Abstract
Objectives:
The aim of this study has been to investigate the possible antihyperlipidemic effect of Salacia chinensis root extract in triton (400mg/kg b.w.)-induced and atherogenic diet-induced hyperlipidemic rats.
Materials and Methods:
Petroleum ether (60-80°C), chloroform, ethanol and aqueous extracts of Salacia chinensis roots were evaluated for antihyperlipidemic activity in triton- and atherogenic diet-induced hyperlipidemic rats. A comparison was also made between the action of Salacia chinensis root extract and a known antihyperlipidemic drug simvastatin (10 mg/kg body wt.). The results of the study were expressed as mean± S.E. and data was analyzed by using one way analysis of variance test (ANOVA) followed by Dunnett's t-test for multiple comparisons. Values with P < 0.05 were considered as significant.
Results:
Oral administration of 500 mg/kg body wt. of the chloroform extract and alcoholic extract of Salacia chinensis root exhibited a significant reduction (P<0.01) in serum lipid parameters like total cholesterol, triglycerides, low density lipoprotein (LDL), very low density lipopreotein (VLDL) and increase in high density lipoprotein (HDL) in hyperlipidemic rats of both models as compared to hyperlipidemic control statistically. These extracts were found to possess better antihyperlipidemic potential as compared to pet ether and aqueous extract.
Conclusions:
Our results demonstrated that chloroform and alcoholic extract of Salacia chinensis roots possessed significant antihyperlipidemic activity and hence it could be a potential herbal medicine as adjuvant with existing therapy for the treatment of hyperlipidemia.
KEY WORDS: Hyperlipidemia, Salacia chinensis, simvastatin, triton
Introduction
Hyperlipidemia is a secondary metabolic dysregulation associated with diabetes. Besides the cause effect relationship with diabetes, elevated serum level of triglycerides, cholesterol and LDL are major risk factors for the premature development of cardiovascular disease like arthrosclerosis, hypertension, coronary heart disease etc.[1] Increased plasma lipid levels mainly total cholesterol, triglycerides and LDL along with decrease in HDL are known to cause hyperlipidemia which is the reason for initiation and progression of atherosclerosis impasse.[2]
Antihyperlipidemic agents having various pharmacological actions are being tested clinically.[3]
Elevated lipid levels result from increased absorption through the gut or enhanced endogenous synthesis and therefore two ways are feasible to reduce hyperlipidemia; to block endogenous synthesis or to decrease absorption. Both factors can be evaluated in normal animals without artificial diets.
Salacia chinensis (Family:Hippocrateaceae) is a woody climbing plant found in the submontane forests in Sri Lanka and India. The roots and stems of this plant have been extensively used in the treatment of diabetes in the ayurvedic system of Indian traditional medicine. Furthermore, other plant species of the Salacia genus (e.g., S. prinoides, S. reticulata) have been historically used in ayurvedic medicine for their antidiabetic properties.[3] Salacia chinensis have been used in India and in other countries as a tonic, blood purifier and to treat amenorrhea and dysmenorrhea.[4] Its root bark is used in treatment of gonorrhoea, rheumatism and skin diseases. Its aqueous extract showed significant hypoglycemic activity. Root bark boiled in oil or as decoction or as powder is used for the treatment of rheumatism, gonorrhoea, itches, asthma, thirst and ear diseases.[5]
Asuti Naveen reported the hepatoprotective activity of ethanolic extract of dried bark in Wistar strain of albino rats of either sex against CCl4-induced hepatic damage.[6] Periyar et al., reported the antihyperglycemic potential of mangiferin purified from methanolic root extract in control and streptozotocin-induced diabetic rats. The activities of lactate dehydrogenase, glucose-6-phosphatase, fructose-1, 6-diphosphatase and glycogen phosphorylase were significantly decreased in liver tissue of diabetic treated rats.[7]
Yoshikawa et al., isolated four dammarane-type, three lupane-type, and an oleanane-type triterpenes named foliasalacins A1, A2, A3, A4, B1, B2, B3, and C from the leaves extract. The structures of new triterpene constituents were characterized on the basis of chemical and physiochemical evidence.[8] Jansakul et al., investigated the hypotensive activity of n-butanol extract in anesthetized female rats in estrus and for vasodilator activities on isolated thoracic aortic rings in vitro and the results suggested its hypotensive effect. The mechanism involved may be an indirect effect by stimulated release of nitric oxide from vascular endothelial cells and causes vasodilatation.[9]
