mg/kg) groups.
Production of Ischemia-Reperfusion Lesions
Ischemia-reperfusion damage was produced in rats by method proposed by Yoshikawa et al.[14] Briefly, under sodium pentobarbital (3%, i.p.) anesthesia, the celiac artery was clamped with a small clamp for 30 min and reperfused by removal of the clamp to obtain the ischemia-reperfusion state. Sixty minutes after the reperfusion, the rat was killed by thoracotomy and the stomach was removed. The stomach was opened along the greater curvature and rinsed with ice-cold phosphate-buffered saline (PBS; 0.1 mol/L), then the stomach was spread out on a cold stand and a paned countering slab (with 1 mm2 panels) for injury index count. The gastric mucosal injury index was determined by an investigator who was unaware of the treatment given. The index is based on a cumulative length scale, in which individual lesions limited to the mucosal epithelium (including pinpoint erosions, ulcers, and hemorrhagic spots) are scored according to their length as follows: 1 for a lesion ≤1 mm; 2 for a lesion >1 mm and <2 mm; 3 for a lesion >2 mm and a 3 mm; etc. For lesions with width >1 mm, the lesion score was doubled.
Preparation of Histological Specimens
Gastric mucosal injury was assessed according to the method described previously.[15] Briefly, the tissue from the gastric mucosa of each animal was fixed with 4% formaldehyde, dehydrated in grade ethanol, and embedded in paraffin wax. Sections were cut at 5μm, mounted on clean glass slides, and dried overnight at 37°C. Sections were cleared, hydrated, and stained with hematoxylin-eosin for light microscopic observation. Blind analysis was performed on all samples in an Olympus BH-2 microscope for characterization of histopathological changes.
Measurement of Myeloperoxidase (MPO) Activity
MPO activity in gastric mucosa was assessed by the o-diansidine method.[7] Briefly, mucosal scrapings were homogenized in 10 volumes 50 mmol/l phosphate buffer containing containing 0.5% hexadecyl trimethyl ammonium bromide (HTAB; pH 6.0). The homogenized samples were subjected to freezing and thawing three times and centrifuged at 10,000 × g for 10 min at 4°C and then the resultant supernatant was used for assay sample. After the addition of 1.9 ml of 10 mM phosphate buffer (pH 6.0) and 145μl of 1.5 mol/l o-dianisidine hydrochloride, containing 0.0005% w/v hydrogen peroxide to the supernatant, changes in the absorbance at 450 nm of each sample were recorded on the spectrophotometer (Hitachi UV2401). Sample protein content was measured by BCA method and the MPO activity was obtained from the slope of the reaction curve based on the following equation: Specific activity (μmol H2O2 /min/mg protein) = (OD/min)/OD/μmol H2O2 × mg protein.
Determination of Malondialdehyde (MDA)
Levels of MDA in the tissues were measured by the method of Ohkawa et al. The reaction mixture consisted of 0.2 ml of 8.1% sodium lauryl sulphate, 1.5 ml of 20% acetic acid solution adjusted to pH 3.5 with sodium hydroxide, and 1.5 ml of 0.8% aqueous solution of thiobarbituric acid was added to 0.2 ml of 10% (w/v) of homogenate. The mixture was heated for 60 minutes at 95C. After cooling with water, 1.0 ml distilled water and 5.0 ml of the mixture of n-butanol and pyridine (15: 1 v/v) was added and centrifuged at 3000 rpm for 10 min. The organic layer was taken out and absorbance at 450 nm of each sample was recorded on the spectrophotometer (Hitachi UV2401). Protein was determined by BCA method. Results are expressed as nmol MDA/mg protein.
