Abstract
Introduction:
Gentamicin is an essential drug for the treatment of sepsis in neonates. The current work aims to optimize the use of gentamicin in neonates during the first week of life.
Materials and Methods:
The study was done at King Abdul-Aziz university hospital. Seventy-three neonates who received gentamicin 4-5 mg/kg and dosing interval at 24-48 hr were enrolled. Peak and trough serum levels of gentamicin were determined by immunoassay. Pharmacokinetic parameters were estimated assuming one compartment model and first order elimination kinetic. Analysis of variance was used to test the difference between means using Statistical Package for the Social Sciences (SPSS) Version 13.
Results:
About 73% of the patients attained peak gentamicin level within therapeutic range (6-12μg/ml), while 12% showed potentially toxic trough level (>2 μg /ml). The incidence of trough level was higher among patients receiving the drug every 24 hr. There was no clear correlation between high trough level and serum creatinine. High volume of distribution (Vd) of gentamicin (0.40-0.45) L/kg was observed. Neonates with proven sepsis showed higher mean Vd. Those with extremely low birth weight showed significantly longer half life of 11.5 h. Other neonates showed half life of (8-9) hr.
Conclusions:
Gentamicin dose of 4.5 mg/kg every 36 hr is recommended as simple empirical regimen during the 1st week of life for neonates with normal or LBW and every 48 hr for those with ELBW.
KEY WORDS: Gentamicin, preterm neonates, pharmacokinetics, therapeutic drug monitoring
Introduction
Neonatal sepsis is a clinical syndrome characterized by systemic signs of infection and accompanied by bacteremia in the first month of life. Neonatal sepsis is always one of the most important causes of neonatal mortality. The incidence of septicemia in neonates is high in both term and preterm neonates weighing less than 1500 g. Neonatal infections occur in the first seven days of life called early-onset infections, but late onset sepsis happen later on.[1,2]
Gentamicin (genta) is one of the most commonly used antibiotics in neonates to control sepsis. It is highly effective against gram negative bacilli. In addition, it has a concentration-dependent antibacterial action and post-antibiotic effect.[3] In neonates, clinical evidences suggest the use of large-dose, extended interval genta regimens to achieve optimum peak and trough concentration when compared to the traditional multiple daily dose regimens. In addition, the adjustment of dose and dosage- interval according to gestational age (GA) and birth weight was found to be suitable for preterm neonates. Based on these rationales, several neonatal genta dosing regimens have been proposed. However, there is a wide variety among these protocols.[4]
There is evidence of a relationship between serum genta levels, efficacy, and incidence of toxic events. Available data on the use of genta in neonates suggested that extended dosing intervals and higher dose ≥ 4 mg/ kg confer favorable pharmacokinetic (PK) profile (high peak and low trough level). This provides the basis for potential enhancement of therapeutic efficacy and reduces toxicity.[4,5] Studies concerned with the efficacy of genta in adults and children showed early achievement of therapeutic genta levels with better clinical outcomes.[3] These finding could be extrapolated to neonates and similar or better outcome is expected as they have reduced immunity.[6]
Therefore, the aim of the present work was to provide a practical guideline to optimize the use of genta in neonates during the first week of life.
Materials and Methods
The present study was conducted at the Neonatal Unit, King Abdulaziz University Hospital (KAUH), Jeddah, KSA from Feb 2008 to November 2008. The study protocol was approved by the Research Ethics Committee of the Faculty of Medicine, KAUH. All neonates aged ≤ 7 days, who received genta as an empirical treatment for clinically suspected or high risk of sepsis, were included in the study.
The exclusion criteria comprised neonates who had missing peak or trough genta levels; received drugs that impair renal function; or have congenital anomalies. All the demographic and clinical data were recorded. These data included demographic characteristics: gestational age (GA), body weight “BW”, sex, etc, certain biochemical, hematological, microbiological investigations, other co-medications, dosing regimen, and sampling time. In all enrolled neonates, both peak and trough levels of genta were determined. The dosing regimen was the sole responsibility of the clinician in charge. Also, serum creatinine level at baseline and at least another two levels during the treatment course were determined.
