Abstract
Objective:
The objective of the present study is to investigate the anti-proliferation activity of Astragalus on human hepatocellular carcinoma (HCC) cells and its mechanism.
Materials and Methods:
Hepatic cancer H22 bearing mice were used to study the anti-hepatocarcinoma activity of Astragalus in vivo. The growth curve and inhibitory rate of tumor growth were measured. Cell apoptosis of each group was measured by flow cytometry (FCM). Protein expression of Bax and Bcl-2 were analyzed by immunohistochemistry (IHC). The Statistical Package for Social Sciences version 13.0 (SPSS Inc, Chicago, IL) was used for standard statistical analysis including one-way ANOVA and Student's t-test. A value of P<0.05 was considered to be statistically significant.
Results:
Astragalus significantly inhibited the growth of H22 carcinoma, with an inhibitory rate of 17.28-52.36%. FCM and immunohistochemical assay show that the cell apoptosis rate and protein expression of Bax and Bax/Bcl-2 ratio of H22 transplanted tumor in Astragalus treated group were significantly higher than the control group (P<0.05). The protein expression of Bcl-2 was significantly lower than control (P<0.05).
Conclusion:
The results of the present study suggest that Astragalus has significant anti-tumor effect in vivo in inducing apoptosis of H22 tumor cells by promoting protein expression of Bax, decreasing protein expression of Bcl-2 gene, and markedly increasing the Bax/Bcl-2 ratio.
KEY WORDS: Antitumor effects, apoptosis, Astragalus, H22
Introduction
Worldwide, hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related death. Liver cancer is a major health problem and its incidence is increasing in China, although several trials have attempted to control its progression.[1] Furthermore, conventional treatments such as chemotherapy and radiotherapy can increase the risk of potential complications.[2] Hence, herbal medicines have attracted considerable interest as a novel anticarcinogenic agent because of their high efficacy, low toxicity and costs.
Radix astragali is a key herb in many traditional formulations in China, which was recorded more than 2000 years ago in Sheng Nong's Materia Medica. In recent years, Astragalus injection (AI), as an extract from Radix astragali, has been extensively used for clinical adjunctive therapy to ameliorate the side effects of antineoplastic drugs.[3] Several clinical studies showed that the active constituents of AI, including polysaccharides, astragalosides and total flavonoids[4] could ameliorate the side effects of chemotherapeutic drugs. Recently, there has been growing evidence that the Astragalus extract may be a potential anti-tumorigenic agent. For instance, Astragalus polysaccharides can prevent hepatocarcinogenesis in rats.[5] On the other hand, the total saponins of Radix astragali (AST) also exhibited anti-carcinogenic effects in HT-29 human colon cancer cells.[6] However, the precise anti-carcinogenic effect and mechanism of AI remains unclear.
In the present study, we aimed to examine the effect of AI on the growth of H22 transplanted tumor in mice, and to further elaborate its growth-inhibitory mechanism. Our key findings demonstrate that AI exerts inhibitory effects on HCC cells in vivo by inducing cell apoptosis. These results suggest that AI is an attractive candidate drug to prevent the tumor proliferation.
Materials and Methods
Reagents
Astragalus injection was obtained from Diao Pharmaceutical Co., Ltd (Chengdu, China). Cyclophosphamide (CTX), (Jiangsu Hengrui Medicine Co., Ltd, batch number - 08122621) was dissolved in distilled water.
Animals
Kunming male mice, five-week old, weighing 18~22 g, were obtained from the Experimental Animal Center at Sichuan University. The mice were housed in plastic cages with hardwood chip bedding in an air-conditioned room at 23±2°C and 55±5% humidity, and with a 12 h light/dark cycle on basal diet (animal center). The animal handlings and experimental procedures were approved by the Animal Ethics Committee of Sichuan University (Permit Number: 2003-149).
