Abstract
Aim:
To compare the efficacy and safety of gabapentin (GBP), duloxetine (DLX), and pregabalin (PGB) in patients with painful diabetic peripheral neuropathy (DPNP).
Methods:
A prospective, randomized, open label, 12-week study was conducted. A total of 152 patients with history of pain attributed to DPNP with a minimum 40-mm score on visual analogue scale (VAS) were randomized to receive GBP, DLX, or PGB. The primary efficacy measure was pain severity as measured on 11 point VAS. Secondary efficacy measures included sleep interference score, Patient Global Impression of Change (PGIC), and Clinical Global Impression of Change (CGIC). Assessment of safety was done by recording the occurrence of adverse drug reactions. Data was analyzed using descriptive statistics, Chi square test, analysis of variance (ANOVA), and repeated measures ANOVA.
Results:
Of total 152 patients, 50 patients received GBP, DLX each while 52 received PGB. A significant reduction in pain score (VAS), sleep interference score, PGIC, and CGIC was seen in all the three treatment groups across time (P<0.05) with no statistically significant difference between the groups. There was a significant interaction between the time and treatment groups (P<0.001) for pain score (VAS), sleep interference score, and PGIC. The improvement in pain scores (VAS) and sleep interference score was higher with PGB compared to DLX and GBP. Adverse drug reactions were mild and occurred in 9.2% of all cases.
Conclusions:
Monotherapy with GBP, DLX, or PGB Produced a clinically and subjectively meaningful pain relief in patients with DPNP with onset of pain relief being faster and superior with PGB.
KEY WORDS: Diabetic peripheral neuropathy, gabapentin, duloxetine, pregabalin
Introduction
Diabetic neuropathy is a common microvascular complication of diabetes mellitus. Symptoms of diabetic neuropathy range from mild dysesthesias to severe unremitting pain that can profoundly affect the quality of life.[1,2] Neuropathic pain in diabetes is defined as pain initiated or caused by a primary dysfunction in the nervous system[3] and occurs in up to 26% of all the patients with diabetes.[4,5] Further, the rising prevalence of type 2 diabetes is anticipated to increase the burden of diabetic peripheral neuropathic pain (DPNP).[6] The main symptoms of DPNP include burning or shooting pain in the lower limbs and feet. Exact pathophysiological mechanism of DPNP is unclear and there are no approved treatments that restore nerve function. Therefore, the treatment in DPNP is primarily aimed at controlling pain.
Apart from glycemic control, current guidelines recommend the use of antidepressants and anticonvulsants in the treatment of DPNP.[7] Gabapentin (GBP), a newer generation anticonvulsant, is licensed for the treatment of neuropathic pain in Europe, and pregabalin (PGB), another gabapentinoid, got approved in 2005 for the same.[7] These are considered to be most effective with supportive evidence for treating DPNP.[8] Duloxetine (DLX), a serotonin-norepinephrine reuptake inhibitor (SNRI), was first approved as an antidepressant for the treatment of major depressive disorder. It has also been reported to be effective in DPNP.[7] However, to the best of our knowledge, no direct head to head comparative studies have been carried out between GBP, DLX, and PGB in the Indian population for DPNP. Therefore, the present study was carried out to evaluate and compare the efficacy and safety of GBP, DLX, and PGB in patients with DPNP to provide an appropriate treatment option in such patients.
Materials and Methods
Ethical clearance was obtained from the Institutional Ethical Review Board (IERB). A written informed consent was obtained from all the patients enrolled in the study. This study is registered in clinical trial registry of India CTRI/2009/091/001058, 12-08-2010.
At screening, the eligibility criteria for inclusion in the study were pain attributed to diabetic neuropathy based on history, clinical examination, biothesiometry, monofilament testing, and Michigan Neuropathy Screening Instrument (MNSI). A pain score of at least 40 mm on the 100 mm visual analogue scale (VAS) was considered for inclusion. Other inclusion criteria were patients should be aged between 18 to 75 years, of both genders with HbA1C less than 10 mg%, duration of diabetes ranging from 1 to 15 years, and that of diabetic neuropathy from 1 month to 5 years. Exclusion criteria of the study were patients with contraindications to the study medications, patients who were already on other medication for the treatment of DPNP one week prior to the study enrolment, those with hepatic, cardiac or renal failure, patients with established neuropathy due to other causes, patients who have undergone amputation of even one lower limb, patients who did not give written informed consent, and pregnant or lactating women. Patients were allowed to continue with their regular medication if there were no interactions with the study drugs.