The dose and dosing interval were as follows: GA < 29 weeks 5 mg/kg/dose every 48 hours, GA=30-34 weeks 4.5 mg/kg/dose every 36 hours, and GA ≥ 35 weeks 4 mg/kg/dose every 24 hours. Genta doses were diluted with 3 milliliters of 5% dextrose-in-water and intravenously infused over 30 minutes with a syringe pump, followed by 1.5 milliliters normal saline solution flush. Samples for the peak genta level were taken after the third dose; 30 minutes after the end of infusion and samples for trough were taken just before the third dose.
Genta level was analyzed by a fully-automated immunoassay procedure using a homogenous particle enhanced turbidimetric inhibitory immunoassay technique (PETINIA) with Dimension Clinical Chemistry System (Stream lab – Dade Behring). Calibration and analysis were performed as specified by the manufacturer. All specimens were tested within one hour otherwise the serum was separated and stored at 1–5°C until analyzed within six hours. Accuracy of analysis was assured by daily running of three samples) low, medium, high concentrations) of genta quality control (Biorad QC program).
The patients were classified in view of birth weight into four groups as follows;
Group 1: Extremely low birth weight, (ELBW) < 1 kg; Group 2: Very low birth weight (VLBW):1 – 1.5 kg; group 3: Low birth weight (LBW) >1.5 –2.5 kg and Normal BW >2.5-4.kg in view of GA (week) into 4 groups as follows:
Group 1: < 29 GA; group 2: 30-33 GA; group 3: 34 –36 GA; group 4: >36 -38.
Groups 1, 2 and 3 were re-categorized as preterm and group 4 as term.
Calculations:
Microsoft Office Excel 2003 program was used to calculate PK parameters: volume of distribution (Vd), elimination rate constant (ke), and half-life (t½).
The following equations were used[7]
Ke = LnC1/C2/ (t2-t1) equation 1
Where C1 = Peak level; C2 = Trough level; t1 = time of sampling for peak (C1); t2 =time of sampling for trough (C2).
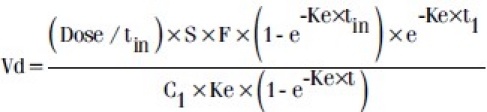
Where dose: mg/kg; S = salt factor =1; F = bioavailability factor =1; τ = dosing interval; tin = infusion time (hr); C1= peak level at Css.
The following equation was utilized for estimation of creatinine clearance (Clcr) in neonates using patient's weight and serum creatinine (Scr) level according to the following equation[8]
Clcr (ml/min/1.73m2) = k × height (cm)/Scr (mg/dL)
Where k = 0.33 for Infant (LBW<1 y), K=0.45 for infants (Term <1y)
Statistical Analysis
Data were statistically analyzed using Statistical Package for the Social Sciences (SPSS) package (version 13). Data were presented as mean ± SD, the statistical significance between means of various parameters was evaluated by analysis of variance test (ANOVA). The difference was considered significant if the P value < 0.05.
Results
Demographic Data of the Patients
Seventy three neonates were enrolled in this study. Their demographic characteristics are summarized in Table 1, preterm neonates were the predominant group (60%), and the prevalence of ELBW was higher in this group. Concomitant illnesses are presented in Table 2. Eleven patients (15%) suffered from sepsis; eight of them were preterm.
Table 1.
Demographic characteristics of patients
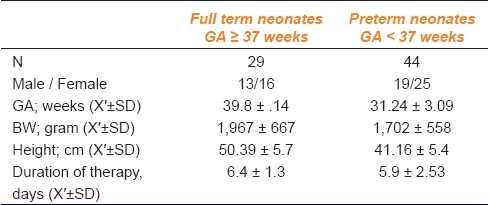
Table 2.
Patients underlying medical conditions (n=73)

In term of neonates, the base line mean Scr was 56.7 ± 18,2 umol/L and increased during the treatment to 69.8 ±16, while in preterm, there was no clear difference between the mean Scr values base line 62.0 ± 15.3 and during the treatment was 59.45 ± 17.2.