In Vivo Anti-Tumor Activity
Five-week old male Kunming mice were inoculated with H22 cell suspension (1×107/ml), 0.1 ml/10 g, through subcutaneous injection at right side axilla. Twenty-four hour after the tumor cell inoculation, the mice were randomized equally into 5 groups of 15 each. The minimum effective dose of Astragalus in treating hepatocellular carcinoma was 4 g/kg. According to the method of Prasad et al.,[7] the positive control group mice received a single dose of cyclophosphamide (i.p., 600 mg/kg) on day 1. The negative control group mice were injected with an equal volume of normal saline (NS) while mice in other three groups were given AI (i.p., 12, 8, 4 g/kg) once every alternate day from day 1 (six times totally). From the fourth day, tumor volume (V) was measured on alternate days, and calculated according to the formula: V (cm3) = ab2/2, where a is largest superficial diameter and b is smallest superficial diameter.
All mice were sacrificed 13 days after inoculation with H22 cells, and the transplanted tumors were excised and weighed. To evaluate the anticancer activity of Astragalus, tumor inhibitory rates were calculated with the following formula: tumor inhibitory rate (%) = 1-(tumor weight of treated group/ tumor weight of control group) ×100.
Flow Cytometry Analysis
Apoptotic rate was assessed by flow cytometry (FCM)after staining with propidium iodide (PI) and annexin VFITC (Sigma, St. Louis, MO), according to the method of Ji et al. Briefly, tumor tissues were immediately removed and the RBCs were lysed using an ammonium chloride lysis buffer and filtered through a double layer of stainless-steel mesh using a syringe plunger to obtain single cell suspension.[8] 1 × 106 cells per sample were incubated in 1× binding buffer containing Annexin V-FITC and PI. At least 5000 cells per sample were analyzed by flow cytometry. Data were analyzed with Flow Cytometry Software (Beckman Coulter) and the gating included all cells.
Measurement of Transplanted Tumors’ BCL-2 and Bax Protein
Sections were cleared of paraffin, and endogenous peroxidases were blocked by incubation with 3% H2O2 and washed. Sections of the tissues were then incubated with rabbit serum for 10 min at ambient temperature. Subsequently, the sections were incubated overnight with a goat polyclonal anti-Bax antibody (Boster, China, 1:50) and anti-Bcl-2 antibody (Boster, China, 1:50) at 4°C, followed by the addition of biotinylated rabbit anti-goat IgG secondary antibody (Jinshan, BJ, China). To verify the binding specificity for Bax and Bcl-2, some sections were also incubated with primary antibody or the secondary antibody. In these situations, no positive staining was found in the sections, indicating that the immunoreactions were positive in all the experiments. Immunohistochemistry staining was processed in accordance with the manufacturer's instructions and visualized by use of diaminobenzidine (DAB) staining. Counterstaining was carried out with Harris hematoxylin (Sigma). Ten sections were analyzed for each animal. Samples were observed in four randomly selected optical fields under microscopy (× 200) and the average optical density was analyzed with Image-pro plus (Media Cybernetics, USA) by an observer blind to the treatment groups.
Statistical Analysis
The data were expressed as mean±standard deviation (SD). The Statistical Package for Social Sciences version 13.0 (SPSS Inc, Chicago, IL) was used for standard statistical analysis including one-way ANOVA and Student's t-test. A value of P <0.05 was considered as statistically significant.
Results
Growth of Mouse Transplantable Hepatoma
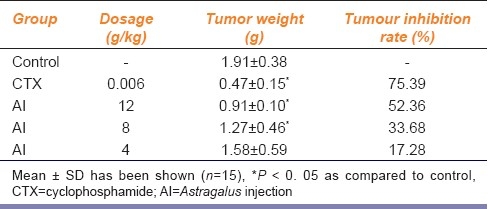
To determine whether Astragalus inhibits tumor growth in vivo, H22 cells were injected into Kunming mice. Growth curve of hepatocarcinoma H22 showed that Astragalus inhibited tumor growth significantly in a dose- and time-dependent manner from 4 to 12 g/kg [Figure 1]. Treated with Astragalus, the tumor inhibition rates ranged from 17.28 to 52.36% [Table 1].
