Abstract
Objective:
To study the analgesic activity of ash of silver used in Indian system of medicine and to explore its safety.
Materials and Methods:
Albino mice of either sex (20-30 gm) were used to investigate the role of ash of silver against noxious stimuli: thermal (Eddy's hot plate and analgesiometer), mechanical (tail clip), and chemical (0.6% acetic acid induced writhing). An effort was made to find nature and site of action of ash of silver following naloxone pre-treatment. Maximum tolerated dose (MTD) and lethal dosage 50 (LD50) were also studied along with toxicological aspects of ash of silver.
Results:
Test drug (ash of silver) at a dose of 50 mg/kg p.o exhibited analgesic activity against thermal, mechanical, and chemical stimuli. Analgesic effects were compared with the standard drug, morphine, in thermal and mechanical noxious stimuli and to aspirin in chemical stimulus. Analgesic activity of the test drug was reduced following naloxone pre-treatment. MTD was found out to be greater than 1.5 g/kg p.o. LD50 was 2 g/kg p.o. Fraction of mice showed symptoms of argyria as explained by autopsy reports.
Conclusion:
Test drug exhibited moderate analgesic activity at 50 mg/kg p.o against all type of noxious stimuli, also suggesting a role of opioidergic system. The ash of silver was been found to be safe upto a dose of 1.5 g/kg p.o. in mice without any untoward toxicity. Further studies are required to explore the effect of ash of silver on pain mediators and excitatory neurotransmitters like glutamate, aspartate, or N-methyl-D-aspartic acid (NMDA).
KEY WORDS: Ash of silver, analgesic, argyria, nanosilver
Introduction
Ayurveda, an ancient system of medicine, advocates the use of herbal and elemental preparations. Many elements which are known to be toxic viz As, Cd, Hg, Fe, Ag, Au etc in modern medicine are also used in ayurvedic preparations.[1–3] One such metal is silver. It is considered to be non-essential accumulative trace element with wide distribution in body including the central nervous system, but with no known biological and physiological function. In ayurveda, ash of silver, also known as Raupya Bhasma, is used to treat many disease conditions like pain, neuralgias, inflammation, anxiety, convulsions, memory loss etc since years.[4,5] Since the safety of ash of silver is already established from traditional experience, the scope of reverse pharmacology is to understand the mechanism of action and to optimize the safety and acceptability of the lead compounds in natural products. In this approach, the candidate travels a reverse path from “clinics to laboratory” rather than classical “laboratory to clinic”.[6]
Ash of silver is prepared by mixing pure silver leaves with equal quantity of sulphur by weight and one half quantity of arsenic trisulphide soaked in lemon juice and subjected to calcination process in sealed earthen containers at a temperature of 300°C.[1,7] The material is scrapped after cooling, triturated with lemon juice, pulverized, and calcined again. It undergoes the same process repeatedly (trituration with lemon juice, pulverization, and calcination) for 14 times to procure the ash of silver.[2–5,7] This process is chemical reduction of silver converting the silver molecule into uniform nano-particles, spherical in shape and with size of 16 nm without change in morphology of silver. Nanosize of silver particle is probably responsible for improving the penetration of silver in brain; hence, ash of silver has been used in past for the treatment of various pain and neurological conditions.[1,4,8,9]
To further confirm its central and peripheral analgesic property, reverse pharmacological studies were planned to find the effect of ash of silver and to explore whether it acts through opioidergic system or not. Safety of silver was also evaluated.
Material and Methods
Swiss strain of albino mice of either sex weighing between 20-30 gm were screened for study following the approval from Institutional Animal Ethics Committee. Mice were maintained under standard pellet diet and water ad libitum, housed in poly propylene cages under similar environmental conditions throughout the experiment. The test drug ash of silver was procured from M/s Baidyanath Ayurved Bhawan Ltd, Jhansi, India. Animals were divided into four groups consisting of six animals in each. Study protocol was as follows:
Group 1: Ash of silver (50 mg/kg) suspended in 1% solution of gum acacia, administered p.o. 20 min before exposure to noxious stimuli.
Group 2: Control, received gum acacia 1% solution p.o.