Study Design
This was a 12-week, randomized, open label, comparative study. Outpatients, based on eligibility criteria who reported at the Department of Endocrinology and Neurology, St. John's Medical College Hospital, Bengaluru, were enrolled in this study.
Randomization
The estimated sample size for the study (including dropouts) was 150 patients (50 in each group). Patients who fulfilled the inclusion / exclusion criteria were randomized by computer generated randomization table into the three treatment groups.
Treatment Schedule
Study patients randomly received either one of the following medications based on their response and tolerability: 1) gabapentin (GBP) 300 mg/day, up to 1800 mg/day 2) duloxetine (DLX) 20 mg/day, up to 120 mg/day 3) pregabalin (PGB) 75 mg/day, up to 300 mg/day as decided by the consultant endocrinologist based on the clinical status. All patients were followed up monthly for a period of three months and were assessed for efficacy and safety. The drop outs or withdrawal if any along with reasons for the same were recorded. Data was collected in a specially designed case record form (CRF) by conducting a personal interview with each patient during the clinic visit.
Efficacy and Safety Assessments
The primary efficacy parameter was reduction in severity of pain rating recorded by patients in daily diaries using 11 point VAS (0 - no pain and 10 - worst possible pain). The monthly mean VAS score for pain was calculated for each patient. The reduction in mean VAS score value from baseline to 12 weeks post treatment was considered as the primary endpoint.
Secondary efficacy endpoints were the monthly mean sleep interference score from daily sleep diary, the Patient Global Impression of Change (PGIC), and Clinical Global Impression of Change (CGIC). Sleep interference was rated on 11 point scale that described how pain had interfered with patients’ sleep during 24 hours in a day (0 = did not interfere; 10 = unable to sleep due to pain). The PGIC was a 7 point scale (1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, or 7 = very much worse). Each patient was rated based on any change in his/her overall status that was experienced since the beginning of study medication. The CGIC was also a 7 point scale (1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, or 7 = very much worse) with similar ratings as of PGIC. Each patient was assessed by clinician and rated the change observed in the patient's overall status since the initiation of the study until 12 weeks of treatment. The secondary efficacy end points were measured as differences in these parameters (sleep score, PGIC, and CGIC) between the different treatment groups from baseline to the end of the treatment period.
The study endpoints considered for analysis were measured at the completion of one month over three consecutive months. The details of dose escalation, if any, between the study visits as reported by the patients were recorded. However, the efficacy end point scores were considered for analysis as pre-specified on the day of completion.
The safety of study medication was assessed in all patients by recording adverse drug reactions (ADRs) as reported by them. The details of occurrence, intensity and causal relationship to the study drug along with the findings of physical and clinical examination were considered.
Sample Size Calculation and Statistical Analysis
Sample size was calculated based on the reduction in pain scores of PGB vs. placebo, GBP vs. placebo, and DLX vs. placebo. We estimated a total sample size of 150 patients accounting for 10% drop out assuming a reduction of 50% of VAS between the groups considering 5% level of significance and 80% power. All analyses were two sided (level of significance P<0.05) and were done using Statistical Package for the Social Sciences (SPSS) version 17. Analyses for all the variables were carried out using intent-to-treat principle, defined as all randomized patients who received at least one dose of study medication and also by using last observation carried forward (LOCF) method. Descriptive statistics were reported as percentages, mean ± SD for continuous parametric variables, and median and inter-quartile range (25th and 75th percentiles) for continuous, non-parametric variables. Chi square test was employed to test the association of study characteristics between the three treatment groups for categorical variables. Analysis of variance (ANOVA) was employed to find the significance in different treatment groups at base line for continuous variables. Comparison for pain score on VAS, sleep score, PGIC, and CGIC across time in all the three groups was carried out using repeated measures ANOVA and using a post-hoc Bonferroni-corrected pair-wise t-test.
Results
Study Profile and Baseline Characteristics
Out of 227 patients screened, 152 were randomized to receive GBP (50), DLX (50), and PGB (52). There were 75 screen failures due to insufficient pain rating score (23), patients taking other groups of medications for pain relief (23), and hemoglobin A1c > 10 mg% (20) and serum creatinine > 1 mg/dl (9). The study profile of enrolled patients is presented in Figure 1.