Figure 1.
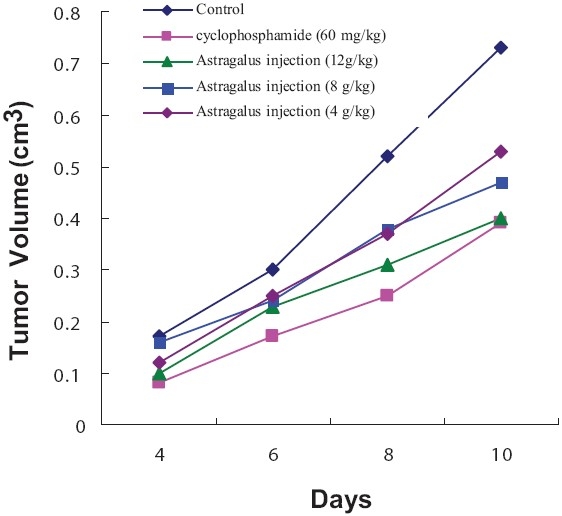
Effects of different dosages of Astragalus injection and cyclophosphamide (CTX) on tumor growth in H22 bearing mice. *P< 0.05 vs control
Table 1.
Inhibitory effect of Astragalus injection on H22 bearing mice
Flow cytometry analysis
After Astragalus treatment for 10 days, a significantly high rate of apoptosis was found, compared with saline controls in hepatocarcinoma H22 bearing mice [Figure 2].
Figure 2.
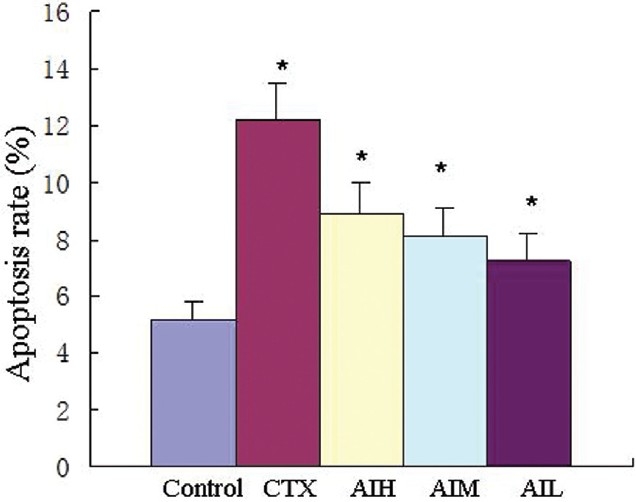
Apoptosis rates of H22 transplanted tumor cells, values are shown as mean ± SD (n=5). *P < 0.05 vs control. CTX=cyclophosphamide (60 mg/kg); AIH=Astragalus injection (12g/kg); AIM=Astragalus injection (8 g/kg); AIL=Astragalus injection (4 g/kg).
Effects of AI onBcl-2 and Bax protein level
To test the effect of AI on the regulation of apoptosis gene, immunohistochemistry staining was performed for Bcl-2 and Bax protein. Figure 3 shows that the protein levels of Bcl-2 and Bax protein changed in Astragalus treated H22 bearing mice.AI treatment decreased the expression of anti-apoptotic protein Bcl-2 compared with the control (P< 0.05). The expression of the pro-apoptotic protein Bax was increased significantly by AI treatment in a dose-dependent manner as compared with control (P < 0.05). A marked enhancement of the Bax/Bcl-2 ratio was observed after AI treatment [Figure 3]. There was no significant difference among the different doses of AI.
Figure 3.