Group 3(a): Morphine hydrochloride (5 mg/kg) i.p. was administered 20 min before exposure to noxious stimuli.
Group 3(b): Aspirin (100 mg/kg) p.o was administered 20 min before exposure to noxious stimuli.
Once analgesic activity of ash of silver (50mg/kg) p.o was confirmed, further experiments were conducted to know its effect on opioidergic receptors as in group 4.
Group 4: Naloxone (1 mg/kg) i.p. was administered 30 min prior to ash of silver to study the mechanism and site of action of ash of silver (50 mg/kg) p.o. The responses of all drugs were assessed at 0, 30, 60, 90, and 120 min using analgesiometer in Tail flick test, Eddy's hot plate method, Haffner's tail clip method, and 0.6% acetic acid induced writhing methods, respectively.[10,11]
Systemic Toxicity Studies
Single dose toxicity studies were conducted in 18 mice of either sex in graded doses of drugs 25, 50, 100, 500, 1500, and 2000 mg/kg, respectively, within 24 hours. Animals were observed for 14 days for any signs of intoxication, any histopathological changes, gross behavioral changes, any weight loss and mortality.
Statistics
All data were expressed as mean±SEM and analyzed using ANOVA followed by Dunnett's “t” test. P-value <0.05 was considered significant.
Results
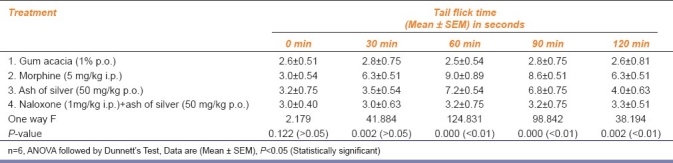
The results have been shown in AMK Tables 1–4. Ash of silver at a dose of 50 mg/kg p.o. produced significant analgesic response (P <0.01) compared to control (gum acacia 1% p.o.) but relatively less compared to standard drug (morphine 5 mg/kg i.p.) in the form of increased reaction time of mice to jump or lick paws in Eddy's hot plate method, increased tail flick latency in tail flick model, increased reaction time of mice to dislodge artery clip in tail clip model, all showing significant central analgesic activity. Ash of silver (50 mg/kg p.o.) suppressed acetic acid (0.6% i.p.) induced writhing episodes in mice (P<0.05) significantly showing peripheral analgesic activity compared to control, but standard drug aspirin (100 mg/kg p.o.) suppressed writhing episodes significantly more compared to ash of silver. Percentage inhibition of writhing episodes over 1 hour was 42.1% using ash of silver in contrast to standard drug aspirin (48.8%).
Table 1.
Assessment of central analgesic action of ash of silver by Eddy's Hot plate in albino mice
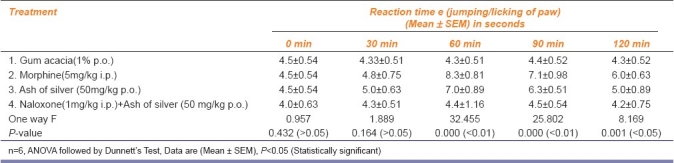
Table 4.
Assessment of peripheral analgesic action of ash of silver by acetic acid (0.6%) induced writhing in albino mice
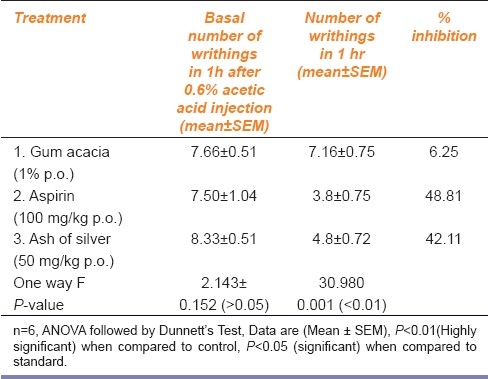
Table 2.