Figure 1.
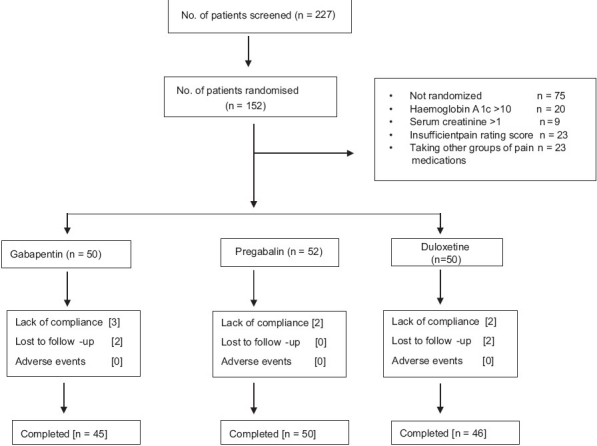
Disposition of study patients
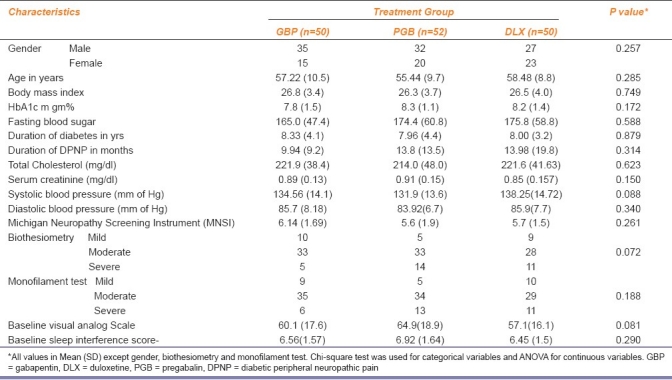
The demographic and baseline clinical characteristics of study patients were similar between the three groups as presented in Table 1.
Table 1.
Patient demographic and baseline characteristics
Mean Pain Score (VAS)
All the three treatment groups, GBP, DLX, and PGB showed a significant reduction in VAS for pain from baseline across time at the end of 12 weeks (P<0.05) with no significant difference between the groups [Figure 2a]. There was a significant interaction between the time and the groups (P<0.001). The reduction in VAS score by PGB was steep and faster compared to GBP and DLX from baseline to 4th week (first follow up) as presented in Figure 2a.
Figure 2.
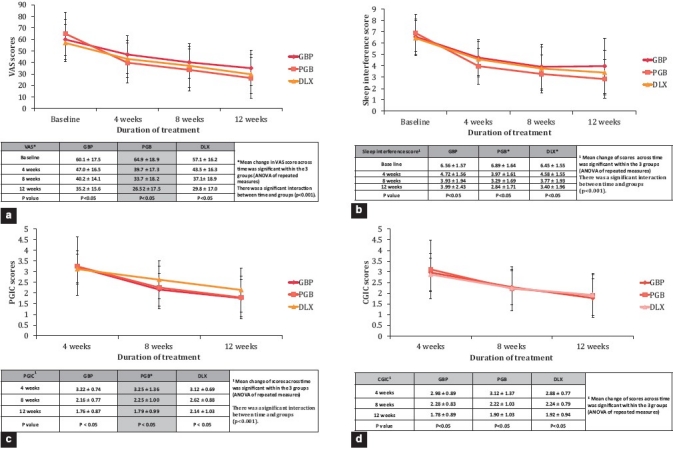
(a) Comparison of pain scores (VAS), (b) sleep interference scores, (c) Patient Global Impression of Change (PGIC) and (d) Clinical Global Impression of change (CGIC) between the three treatment groups across time
Sleep Interference Score
There was a significant reduction in sleep interference score over the study period of 12 weeks (P<0.05) in all the three treatment groups [Figure 2b] with a significant interaction between the time and the groups (P<0.001). PGB showed better improvement in sleep interference score compared to DLX and GBP.
Patient Global Impression of Change (PGIC)
The analysis of PGIC score showed a significant reduction in PGIC across time (P<0.05) in all the treatment groups [Figure 2c] with a significant interaction between the time and the treatment groups (P<0.001). The PGB and GBP demonstrated a higher response than DLX treated group in PGIC scores, indicating better improvement from the patient's point of view [Figure 2c].