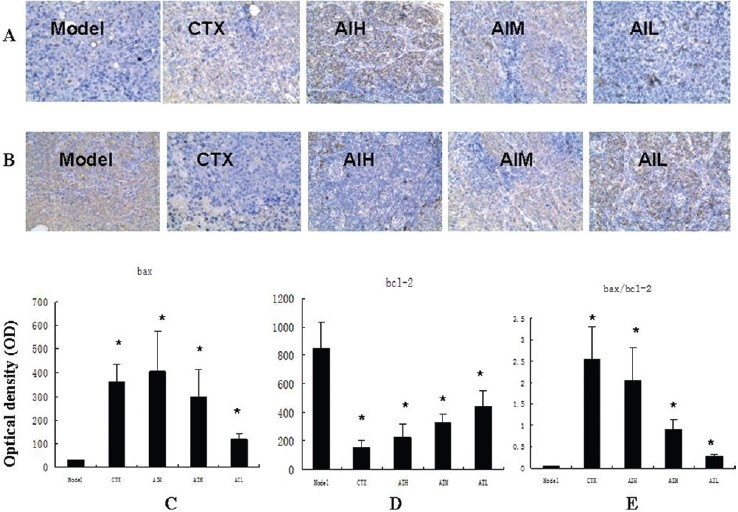
Expression of Bax and Bcl-2 proteins in transplanted tumor cells of H22 bearing mice (a) Representative immunostaining for Bax proteins in H22 bearing mice (×200. (b) Representative immunostaining for Bcl-2 proteins in H22 bearing mice (×200. (c) Semi-quantitative analysis of the optical density of Bax. (d) Semi-quantitative analysis of the optical density of of Bcl-2. (e) The calculated Bax/Bcl-2 ratio. Values are shown as mean±SD (n=10). *P< 0.05 vs control. CTX=cyclophosphamide (60 mg/kg); AIH = Astragalus injection (12 g/kg); AIM = Astragalus injection (8 g/kg); AIL = Astragalus injection (4 g/kg).
Discussion
Apoptosis, the program of cellular suicide, is critical in tumorigenesis and tumor progression.[9] Thus, targeting apoptosis is considered as an effective therapeutic strategy for treatment of cancer.[10,11] Astragalus, a traditional Chinese medicine, has so far been used as a modulating agent for all kinds of immunological diseases and in combination with cytotoxic drugs to ameliorate their side effects.[3] Recently, it has been demonstrated that the anti-tumor mechanism of Astragalus may be an induction of the apoptosis of cancer cell.[12–14] However, there has been little scientific advancement with regard to the effect of Astragalus on cancer. Among the effective components of Astragalus mongholicus, total Astragalus saponins (AST) possess anti-tumor properties on human colon cancer cells and human hepatocellular HepG2 cell line by inducing apoptosis in vitro.[6,13,15] Astragalus injection is the water extract of Astragalus mongholicus. Astragalus saponins are the main component of Astragalus injection. Our study demonstrated that both the weight and volume of tumor were inhibited by Astragalus in a dose- and time-dependent manner from 4 to 12 g/kg. Moreover, Astragalus injection could induce apoptosis of H22 transplanted tumors cells. The apoptosis rate also exhibited a dose- and time-dependence.
To obtain further information regarding apoptotic modulation, we have examined the immunoreactivity of Bax and Bcl-2 proteins in H22 transplanted tumors. Bcl-2 and Bax function as tumor anti-apoptotic and pro-apoptotic factors and are the two main members of the Bcl-2 protein family, which might interact with each other to form homodimers and heterodimers in regulating apoptosis.[16,17] Therefore, alteration in the levels of Bax and Bcl-2 protein influences apoptosis[18] and the ratio between them is the key to determine the cell survival or death.[19,20] In our study, we showed the Astragalus injection induced apoptosis in H22 transplanted tumors and it was accompanied by up-regulation of Bax and the down-regulation of Bcl-2. The ratio of Bax/Bcl-2 also increased after Astragalus injection. These results indicate that imbalance of Bcl-2 family protein expression plays a critical role in this anti-tumor treatment. Astragalus injection induced apoptosis in H22 transplanted tumors by modulating Bcl-2 family proteins.