Several studies showed that systematic administration of triton WR1339 (ionic surfactant) in fasted rats causes elevation in plasma lipid level. Initially, there is a sharp increase in lipid level reaching a peak two to three times the control value by 24 h after the administration of triton injection phase I (synthetic phase),this hyperlipidemia falls within next 24 h i.e. 48 h after triton administration, phase II (Excretion phase). This increase in plasma lipid by triton is thought to be due to one of the following mechanisms; as due to increased hepatic synthesis of cholesterol or removal of very low density protein (VLDL) from the blood due to their physical alteration by triton. Antihyperlipidemic drugs interfering with cholesterol synthesis were shown to be active in phase I while drug interfering with cholesterol excretion and metabolism were active in phase II. Triton-induced hyperlipidemia is rather simple and rapid for evaluation of test substance and can be considered as the useful method for preliminary screening of antihyperlipidemic drugs.[2] The search for new drug with the ability to reduce or regulate serum cholesterol and triglyceride concentrations has gained momentum over the years, resulting in a plethora of publications reporting significant activity of a variety of natural and synthetic agents. Molecular modification of naturally occurring compounds has also given rise to potent agents like pravastatin and simvastatin; the former prepared by replacement of the methyl group of naturally occurring lovastatin by a hydroxyl group and the latter by a methylated derivative of compaction. In continuation of our search for plant-derived antihypercholesterolemic and hypolipidemic agents, we directed our attention to some Indian medicinal plants for which antihyperlipidemic activity has not been scientifically validated.[5]
Materials and Methods
Plant material
Roots of Salacia chinensis were collected in and around local forest area of Sirsi in Western Ghats, Karnataka and authenticated by the Botanist Prof. G. S. Naik, Department of Botany, G. C. Science and Art College, Ankola. A voucher herbarium specimen number GCSAC/SC/01 was also preserved in the same college. The collected roots were dried and powdered to coarse consistency in cutter mill. The powder was passed through 40 # mesh particle size and stored in an airtight container at room temperature.
Atherogenic diet and chemicals
Experimental hyperlipidemic diet: Experimental diet consists of well pulverized mixture of cholesterol (2%), cholic acid (1%), peanut oil (10%), sucrose (40%) and normal laboratory diet (47%).
Experimental hyperlipidemic agent: A suspension of triton -WR 1339 (SDFine chemicals) in 0.15 M NaCl was used for inducing hyperlipidemia in experimental rats.
Simvastatin (Dr. Reddy's Laboratories, Hyderabad), Diagnostic kits for estimation were purchased from Merck Diagnostics India Ltd. Anesthetic ether (Ozone International, Mumbai), and all other chemicals were of analytical grade.
Plant extract
2.5 kg of the fresh air-dried, powered crude drug of Salacia chinensis roots were extracted with petroleum ether (60-80° C), chloroform, 95% ethanol and chloroform water I. P.(0.1%) in the ratio of 1:8) by adopting simple maceration procedure at room temperature for seven days in a conical flask with occasional shaking and stirring. The extract was filtered and concentrated to dryness at room temperature to avoid the decomposition of the natural metabolites.[10] All the extracts were preserved in a refrigerator till further use. Preliminary phytochemical analysis was carried out in all 4 extracts by different methods of phytochemical analysis.[11] A known volume of extract was suspended in distilled water and was orally administered to the animals by gastric intubation using a force feeding needle during the experimental period.
Animals
Adult albino rats of Wistar strain (150-200g) of either sex were procured and housed in the animal house of K L E S College of Pharmacy, Ankola with 12 h light and 12 h dark cycles. Standard pellets obtained from Goldmohar rat feed, Mumbai, India, were used as a basal diet during the experimental period. The control and experimental animals were provided food and drinking water ad libitum. All the animal experiments were conducted according to the ethical norms approved by CPCSEA, Ministry of social justice and empowerment, Government of India and ethical clearance was granted by institutional ethical committee in resolution no. 1/18/2007 held on 23rd November 2007 at J N Medical college, Belgaum (Ethical committee IAEC reg. no.: 627/02/a/CPCSEA).
Preparation of dose for dried extracts
Petroleum ether (60-80°C), chloroform, alcohol and aqueous extracts (500 mg/kg) of the selected plants were formulated as suspension in distilled water using Tween-80 as suspending agent. The strength of the suspension was according to the dose administered and was expressed as weight of dried extract.[12]
Preparation of standard drugs
Simvastatin 10 mg/kg was used as the reference standard drug for evaluating the antihyperlipidemic activity which was made into suspension in distilled water using Tween-80 as a suspending agent.