Genta Serum Level
Figure 1 shows the peak and trough levels of all neonates. Out of the 73 patients, 53 (73%) had a peak within the therapeutic range (6-12 μg/ml), 18 (25%) had a peak level (>12 μg/ml), and 9 (12%) had a potentially toxic trough (> 2 μg/ml).
Figure 1.
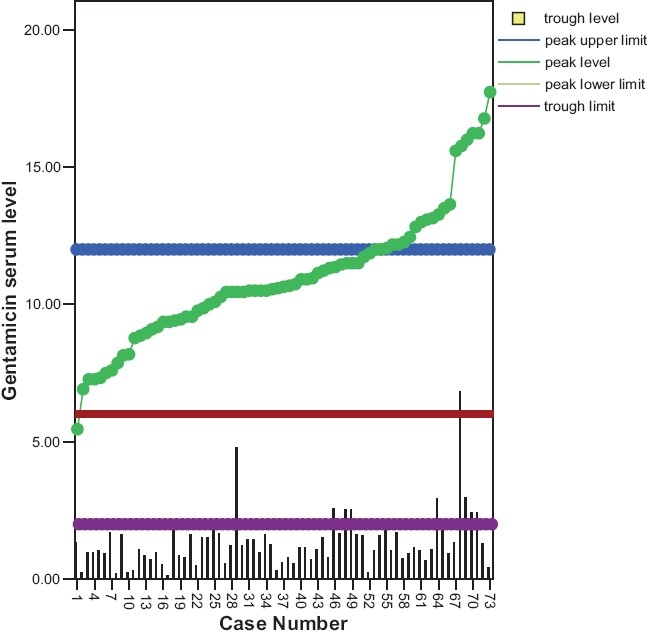
Genatmicin level in term and preterm neonates
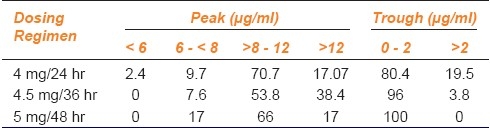
The peak and trough levels in view of dosing regimen are shown in Table 3. The incidence of potentially toxic trough level was clearly higher among the patients who received genta in the regimen 4 mg/24 hr. In case of patients who received genta in the regimen 4 mg/36 hr, all the peak levels were within the therapeutic range and none showed trough level > 2 μg/ml.
Table 3.
Peak and trough levels (% of total samples) in view of dosing regimen
Genta PK Parameters
Tables 4 and 5 show the PK parameters (PK) of genta in neonates classified according to GA and BW, respectively. The mean value of all the tested PK parameters showed no significant difference among different groups classified in view of GA. Neonates with ELBW showed significantly (P <0.05) and higher t½(11.5 ± 0.5 h) compared to other weight groups. Extreme variability was observed in all the PK parameters especially in ELBW. Neonates with documented sepsis showed significantly (P<0.05) higher mean Vd ± SD (0.49 ± 0.069) compared to the non-septic patients (0 0.42 ±0.013 [Figure 2].
Table 4.
Pharmacokinetic parameters mean ± SD, (CV%) of genta in neonates classified according to GA
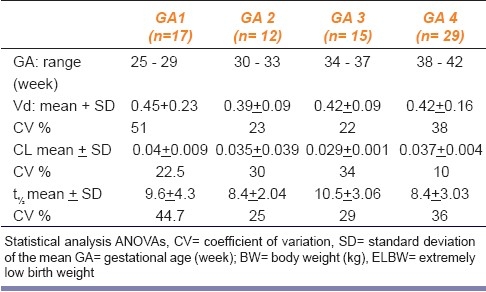
Table 5.