Determination of the Expression of iNOS, nNOS, and eNOS
Protein of gastric mucosal tissue was extracted using protein extraction buffer consisting of 1% Nonidet P-40, 50 mM Tris-HCl (pH 7.4), 120 mM NaCl, 1 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, and containing protease inhibitor cocktail. Following centrifugation, protein contents were quantified by BCA method. Subsequently, lysates (200 μg of total protein) were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and transferred onto a nitrocellulose membrane according to the method of Towbin et al.[17] Blotting filters were blocked with 3% BSA in TBS/Tween-20 buffer (137 mmol NaCl, 20 mmol Tris-HCl, Ph 7.4, 0.1% Tween-20). After washing with TBST, the membranes were incubated with a 1:1,000 dilution of anti-iNOS, anti-nNOS, and anti-eNOS over night at room temperature with constant agitation. The filters were then washed and subsequently probed with horseradish peroxidase-conjugated anti-rabbit for iNOS, nNOS, and eNOS at a dilution of 1: 20,000. Immunocomplexes were detected by the Supersignal West Pico Chemiluminescent Kit (Pierce, USA). Comparison between different treatment groups was performed by determination of the examined protein/β-actin protein ratio of the immunoreactive area by densitometry.
Statistical Analysis
Results were expressed as mean ± S.E.M. The data were evaluated with Statistical Package for the Social Sciences (SPSS) 13.0. The statistical significance of differences for each parameter among the groups was evaluated by one-way ANOVA, followed by Dunnett's t-test. P values of < 0.05 were considered to be statistically significant.
Results
Effect of L-citrulline on Gastric Mucosal Injury Induced by I/R
When rats were subjected to I/R, gastric mucosal lesions developed as shown in Figure 1. In contrast, pretreatment with L-citrulline (300, 600, and 900 mg/kg, i.g.) significantly prevented the lesion development due to I/R. Moreover, the protective effect of L-citrulline was comparable with the effect of 1400W (100 mg/kg, s.c.). L-citrulline (900 mg/kg, i.g.) showed the most effective protection.
Figure 1.
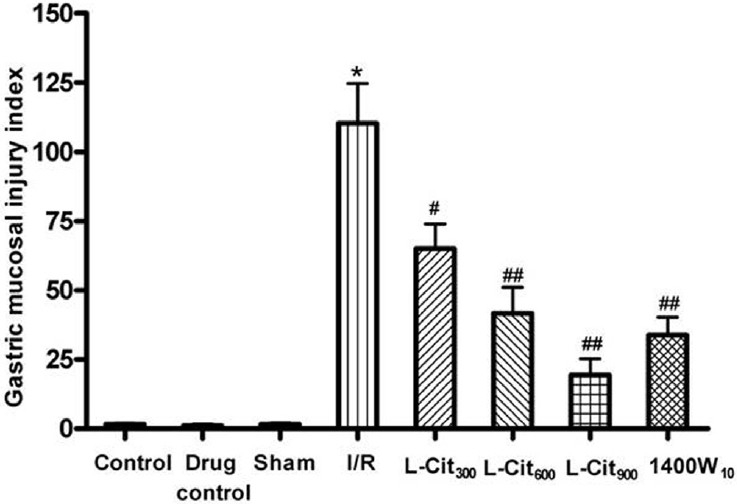
Effect of L-citrulline (L-Cit, 300, 600, 900 mg/kg, i.g.) on the gastric damage induced by ischemia-reperfusion (I/R) injury in rats. Values are mean ± S.E.M. for six to eight animals. *P< 0.001 compared with the sham group; #P< 0.01, ##P< 0.001 compared with the I/R group
Histological Observations
No damage was observed in the gastric mucosa of sham-operated rats [Figure 2A]. Nevertheless, as light microscopy showed I/R injury produced severe damage in the stomach, mostly in the surface epithelial cells, but some damage occurred in the mucosa extending to the region of pits and glands [Figure 2B]. After pretreatment with L-citrulline, the pathological characteristics were markedly reduced and only slight damage was observed in the surface epithelium [Figure 2C].
Figure 2.