Assessment of central analgesic action of ash of silver by Tail flick method in albino mice
Safety Evaluation
Three out of eighteen mice died on day 11. No gross behavioral changes were observed in rest of the animals (up to a dose of 1500 mg/kg p.o.) except for some bluish black pigmentation of skin in four mice at the end of tenth day. Autopsy specimens from skin, spleen, liver, kidneys, and lymph nodes of dead mice showed deposits of silver granules in malpighian body of spleen, histiocytes of liver, renal glomerulus [Figures 1–3], and in the epidermal layer of skin without any changes in the morphology of skin. Maximum tolerated dose (MTD) was found to be >1.5g/kg. Lethal dosage 50 (LD50) was found to be 2 g/kg p.o as per the method described by Reed and Meuch.[10]
Figure 1.
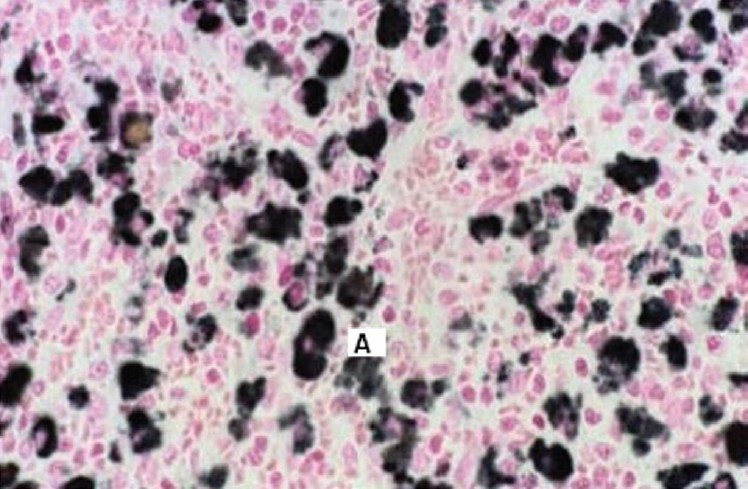
Section of spleen of mouse showing silver deposits (A) In reticulo-endothelial meshwork. (Magnification 40 ×)
Figure 3.
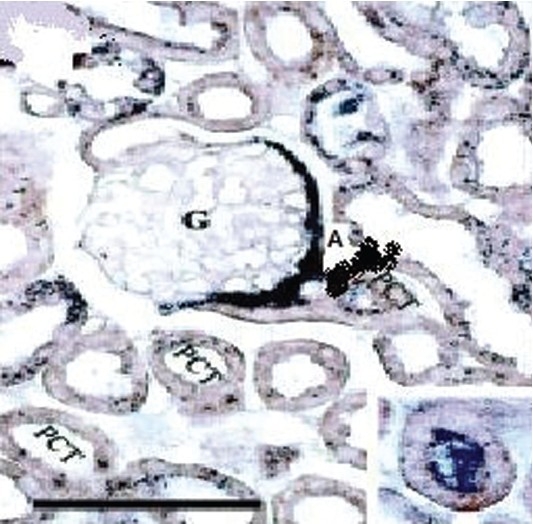
Section of kidney of mouse showing Argyrosis (A) in glomerular basement (G) and around proximal convulated tubules (PCT) (Magnification 100 ×)
Figure 2.