Acute oral toxicity studies
The acute oral toxicity studies of extracts were carried out as per the OECD guidelines, draft guidelines 423 adopted on 17th December 2001 received from CPCSEA, Ministry of social justice and empowerment, Govt. of India. Administration of the stepwise doses of all 4 extracts of Salacia chinensis from 50 mg/kg up to the dose 5000 mg/kg caused no considerable signs of toxicity in the tested animals. One tenth of upper limit dose were selected as the levels for examination of antihyperlipidemic activity.[13]
Diet-induced hyperlipidemic model
The animals were selected, weighed then marked for individual identification. In this model, rats were made hyperlipidemic by the oral administration of atherogenic diet was for 20 days by mixing with regular pellet diet and rats were given free access to the feed ad libitum. The rats were then given plant extracts suspended in 0.2% tween 80 at the dose of 500mg/kg b.w once daily in the morning through gastric intubation for 14 consecutive days. During these days, all the groups also received atherogenic diet in the same dose as given earlier. The control animals received the hyperlipidemic diet and the vehicle. At the end of treatment period, the animals were used for the study of various biochemical parameters. Blood was collected by orbital plexus of rat under ether anesthesia and centrifuged by using centrifuge at 2000 rpm for 30 min to get serum.[14]
Triton-induced hyperlipidemic model
Animals were kept for fasting for 24 h and injected a saline solution of Triton at the dose of 400 mg/kg intraperitoneally. The plant extracts, at the dose of 500 mg/kg., were administered orally through gastric intubation, the first dose being given immediately after triton injection and second dose 20 h later. After 4 h of second dose, the animals were used for the study of various biochemical parameters. Blood was collected by orbital plexus of rat under ether anesthesia and centrifuged by using centrifuge at 2000 rpm for 30 min to get serum.[1]
Experimental design
Animals were divided into seven different groups with six animals in each group. Group I served as normal control and this group did not receive atherogenic diet and triton except regular standard pellet diet. Group II was positive control which was given standard antihyperlipidemic drug simvastatin (10mg/kg/day p.o.). Group III was hyperlipidemic control and this group did not receive any treatment except atherogenic diet in case of diet induced and triton in case of triton induced hyperlipidemia. Group IV, V, VI and VII received different extracts of Salacia chinensis root (500 mg/kg/day, p.o.). Treatment period for all these groups was 14 days in atherogenic diet induced hyperlipidemia and 48 h in case of triton-induced hyperlipidemia.
Collection of blood
Blood was collected by retro-orbital sinus puncture, under mild ether anesthesia. The collected samples were centrifuged for 10 min.
Biochemical analysis
The serum was assayed for total cholesterol, triglycerides, phospholipids, high-density lipoprotein (HDL), low density lipoprotein (LDL), and very low density lipoprotein (VLDL) using standard protocol method. Serum total cholesterol, triglyceride were estimated by the method of CHOD-PAP and high density lipoprotein by the method of GPO-PAP. Low density and very low density cholesterol were calculated by using Friedewald formula and VLDL: TG/5 respectively.[15,16]
Statistical analysis
The results of the study were expressed as mean± S.E. Data was analyzed by using one way analysis of variance test (ANOVA) followed by Dunnett's t-test for multiple comparisons. Values with P < 0.05 were considered as significant.[17]
Results
Phytochemical analysis of Salacia chinensis root extracts showed the presence of steroids, triterpenoids, tannins, saponins and flavonoids.
Acute toxicity study
As per (OECD) draft guidelines 423 adopted on 17th December 2001 received from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), young female albino rats were given 50-5000 mg/kg b.w. of Salacia chinensis extract for the purpose of toxicity study. Animals were observed at regular time intervals at least once during the first 30 min of initial dosing during the first 24 h. In all the cases, no death was observed within first 24 h. Additional observations like behavioral changes in skin, fur, eyes, mucous membranes, respiratory, circulatory, autonomic and central nervous systems and somato motor activity and behavior pattern were also found to be normal. Attention was also given to observation of tremors and convulsions. Overall results suggested the LD50 value as 5000 mg/kg. Hence therapeutic dose was calculated as 1/10th (500 mg/kg of the lethal dose for the purpose of antihyperlipidemic investigations.
The effect of various extracts, obtained from simple maceration of roots of Salacia chinensis were studied on serum lipids and lipoproteins level of triton (400mg/kg induced hyperlipidemic rats and results are expressed as change in serum lipid and lipoprotein levels.
Triton-induced hyperlipidemic model
As expected, administration of triton WR1339 led to elevation of serum lipid and lipoprotein levels, which were maintained over a period of study in hyperlipidemic control group and these rats were given treatment with aqueous, ethanolic, chloroform and petroleum ether root extracts of Salacia chinensis. The results were comparable with reference standard simvastatin. There was a significant elevation in serum lipids and lipoproteins in triton-induced hyperlipidemic control rats when compared with normal control. At this time, an increased level of HDL-cholesterol was also observed. Salacia chinensis root chloroform extract showed significant serum lipid lowering effects in hyperlipidemic rats and reduced serum lipids significantly (P<0.001) as compared to hyperlipidemic control statistically [Tables 1 and 2].