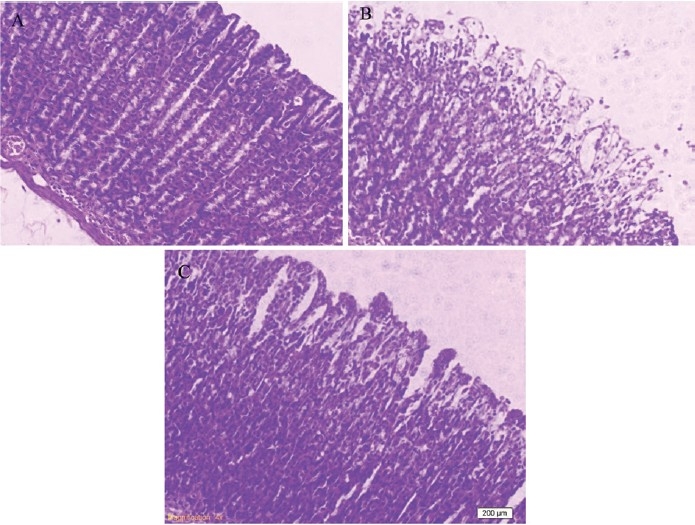
Effects of L-citrulline on gastric damage induced by ischemia-reperfusion (I/R) in rats (Haematoxylin and Eosin stain) sham (A), I/R control (B), L-citrulline 900 (mg/kg) (C)
Effect of L-citrulline on MPO Activity in the Gastric Mucosa
MPO activity was measured in the gastric mucosa as a marker for neutrophil infiltration [Figure 3]. The activity of MPO in the mucosa of sham group (2.62 ± 0.31 μmol H2O2/min/mg protein) was increased almost three-fold by the I/R procedure. L-citrulline pretreatment (300, 600, and 900 mg/kg i.g.) attenuated this increase in MPO activity.
Figure 3.
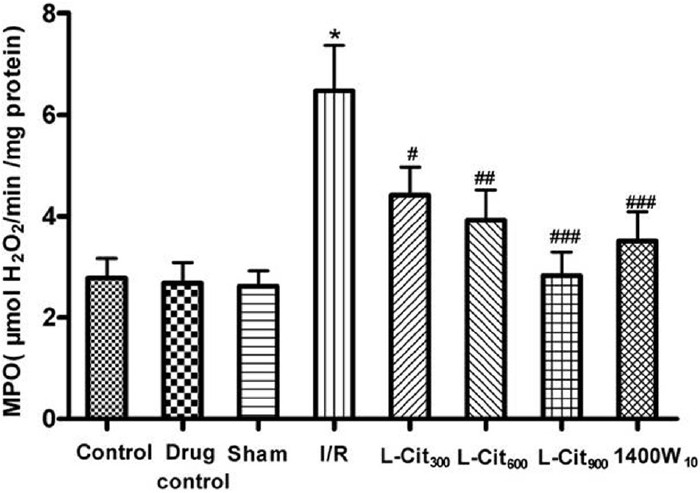
Effects of L-citrulline (L-Cit, 300, 600, 900 mg/kg, i.g.) on the myeloperoxidase (MPO) activity in the gastric mucosa after ischemia-reperfusion (I/R) injury in rats. Data are the mean ± S.E.M. for six to eight animals. *P< 0.001 compared with the sham group; #P< 0.05, ##P< 0.01, ###P< 0.001 compared with the I/R group
Effects of L-citrulline on MDA Content in the Gastric Mucosa
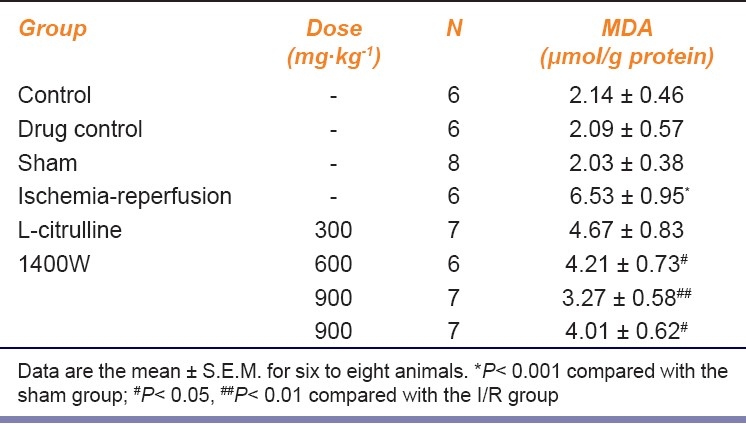
Ischemia-reperfusion significantly increased the MDA level in the gastric mucosa [Table 1]. Pretreatment with L-citrulline (600 and 900 mg/kg) significantly reduced the increase in MDA level, particularly at the dose of 900 mg/kg. The inhibition by L-citrulline (600 and 900 mg/kg, i.g.) was more effective than that by 1400W (10 mg/kg, s.c.).