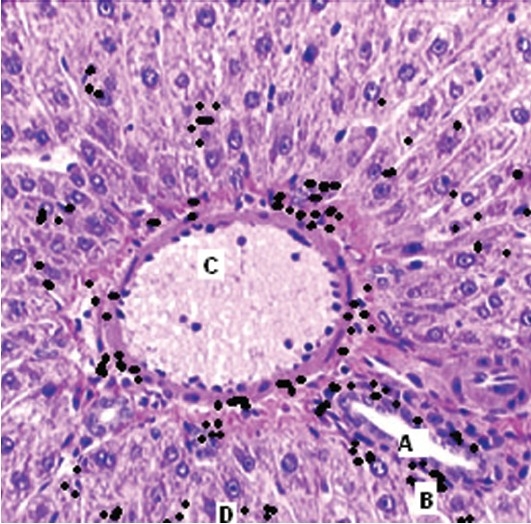
Section of mouse showing silver deposits (B) around whole thickness of portal vein (A) and hepatic artery (C) along with reticulum (D). (Magnification 40 ×)
Discussion
In the present study, we have tried to explore the mechanism of action, therapeutic use, and safety of ash of silver (which is in use since traditional times as analgesic without any documented evidence) by reverse pharmacological study through screening in selected model to fast track drug discovery and development. Ayurvedic knowledge and experimental database can provide new functional leads to reduce time, money, and toxicity.[6]
Ash of silver (50 mg/kg p.o.) has shown to exhibit significant analgesic activity at 60 min (P<0.01) compared to control and significantly less than standard drug morphine (5 mg/kg i.p.) in Eddy's hot plate, Haffner tail clip, Tail flick methods in central pain producing models and comparable to aspirin (100 mg/kg p.o.) in peripheral pain producing method viz writhing episodes induced by using 0.6% acetic acid.[10,11] Ash of silver exhibited maximum analgesic effect at 60 min in contrast to morphine which exhibited maximum analgesic effect between 60 to 90 min AMK [Tables 1–3] after which the effects started declining. Analgesic effect was observed in the form of increase in the reaction time of mice as paw licking or jump response, dislodging or biting of clip and tail flicking response in Eddy's hot plate, Haffner's tail clip and tail flick methods, respectively. Peripheral analgesic effect was tested by using 0.6% acetic acid [Table 4]. Number of writhing episodes before and after drugs administration was observed and percentage inhibition of writhing using ash of silver was compared with standard (aspirin 100mg/kg p.o.) and control (gum acacia) in the initial 1 hour. Percentage inhibition of writhing using ash of silver (42.1%) was comparable to that of the standard drug aspirin (48.8%), but not with control (gum acacia 6.25%) indicating that gum acacia does not have analgesic action of its own and does not potentiate the action of ash of silver in which it is suspended. The reduction in writhing episodes compared to basal number of writhings in first 60 min indicates an analgesic response.
Table 3.
Assessment of central analgesic action of ash of silver by nociception induced by Haffner's Tail Clip method
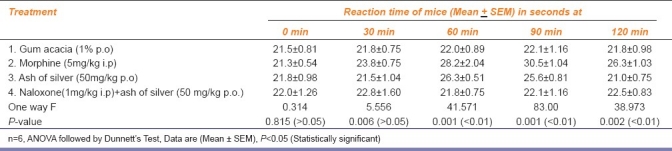
To find the nature and site of action of analgesic action of ash of silver, we tried to block opioid receptors by pre treatment of mice with naloxone (1 mg/kg i.p.) in all central methods of nociception (Eddy's hot plate, Tail flick, and Tail clip methods) followed by the administration of ash of silver (50 mg/kg p.o.), morphine (5 mg/kg i.p.), and gum acacia (1% p.o.). There was significant reduction in analgesic responses elicited by drug ash of silver.
Ash of silver exhibited potent analgesic effect against thermal and mechanical noxious stimuli indicating that it as a predominant central analgesic, but also inhibited peripheral pain components as shown in writhing method. This is further supported by the fact that when we blocked opioidergic system using naloxone in central pain producing methods, the analgesic effect was reduced significantly. Any injury or tissue damage is associated with pain. Analgesics can act on peripheral or central nervous system to block pain perception. Peripherally acting analgesics act by inhibiting PGs and bradykinins at the site of pain, thereby suppressing the generation of impulses at chemoreceptor site of pain as shown in chemical method, while centrally acting analgesics not only raise the threshold of pain, but also alter the physiological response to pain by acting on CNS and suppress patient anxiety and apprehension.[12–14] Further, it can be hypothesized that ash of silver may be having an inhibitory action on excitatory neuro-transmitters glutamate, aspartate, or N-methyl-D-aspartic acid (NMDA) receptors which needs to be evaluated. As far as traditional system of medicine is concerned, ash of silver is being used for treating pain and inflammatory conditions since ancient times,[15] It might be having inhibitory action on certain pain and inflammatory mediators e.g. PGs, substance-p, bradykinin, LTs etc.