Clinical Global Impression of Change (CGIC)
There was a significant reduction in CGIC in all the three drug treatment groups across time (P<0.05). However, in contrast, there was no significant interaction between the time and the treatment groups [Figure 2d].
Dose Evaluation
All the 45 patients who completed the study receiving GBP responded to a dose between 300 mg/day to 1200 mg/day in divided doses. The DLX group showed response between 20-80 mg/day and PGB group showed response between 75 to 300 mg/day.
Safety
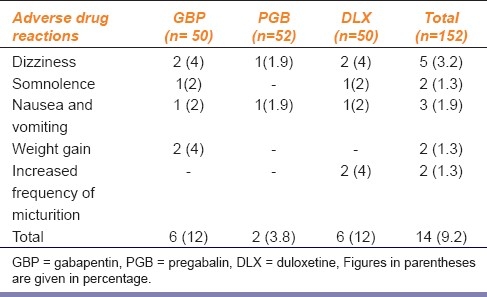
A total of 14 (9.2%) adverse drug reactions were reported in 152 patients. All were mild, self limiting, and did not require the discontinuation of therapy [Table 2]. Weight gain was observed in 4% of patients treated with GBP. There was a gain of 5 kg in one patient (from 47 to 52 kg) and 4 kg in the second patient (from 52 to 56 kg). Two patients in the DLX group reported an increased frequency of micturition and causes of this symptom, including uncontrolled diabetic status, were ruled out . Neurological examination did not reveal differences in the rate of progression of neuropathy.
Table 2.
Adverse drug reactions reported with drugs used in the treatment of diabetic peripheral neuropathy
Discussion
There are limited number of direct head-to-head comparison studies reported for drugs used in the treatment of DPNP. In the present study, safety and efficacy of GBP, DLX, and PGB were compared in a natural setting in patients with DPNP. While, all the three drugs are approved for the treatment of DPNP, it is for the first time that a head to head comparison is being presented to the best of our knowledge.
The two large clinical trials with mexiletine for the symptomatic relief of DPNP have demonstrated that a detailed history and physical examination are adequate for the diagnosis of neuropathy in patients with diabetes mellitus using MNSI.[9,10] In our study also, patient diagnosis was more dependent on symptoms and clinical findings rather than the electrophysiological data. This appears to be appropriate since treatment was generally designed to affect symptoms rather than the disease process.[11]
The present study showed a significant reduction in pain score as assessed on VAS in all three drug treated groups across the time indicating similar therapeutic benefits. A significant interaction between the time and the treatment groups was noticed [Figure 2a] indicating that all three drugs were effective for the symptomatic relief from DPNP. The mean reduction in pain score during the initial phase of treatment was more with PGB compared to GBP or DLX [Figure 2a]. This finding suggests that treatment with PGB could be ideal for patients in whom a faster reduction in pain intensity is necessary.
A large placebo controlled study has reported that GBP is effective in alleviating DPNP and the calculated Number Needed to Treat (NNT) for 50% pain relief for GBP was 3.7.[7,11] A similar placebo controlled study conducted with PGB for a period of five weeks has documented a significant reduction in pain compared to placebo (P<0.001).[12] Both GBP and PGB bind to the α2δ site of L type voltage gated calcium channel and modulate the influx of calcium during neuronal depolarization in the central nervous system. Further, a recent placebo controlled, multicentric, randomized study demonstrated that DLX was superior to placebo with 50% reduction in the 24-h average pain score.[13] DLX is considered balanced and potent dual reuptake inhibitor of serotonin and norepinephrine which is thought to inhibit pain via descending pain pathways.
Although, GBP, DLX, and PGB have not been compared in patients with neuropathic pain in a head-to-head trial to date, available evidence supports that GBP monotherapy is efficacious in reducing sleep interference associated with DPNP.[11] However, in this study, the analysis of the sleep interference score indicated a reduction in scores among all the treatment groups. There was a better reduction in sleep interference score in patients receiving PGB compared to DLX and GBP [Figure 2b].