Table 1.
Effect of L-citrulline on the MDA production in gastric mucosa after gastric ischemia-reperfusion injury
Effect of L-citrulline on Expression of iNOS, nNOS and eNOS in the Gastric Mucosa
As shown in Figure 4, a significant increase in iNOS expression was observed in the I/R group with respect to control (P< 0.001); L-citrulline (600 and 900 mg/kg) pre-administration significantly attenuated this increase (P< 0.01 and P< 0.001) [Figure 4]. However, the expression of nNOS and eNOS were not significantly affected by I/R or L-citrulline.
Figure 4.
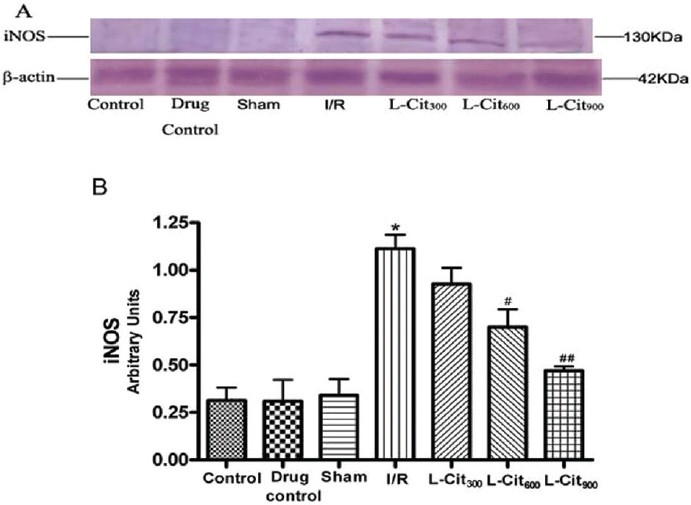
Effect of L-citrulline (L-Cit, 300, 600, 900 mg/kg, i.g.) on iNOS expression in the gastric mucosa after ischemia-reperfusion (I/R) injury in rats. (A) Inducible nitric oxide synthase (iNOS) protein levels in the gastric mucosal homogenate as visualized by immunoblotting with antibodies against iNOS. (B) Densitometric analysis after normalization with β-actin. Data represents mean ± S.E.M., n=4 *P< 0.001 compared with the sham group; #P< 0.01, ##P< 0.001 compared with the ischemia-reperfusion (I/R) group
Discussion
Previous studies have reported that L-citrulline could increase plasma levels of L-arginine concentration by the L-citrulline-NO cycle.[8] Although different authors have focused on the protective effect of L-arginine against gastric ulcers by maintaining tissue contents of gastric mucus and inhibiting neutrophils infiltration into gastric mucosa,[18,19] as far as we know, there is no information on whether or not L-citrulline administration could inhibit the development of the gastric mucosal lesions. In the present work, we investigated the effect of L-citrulline, the co-product of NO synthesis, on gastric mucosal lesions induced by I/R in rats. Our results indicate that L-citrulline reduced the development of gastric mucosal lesions induced by I/R in rats in a dose dependent manner. Macroscopic observations also showed a protective effect of L-citrulline against the gastric mucosal lesions induced by I/R.
In the present study, we observed a significant increase in mucosal MPO activity in the stomach after I/R confirming the infiltration/activation of neutrophils in the gastric mucosa during I/R. Moreover, previous reports have demonstrated that superoxide radicals play an important role in the pathogenic mechanism of I/R-induced damage.[20] In this experimental model, neutrophils appeared to be an important source of reactive oxygen metabolites and play an important role in the development of gastric damage by their aggregation and release of tissue disrupting substances, such as oxygen free radicals and proteases.[18] In our study, gastric damage with the increase of MPO activity and MDA level during reperfusion in rats were prevented by prior administration of 1400W, which is consistent to previous reports.[7] These results further demonstrated that the NO derived from the iNOS is proulcerogenic in the stomach during I/R.