PK parameters of genta in neonates classified according to BW (n= 73)
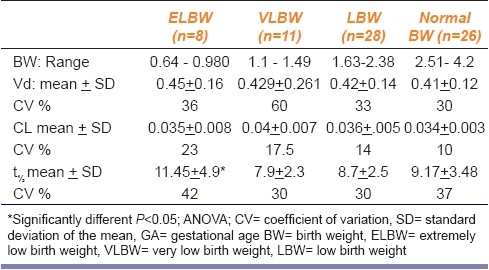
Figure 2.
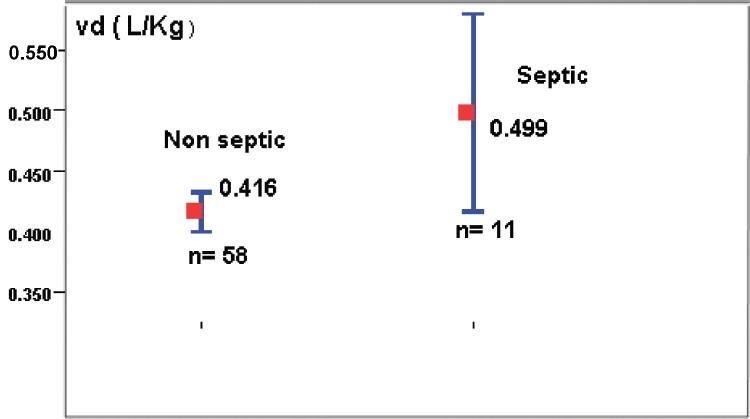
Effect of sepsis on volume of distribution (P<0.05)
Discussion
Many studies on neonates defined the therapeutic range of peak gentamicin as a marker for efficacy and trough level as a probe for potential toxicity. Many of these studies have set the upper limit of the peak as 12 μg/ml and the lower limit as 4-6 μg/ml; some studies considered higher peaks (18 -20 μg/ml) as acceptable if lower trough (< 1.5 μg/ml) is ensured.[9] Most studies considered a trough level 0- 2 μg/ml as acceptable prediction of medication safety through TDM application as it is an important issue in case of genta administration. In neonates, trough level <2 μg/ml (or <1.5 μg/ml) in case of peak (>12 ug/ml) has been used as a measure to avoid potential renal or ototoxicity.[9]
In the present study, the dosing guidelines of genta during the first week of life in neonates were applied and re-evaluated. The present results provided the evidence that current dosing regimen (high dose and extended interval) allowed achievement of therapeutically effective peak levels > 6 μg/ml in almost all of the patients. In about 88% of the patients, peak level (> 8μg) was attained. These concentrations seem to be sufficiently high for treatment of most infections by microorganisms sensitive to genta. We consider that all patients received concomitantly adequate β-lactam antibiotics which have synergistic effect with aminoglycoside[10] (mixing of β-lactam with aminoglycosides was avoided).
In this study, 12% of neonates showed trough genta level >2 μg/ml. The incidence of high trough level is greater in those who received 4 mg/24 hr. On the other hand, the present findings fail to find a correlation between trough genta level and serum creatinine level. In neonates, Scr level has been reported to be inaccurate during the first week(s) of life . Immediately after birth, the high Scr of neonates represent maternal level. Shortly thereafter, tubular re-absorption of creatinine seems to be responsible for elevation of creatinine levels. In view of this, it was suggested that patients with trough level >2 μg/ml are liable for genta nephrotoxicity even when Scr level appear within normal range.[11] In clinical practice, it is preferable to ensure accuracy of sampling time, wherever genta trough level (> 2 μg/ml) is observed.
Similar result were reported by Verelde et al., where they measured the peak and trough genta levels in neonates during the first week of life after a dose of 3.5 mg/kg/24hr. They considered peaks (5-10) μg/ml and a trough (<2 μg/ml) as target concentrations. They revealed that 87% and 95% of patients have attained the target peak and trough levels, respectively.[12]
Neonates display a large interindividual difference in the PK of aminoglycosides due to the developmental differences early in life. The volume of distribution of aminoglycosides shows a strong relationship with body weight, which tends to be larger in premature infant and those with sepsis. This variability will affect the peak/trough levels and hence efficacy and or toxicity pattern[5] . TDM and PK principles were appropriately used to support clinicians and to keep genta trough concentration within the acceptable (< 2ug/ml) level. In addition, the newborn's weight and gestational age were among the important variables which influenced genta peak in neonates and hence its serum level.[10]
In the present study, individual PK parameters of neonates were estimated using peak and trough levels and determined during routine clinical practice. The aim was to provide simple and accurate approach for both empirical dosing and dosage adjustment that do not involve complicated calculations.