The previous studies by Khanna et al. suggest moderate to marked analgesic activity against chemical and electrical pain, but not against mechanical pain in contrast to our study which has shown moderate analgesic effect against mechanical pain which is also centrally mediated. The authors suggested a role of opioidergic system in pain suppression by silver. One of the studies has proposed that ashes of heavy metals used in traditional system of medicine act as catalyzer by their presence in intestine, plasma and blood, thereby acting as free radical scavengers.[16] Ash particles of heavy metals (gold, silver) in calcined form, being insoluble, exist as nanoparticles (16 nm) which are very tiny particles and found biocompatible, therefore, can cross blood brain barrier to produce various central actions viz analgesic, anti-inflammatory, anti-anxiety, cognitive, antidepressant, neuroleptic, and antiepileptic for which they are used in traditional Indian system of medicine.[1,3,4,17]
Use of metals in medicine is associated with toxicity.[18] The use of metal based medicines can be attributed to various causes including a need to revive a rich tradition, the dependency of 80% population of India on these drugs, their easy availability, comparatively low cost, and therefore, increasing world wide use. Calcined form of silver has been found to be non-toxic and exhibiting free radical scavenging activity by virtue of their antioxidant activity as shown in some studies. The quantitative analysis of ash of silver by spectroscopy has detected traces of heavy metals (in parts per million) like arsenic, lead, chromium, and iron. Ashes are associated with organic macromolecules show increased superoxide dismutase and catalase activity which reduce free radical concentration.[1–3] Nitrate based salt of silver has found to be toxic at 50 mg/kg p.o. in rats and mice leading to hepatic necrosis and deaths.[19–21] As far as safety of ash of silver is concerned, in our study, three mice died on day 11. Rest of mice did not show any weight loss or any morbidity. Ash of silver has been found to be safe up to 1.5 g/kg p.o. which is its maximum tolerated dose (MTD). Four among 18 mice showed characteristic bluish black pigmented patches over the skin. Autopsy reports of skin, spleen, liver, and lymph nodes from two dead mice have shown deposits of silver in these sites owing to their very small particle size. LD50 was found to be 2 g/kg p.o. The characteristic bluish black pigmentation is known as argyria is due to deposition of silver particles and melanocyte stimulating effect of silver.[22] Few studies have shown cyanocobalamin, vitamin E, and selenium antagonize the toxic effects of silver.[23,24] Such reports indicate the possibility that silver as such is not harmful in humans, but may be toxic in those deficient in vitamins and trace minerals.[25] A correlation between silver and vitamins like vitamin E and trace minerals need to be explored.
Further studies are required to establish the safety and efficacy of ash of silver. Metallic and herbal preparations offer advantages over plant drugs and allopathic preparations by virtue of their stability over a period, lower dose, easy stability, sustained availability, and fewer adverse effects.[4] The ashes of metals need to be thoroughly investigated with regard to their elemental content speciation and organic constituents so as to develop an understanding of their therapeutic effect.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Khanna AT, Silvaraman R, Vohora SB. Analgesic activity of silver preparations used in Indian system of medicine. Indian J Pharmacol. 1997;29:393–8. [Google Scholar]
- 2.Gogtay NJ, Bhatt HA, Dalvi SS, Kshirsagar NA. The use and safety of non-allopathic Indian medicines. Drug Safety. 2000;25:1005–19. doi: 10.2165/00002018-200225140-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Nair AG, Reddy AV, Garg AN. Availability of essential elements in bhasmas: Analysis of ayurvedic metallic preparations by INAA. J Radioanalyt Nucl Chem. 2006;270:173–80. [Google Scholar]