Moreover, in the present study, pre-administration of L-citrulline significantly inhibited the neutrophil infiltration into the gastric mucosal tissue after I/R. Recently, it has been indicated that NO derived from iNOS contributes the infiltration/activation of neutrophils in the gastric mucosa.[7] It is considered that NO interacts with superoxide radicals to produce cytotoxic peroxynitrite which has a deleterious influence on the gastrointestinal mucosal integrity.[21,22] Rachmilewitz et al.[23] showed tissue inflammation after applying the peroxynitrite generating system locally to the rat colon. Kobata et al.[7] reported that the occurrence of gastric ulcer was associated with the up-regulation of iNOS expression and neutrophils infiltration in rats. In our experiment, the gastric damage with the increase in iNOS expression was prevented by L-citrulline. There is a growing evidence that L-citrulline can be readily converted to L-arginine in the kidney, vascular endothelium, and other tissues, thus raising plasma and tissue levels of L-arginine.[8] Moreover, L-arginine could inhibit the increase of iNOS expression induced by myocardial ischemia-reperfusion.[24] Therefore, the inhibitory effect of L-citrulline on iNOS expression might be mediated via conversion into L-arginine in vivo. In the present study, we also determined the expression of nNOS and eNOS. However, the expression of nNOS and eNOS was not affected by I/R or L-citrulline. It is possible that L-citrulline produces a protective effect against I/R-induced gastric mucosal lesions in rats through inhibition of neutrophil infiltration into gastric mucosal tissues and increase in MDA content in rats with I/R, which are closely related to preventing the NO production via iNOS in gastric mucosal tissue with I/R.
In conclusion, we have demonstrated that L-citrulline has a protective effect on the I/R-induced gastric mucosal damage. This protective effect occurs, at least in part, through inhibition of the neutrophil infiltration into gastric mucosa and the increase in MDA content, which might be related to preventing the increase of iNOS expression. However, further investigations are required to clarify the mechanisms in detail for the protective effect of exogenous L-citrulline against gastric mucosal lesions in rats.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK2009253), The Foundation of Xuzhou Medical College Key Laboratory of Tumor Biology Therapy (C0904), The Foundation of Xuzhou Medical College for postgraduate (No.XYCX 200913).
Footnotes
Source of Support: The Foundation of Xuzhou Medical College Key Laboratory of Tumor Biology Therapy (C0904) and The Natural Science Foundation of Jiangsu Province (No. BK2009253).
Conflict of Interest: None declared.
References
- 1.Kim CD, Hong KW. Preventive effect of rebamipide on gastric lesions induced by ischemia-reperfusion in the rat. J Pharmacol Exp The. 1995;275:340–4. [PubMed] [Google Scholar]
- 2.Wallace BJ, Keen CN, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462–G7. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- 3.Cutin J, Haase H, Moura MA. Evaluation of gastric mucosal potential differences across gastric mucosa in patients with chronic gastritis according to histology and degree of inflammation. Dig Dis Sci. 1987;32:239–43. doi: 10.1007/BF01297047. [DOI] [PubMed] [Google Scholar]
- 4.Whittle BJ, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: Interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99:607–11. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg SS, Xie J, Ouyang J, Zhao X. Ethanol metabolism is not required for inhibition of LPS-stimulated transcription of inducible nitric oxide synthase. Alcohol. 1999;17:203–13. doi: 10.1016/s0741-8329(98)00048-2. [DOI] [PubMed] [Google Scholar]