These findings confirmed the long half life (9-11 hr) of genta in all groups of neonates. These findings support the extended dosing intervals regimen. The longest half life was demonstrated in preterm with ELBW (less than 1000 g). This suggests that dosing interval of 48 is appropriate for those subgroups of neonates.
Multiple regression analysis was utilized to identify covariates which might significantly affect these parameters. This study was limited to focus on PK of genta during the first week of life, since most studies have been focused on the relevance of PCA and PNA on these parameters.[13]
Aminoglycosides are water soluble compounds, minimally bound to plasma proteins; so that they are readily distributed mainly through extra cellular fluids and prevent them from readily crossing cell membrane into intracellular fluids. Therefore, volume of distribution of aminoglycosides corresponds approximately to extracellular fluid volume[14]
In preterm neonates, water makes up to 85% of the body weight and 75% in case of neonates. This decreases to approximately 60% at five months and then remains relatively constant from this age on ward. The percentage of body weight contributed by fat is 3% in 1.5 kg premature neonates compared with 12% in a term of neonates and these proportions doubled by five months of age. Besides, baby fat is lost when infant starts walking and also protein mass as well as muscle bulk increase.[15] These facts explain the observed relatively high Vd and low clearance of aminoglycosides in neonates compared with children and adults.
In the present study, an increase in the mean Vd with decrease in the mean BW was observed. Sepsis was found to affect Vd in this study population; about 17% increase in Vd and this finding is approximately similar to study done by Lingvall et al.,[16]
In the present study, the mean Vd range was between 0.39 and 0.46 L/kg. Furthermore, a greater variability was observed in neonates with lower GA (25-29 week). Similar values of mean Vd ±SD of genta in neonates during first week of life were reported by Rocha et al.;[17] since they found VD in the preterm (0.46 ±0.091) and in term (0.43 ±0.094), respectively.
Many drugs that are used for the management of infectious disease in neonates are mainly eliminated by the kidneys. These include penicillins, cephalosporins, and aminoglycosides. In neonates, especially preterm, both glomerular filtration rate (GFR) and tubular secretion are reduced. In contrast, filtration is relatively more developed. Improvement of GFR after birth depends on both gestational age and postnatal age.[15] The lower GFR seen in neonates is expected to cause less drug clearance of renal eliminated drugs such as genta.
In the present study, we couldn’t document the effect of GA on mean genta clearance or other PK parameters. We found that CL range (0.035- 0.041) L/Kg/hr; and half life range (8 - 11.5) hr. Half life was significantly (P<0.05) higher in ELBW (11.45 ± 4.9) hr compared to other groups. Similar values of mean Cl and t½ in preterm neonates during the first week of life were reported;[18] Cl ± SD (0.030 ± 0.008); t½ ± SD (11.17 ± 2.89) hr. Other study done by Veltkamp et al.,[18] showed that the mean CL± SD of genta was 0.046 ± 0.0015 and the mean t½ of genta was 10.2 ±3.7.
Acknowledgment
The present work was supported by a grant from King Abdul-Aziz City of Science and Technology garnt AT -17-122. The authors acknowledge MRs Randa Moumena biochemistry lab KAUH for her efforts in analysis of genatmicin samples
Footnotes
Source of Support: Grant from King Abdul-Aziz City of Science and Technology garnt AT -17-122.
Conflict of Interest: None declared.