- 4.Nadeem A, Khanna T, Vohora SB. Silver preparations used in Indian system of medicine: Neuropsychobehavioural effects. Indian J Pharmacol. 1999;31:214–21. [Google Scholar]
- 5.Hamilton EJ, Minski MJ, Cleary JJ. The concentration and distribution of some stable elements in healthy human tissues from United Kingdom. Sci Total Environ. 1972;1:341–74. [Google Scholar]
- 6.Patwardhan B, Vaidya AD, Chorghade M, Joshi SP. Reverse pharmacology and system approach for drug discovery and development. Curr Bioact Comp. 2008;4:1–12. [Google Scholar]
- 7.Durucan C, Akkopru B. Effect of calcinations on microstructure and antibacterial activity of silver containing silica coatings. J Biomed Mater Res Applied Biomaterials. 2010;93B:448–58. doi: 10.1002/jbm.b.31602. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature. 2005;431:3968. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]
- 9.Chou CW, Chu SJ, Chiang HJ, Haung CY, Lee CJ, Sheen SR, et al. Temperature programmed reduction study on calcinations of nano palladium. J Phys Chem B. 2001;38:9113–7. [Google Scholar]
- 10.Ghosh MN. 4th ed. Kolkatta: Hilton and co; 2008. Fundamentals of experimental pharmacology; pp. 243–56. [Google Scholar]
- 11.Turner RA. New York: Academic press; 2009. Screening methods in pharmacology; pp. 152–63. [Google Scholar]
- 12.Sharma S, Jain NK, Kulkarni SK. Inhibition of COX-1 enzyme potentiates opioid induced antinociception in animal model of central nociception. Indian J Pharmacol. 2003;35:21–6. [Google Scholar]
- 13.Shreedhara CS, Vaidya VP, Vagdevi HM, Latha KP, Muralikrishna KS, Krupnidhi AM. Screening of Budhinia purpurea linn.for analgesic and anti-inflammatory activities. Indian J Pharmacol. 2009;41:75–9. doi: 10.4103/0253-7613.51345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhutia YD, Vijayaraghavan R, Pathak U. Analgesic and anti-inflammatory activity of amifostine, DRDE-07, and their analogs, in mice. Indian J Pharmacol. 2010;42:17–20. doi: 10.4103/0253-7613.62401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra RN, Chopra IC, Handa KL, Kapur LD. 2nd ed. Calcutta: Academic publishers; 1982. Chopra's indigenous drugs of india; pp. 454–5. [Google Scholar]
- 16.Sharma DC, Budania R, Shah M, Jain P, Gaur BL. Hypolipidemic activity of silver preparations in chicks, Gallus serregineus. Indian J Exp Biol. 2004;42:504. [PubMed] [Google Scholar]
- 17.Bajaj S, Vohora SB. Anticataleptic, antianxiety and antidepressant activity of Gold preparations used in Indian systems of medicine. Indian J pharmacol. 2000;32:339–46. [Google Scholar]
- 18.Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52:1361. doi: 10.1016/S0045-6535(03)00471-5. [DOI] [PubMed] [Google Scholar]
- 19.Nishioka H. Mutagenic activity of metal compounds in bacteria. Mutat Res. 1975;31:185–9. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- 20.Ham KN, Tange JD. Silver deposition in rat glomerular basement membrane. Aust J Biol Med Sci. 1972;50:423–34. doi: 10.1038/icb.1972.36. [DOI] [PubMed] [Google Scholar]
- 21.La Torraea F. Anatomic histopathological and histochemical aspects of acute experimental intoxication with silver salts. Folia Med (Naples) 1996;45:1065–9. [Google Scholar]
- 22.Rich LL, Epinette WW, Nasser WK. Arygria presenting as cyanotic heart disease. Am J Cardiol. 1972;30:290–2. doi: 10.1016/0002-9149(72)90075-6. [DOI] [PubMed] [Google Scholar]
- 23.Grasso P, Abhram R, Handy R, Diplock AT, Goldberg L, Green L. Role of dietry silver in production of liver necrosis in vitamin E deficient rats. Exp Mol Pathol. 1969;11:186–99. doi: 10.1016/0014-4800(69)90007-0. [DOI] [PubMed] [Google Scholar]
- 24.Swanson AB, Wagner PA, Ganther HE, Hoekstrc WG. Antagonistic effects of silver and tri-o-cresyl phosphate on selenium and glutathione peroxidase in rat liver and erythrocytes. Fed Proc. 1974;33:639. [Google Scholar]
- 25.Klasing KC, Golf JP, Greger JL, King JC, Lei XG. 2nd ed. USA: National Academies;; 2005. Mineral tolerance of animals; p. 452. [Google Scholar]