- 6.Nishio H, Hayashi Y, Terashima S, Takeuchi K. Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci. 2006;79:1523–30. doi: 10.1016/j.lfs.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kobata A, Kotani T, Komatsu Y, Amagase K, Kato S, Takeuchi K. Dual action of nitric oxide in the pathogenesis of ischemia/reperfusion-induced mucosal injury in mouse stomach. Digestion. 2007;75:188–97. doi: 10.1159/000108590. [DOI] [PubMed] [Google Scholar]
- 8.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol. 2003;206:2083–7. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan BC, McIntyre RC, Jr, Meldrum DR, Fullerton DA. L-Arginine prevents lung neutrophil accumulation and pulmonary endothelial function after endotoxin. Am J Physiol. 1998;274:L337–L42. doi: 10.1152/ajplung.1998.274.3.L337. [DOI] [PubMed] [Google Scholar]
- 10.Izhar U, Schwalb H, Borman JB, Merin GJ. Cardioprotective effect of L-arginine in myocardial ischemia and reperfusion in an isolated working rat heart model. J Cardiovasc Surg. 1998;39:321–9. [PubMed] [Google Scholar]
- 11.Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, et al. L-Citrulline and L-arginine supplementation retartds the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc Natl Acad Sci USA. 2005;102:13681–6. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida K, Ohta Y, Ishiguro I. Role of gastric mucosal constitutive and inducible nitric oxide synthases in the development of stress-induced gastric mucosal lesions in rats. Biochem Biophys Res Commun. 1997;236:275–9. doi: 10.1006/bbrc.1997.6972. [DOI] [PubMed] [Google Scholar]
- 13.Sureda A, Cordova A, Ferrer MD, Tauler P, Perez G, Tur JA, et al. Effects of L-citrulline oral supplementation on polymorphonuclear neutrophils oxidative burst and nitric oxide production after exercise. Free Radic Res. 2009;43:828–35. doi: 10.1080/10715760903071664. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Ueda S, Naito Y, Takahashi S, Oyamada H, Morita Y, et al. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radical Res Commun. 1989;7:285–91. doi: 10.3109/10715768909087953. [DOI] [PubMed] [Google Scholar]
- 15.Wada K, Kamisaki Y, Kitano M, Nakamoto K, Itoh T. Protective effect of cystathionine on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Eur J Pharmacol. 1995;294:377–82. doi: 10.1016/0014-2999(95)00558-7. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H, Hwan Kim K. Role of nitric oxide and mucus in ischemia/reperfusion-induced gastric mucosal injury in rats. Pharmacology. 2001;62:200–7. doi: 10.1159/000056095. [DOI] [PubMed] [Google Scholar]
- 19.Ohta Y, Nishida K. L-arginine protects against stress-induced gastric mucosal lesions by preserving gastric mucus. Clin Exp Pharmacol Physiol. 2002;29:32–8. doi: 10.1046/j.1440-1681.2002.03607.x. [DOI] [PubMed] [Google Scholar]
- 20.McCord JM. Oxygen-derived free radicals in postischemic Tissue injury. N Eng J Med. 1985;12:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka A, Kunikata T, Mizoguchi H, Kato S, Takeuchi K. Dual action of nitric oxide in pathogenesis of idomethacin-induced small intestinal ulceration in rats. J Physiol Pharmacol. 1999;50:405–17. [PubMed] [Google Scholar]
- 22.Lamarque D, Whittle BJ. Role of oxygen-derived metabolites in the rat gastric mucosal injury induced by nitric oxide donors. Eur J Pharnmacol. 1995;24:187–94. doi: 10.1016/0014-2999(95)00075-v. [DOI] [PubMed] [Google Scholar]
- 23.Rachmilewitz D, Stamler JS, Karmeli F, Mullins ME, Singel DJ, Loscalzo J, et al. Peroxynitrite-induced rat colitis-a new model of colonic inflammation. Gastroenterology. 1993;105:1681–8. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- 24.Liang F, Gao E, Tao L, Liu HR, Qu Y, Christopher TA, et al. Critical timing of L-arginine treatment in post-ischemic myocardial apoptosis-role of NOS isoforms. Cardiovasc Res. 2004;62:568–77. doi: 10.1016/j.cardiores.2004.01.025. [DOI] [PubMed] [Google Scholar]