References
- 1.Gomella TL, Cunningham MD, Eyal FG. 5th ed. New York: Lange Medical Books; 2004. Neonatology: Management, Procedures, On-Call Problems, Diseases, and Drugs. [Google Scholar]
- 2.Levene MI, Tudehope DI, Thearle MJ. 3rd ed. Oxford: Blackwell Science; 2000. Essentials of Neonatal Medicine. [Google Scholar]
- 3.Thingvoll ES, Guillet R, Caserta M, Dicenzo R. Observational trial of a 48-hour genatmicin dosing regimen derived from Monte Carlo simulations in infants born at less than 28 weeks’ gestation. J Pediatr. 2008;153:530–4. doi: 10.1016/j.jpeds.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 4.Darmstadt GL, Miller-Bell M, Batra M, Law P, Law K. Extended- interval dosing of genatmicin for treatment of neonatal sepsis in developed and developing countries. J Health Popul Nutr. 2008;26:163–82. [PMC free article] [PubMed] [Google Scholar]
- 5.Touw DJ, Westerman EM, Sprij AJ. Therapeutic drug monitoring of aminoglycosides in neonates. Clin Pharmacokinet. 2009;48:71–88. doi: 10.2165/00003088-200948020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Testa M, Fanos V, Martinelli V, Mussap M, Zompo M. Therapeutic drug monitoring of genatmicin in neonatal intensive care unit: Experience in 68 newborns. J Chemother. 2007;19(Suppl 2):39–41. doi: 10.1080/1120009x.2007.11782443. [DOI] [PubMed] [Google Scholar]
- 7.Zaske D, Shikoma L, Tholl D. Aminoglycosides. In: Taylor W, Diers CM, editors. A Textbook for Theclinical Application of Therapeutic Drug Monitoring. Irving: Abbott Laboratories; 1986. pp. 285–320. [Google Scholar]
- 8.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–6. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 9.Nestaas E, Bangstad HJ, Sandvik L, Wathne KO. Aminoglycoside extended interval dosing in neonates is safe and effective: A meta-analysis. Arch Dis Child. 2005;90:F294–300. doi: 10.1136/adc.2004.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy J. Prediction of gentamicin peak and trough concentrations from six extended-interval dosing protocols for neonates. Am J Health Syst Pharm. 2005;62:823–7. doi: 10.1093/ajhp/62.8.823. [DOI] [PubMed] [Google Scholar]
- 11.Locksmith G, Chin A, Vu T, Shattuck K, Hankins G. High compared with standard genta dosing for chorioamnionitis: A comparison of maternal and fetal serum drug levels. Obstet Gynecol. 2005;105:473–9. doi: 10.1097/01.AOG.0000151106.87930.1a. [DOI] [PubMed] [Google Scholar]
- 12.Vervelde ML, Rademaker CM, Krediet TG, Fleer A, Van Asten P, Van Dijk A. Population pharmacokinetics of genta in preterm neonates: Evaluation of a once-daily dosage regimen. Ther Drug Monit. 1999;21:514–9. doi: 10.1097/00007691-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Stolk LM, Degraeuwe PL, Nieman FH, De Wolf Mc, De Boer A. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of genta in neonates. Ther Drug Monit. 2002;24:527–31. doi: 10.1097/00007691-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Brunton LL, Lazo JS, Parker KL. The pharmacological basis of therapeutics. In: Goodman, Gilmans, editors. 11th ed. New York: McGraw-Hill; 2006. [Google Scholar]
- 15.Anderson B, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 16.Lingvall M, Reith D, Broadbent R. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br J Clin Pharmacol. 2005;59:54–61. doi: 10.1111/j.1365-2125.2005.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha MJ, Almeida AM, Afonso E, Martins V, Santos J, Leitão F, et al. The kinetic profile of genta in premature neonates. J Pharm Pharmacol. 2000;52:1091–7. doi: 10.1211/0022357001775010. [DOI] [PubMed] [Google Scholar]
- 18.Veltkamp SA, Westrman E, Sprij AJ, Sum BL, Touw DJ. Genta in preterm neonates: An extended interval dosing scuduale. Eur J Hosp Pharm Sci. 2007;13:92–7. [Google Scholar]