Conclusion
In the present study, the antitumor effect and the preliminary growth-inhibitory mechanism of Astragalus injection on H22 bearing mice were investigated. From the results, it is concluded that Astragalus injection can inhibit tumor growth by inducing apoptosis via up-regulating Bax expression and the ratio of Bax/Bcl-2 and down-regulating Bcl-2 expression. It is suggested that Astragalus injection can be an attractive candidate for cancer therapy. Further investigation of Astragalus injection as an anti-tumor compound is warranted.
Acknowledgments
The authors gratefully acknowledge the encouragement of Pro. Liming Zhou, Director, Department of Pharmacology, West China Centre of Medical Sciences, Sichuan University, Chengdu, China.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: Current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353–67. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- 2.Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319–28. doi: 10.1111/j.1440-1746.1997.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 3.Zee-Cheng RK. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT.A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find Exp Clin Pharmacol. 1992;14:725–36. [PubMed] [Google Scholar]
- 4.Zhang RP, Zhang XP, Ruan YF, Ye SY, Zhao HC, Cheng QH, et al. Protective effect of Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J Gastroenterol. 2009;15:2862–9. doi: 10.3748/wjg.15.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui R, He J, Wang B, Zhang F, Chen G, Yin S, et al. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51:75–80. doi: 10.1007/s00280-002-0532-5. [DOI] [PubMed] [Google Scholar]
- 6.Tin MM, Cho CH, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumour xenograft. Carcinogenesis. 2007;28:1347–55. doi: 10.1093/carcin/bgl238. [DOI] [PubMed] [Google Scholar]
- 7.Prasad SB, Rosangkima G, Nicol BM. Cyclophosphamide and ascorbic acid-mediated ultrastructural and biochemical changes in Dalton's lymphoma cells in vivo. Eur J Pharmacol. 2010;645:47–54. doi: 10.1016/j.ejphar.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Ji DB, Ye J, Jiang YM, Qian BW. Anti-tumor effect of Liqi, a traditional Chinese medicine prescription, in tumor bearing mice. BMC Complement Altern Med. 2009;9:20. doi: 10.1186/1472-6882-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Macha MA, Matta A, Sriram U, Thakkar A, Shukla NK, Datta Gupta S, et al. Clinical significance of TC21 overexpression in oral cancer. J Oral Pathol Med. 2010;39:477–85. doi: 10.1111/j.1600-0714.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhar M, Sen S, Singh N. Role of mitochondria in quercetin-enhanced chemotherapeutic response in human non-small cell lung carcinoma H-520 cells. Anticancer Res. 2006;26:1297–303. [PubMed] [Google Scholar]
- 12.Song Y, Yang J, Bai WL, Ji WY. Anti-tumour and immunoregulatory effects of Astragalus on nasopharyngeal carcinoma In Vivo and In Vitro. Phytother Res. 2011;25:909–15. doi: 10.1002/ptr.3354. [DOI] [PubMed] [Google Scholar]
- 13.Auyeung KK, Mok NL, Wong CM, Cho CH, Ko JK. Astragalus saponins modulate mTOR and ERK signaling to promote apoptosis through the extrinsic pathway in HT-29 colon cancer cells. Int J Mol Med. 2010;26:341–9. [PubMed] [Google Scholar]
- 14.Hu YW, Liu CY, Du CM, Zhang J, Wu WQ, Gu ZL. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol. 2009;123:293–301. doi: 10.1016/j.jep.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Auyeung KK, Law PC, Ko JK. Astragalus saponins induce apoptosis via an ERK-independent NF-kappaB signaling pathway in the human hepatocellular HepG2 cell line. Int J Mol Med. 2009;3:189–96. [PubMed] [Google Scholar]
- 16.Kim KW, Moretti L, Mitchell LR, Jung DK, Lu B. Combined Bcl-2/mammalian target of rapamycin inhibition leads to enhanced radiosensitization via induction of apoptosis and autophagy in non-small cell lung tumor xenograft model. Clin Cancer Res. 2009;15:6096–105. doi: 10.1158/1078-0432.CCR-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS One. 2009;4:e7059. doi: 10.1371/journal.pone.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2007;7:249–57. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–53. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–700. [PubMed] [Google Scholar]