PGB had a significant effect on the improvement in overall status rated by patient (P<0.05) as indicated by PGIC scale in our study. Indirect comparison results of a recently published meta-analysis also documented similar findings for the patient global impression outcomes, in which PGB showed a significant improvement over DLX (95% CI: 0.016; 1.060).[14] The CGIC rated by the clinician based on the change observed in patient's overall status showed a significant reduction in the score in all the three treatment groups.
Previous studies have reported efficacy with PGB at dose range between 150 to 600 mg/day administered twice or thrice a day for the treatment of painful DPN.[15,16] The dose administered was lower (75-150 mg/day) in our study. This difference is difficult to explain and may need another study in larger number of patients.
Dose limiting ADRs remain a problem for patients’ with neuropathic pain. The tolerability profile in this study was generally consistent with previous studies.[8,14] The effectiveness of therapy with tricyclic antidepressants is often limited by intolerable adverse effects like sedation, urinary retention, orthostatic hypotension, or cardiac arrhythmias. ADRs reported in our study were mild [Table 2] and no discontinuation was needed. Two patients in GBP group reported weight gain and two patients from DLX group had increased frequency of micturition.
There have been a few limitations in our study e.g. a) This was an open label design; b) we were not able to include a placebo arm because of logistic reasons; and c) follow-up of patients was only for 12 weeks and therefore, the long term efficacy and safety of the study drugs could not be assessed.
In conclusion, monotherapy with GBP, DLX, or PGB produced clinically meaningful pain relief with minor side effects in patients with DPNP and the onset of pain reduction was superior with PGB compared to GBP and DLX. Therefore, it is suggested that PGB could be a treatment option for early pain relief in the patients with DPNP. However, further studies in larger number of patients and for longer duration are necessary to confirm the benefits of PGB.
Acknowledgement
This study was funded by RSSDI (Research Society for the Study of Diabetes in India). Authors would like to thank Nicholas Piramal, Bangalore for providing the instrument for the measurement of Biothesiometry. We thank Mrs Sumithra, Biostatistician for helping us with statistical analysis.
Footnotes
Source of Support: RSSDI.
Conflict of Interest: None declared.
References
- 1.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–8. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 3.Classification of Chronic Pain. 2. Seattle: IASP Press; 1994. IASP Task Force on Taxonomy. [Google Scholar]
- 4.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 5.Young MJ, Boulton AJ, Macleod AF, Williams DR, Sonksen PH. A multicentre study of prevalence of diabetic neuropathy in patients attending UK diabetic clinics. Diabetologia. 1993;36:150–4. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 6.Leibson CL, Williamson DF, Melton LJ, III, Palumbo PJ, Smith SA, Ransom JE, et al. Temporal trends in BMI among adults with diabetes. Diabetes Care. 2001;24:1584–9. doi: 10.2337/diacare.24.9.1584. [DOI] [PubMed] [Google Scholar]
- 7.Chong MS, Hester J. Diabetic painful neuropathy: current and future treatment options. Drugs. 2007;67:569–85. doi: 10.2165/00003495-200767040-00006. [DOI] [PubMed] [Google Scholar]
- 8.Frampton JE, Foster RH. Pregabalin: in the treatment of postherpetic neuralgia. Drugs. 2005;65:111–8. doi: 10.2165/00003495-200565010-00011. [DOI] [PubMed] [Google Scholar]
- 9.Stracke H, Meyer UE, Schumacher HE, Federlin K. Mexiletine in the treatment of diabetic neuropathy. Diabetes Care. 1992;15:1550–5. doi: 10.2337/diacare.15.11.1550. [DOI] [PubMed] [Google Scholar]
- 10.Oskarsson P, Ljunggren JG, Lins PF. Efficacy and safety of mexiline in the treatment of painful diabetic neuropathy. Diabetes Care. 1997;20:1594–7. doi: 10.2337/diacare.20.10.1594. [DOI] [PubMed] [Google Scholar]
- 11.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus; a randomized controlled trial. JAMA. 1998;280:1831–6. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 12.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–10. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Quilici S, Chancellor J, Löthgren M, Simon D, Said G, Kim Le T, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. doi: 10.1186/1471-2377-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arezzo JC, Rosenstock J, LaMoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/dl for treating painful diabetic peripheral neuropathy: A double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. doi: 10.1186/1471-2377-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful DN with pregabilin: A randomized, placebo-controlled trial. J Pain. 2005;6:53–60. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]