Table 1.
Effect of Salacia chinensis root extracts on serum total cholesterol, triglycerides and phospholipids level in triton-induced hyperlipidemic rats
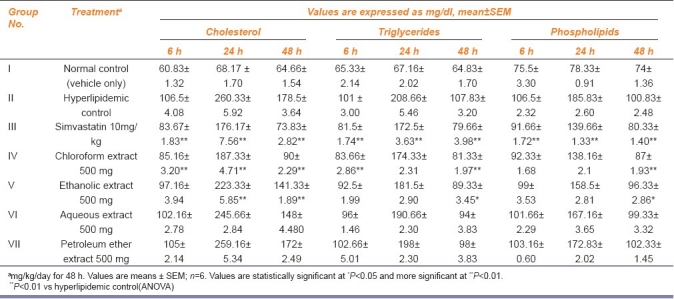
Table 2.
Effect of Salacia chinensis root extracts on LDL, VLDL and HDL level in triton-induced hyperlipidemic rats
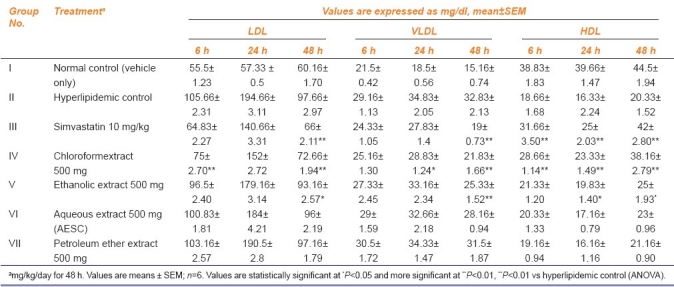
Diet-induced hyperlipidemic model
Salacia chinensis chloroform extract showed significant serum lipid lowering effects in hyperlipidemic rats which brought down total cholesterol level till 85±2.160, triglycerides 76.5±2.975, phospholipids 89.5±3.810, LDL 57±2.129, VLDL 28.66±1.206 and increased level of HDL 26±1.693 in comparison of diet-induced hyperlipidemic control, total cholesterol 101.16±2.613, triglycerides 86±2.280, phospholipids 107.66±2.642, LDL 81±2.556, VLDL 35±1.141 and HDL 21.08±1.172 at 14th day [Figure 1].
Figure 1.
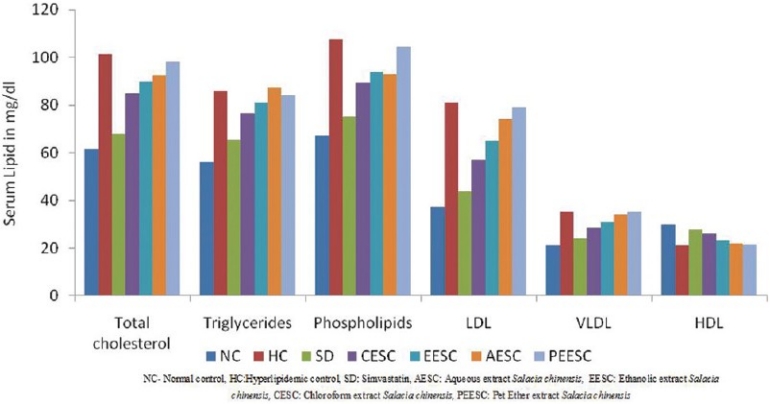
Effect of Salacia chinensis root extract on serum lipid parameters in diet-induced hyperlipidemic rats
Standard antihyperlipidemic agent simvastatin 10 mg/kg body weight also able to reduce the elevated serum lipid level toward the normal. It brought down total cholesterol 68±2.864, triglycerides 65.33±1.801, phospholipids 75±1.528, LDL 43.83±2.182, VLDL 24±1.461 and increased level of HDL 28±1.571 when compared to diet-induced hyperlipidemic control, total cholesterol 101.16±2.613, triglycerides 86±2.280, phospholipids 107.66±2.642, LDL 81±2.556, VLDL 35±1.141 and HDL 21.08±1.172 at 14th day.
Discussion
There was marked increase in the level of serum total cholesterol, triglycerides, phospholipids, LDL, VLDL and decrease in the level of good cholesterol carrier HDL in the animals treated with triton and atherogenic diet. Elevated level of blood cholesterol especially LDL was the major risk factor for the coronary heart disease and HDL as cardio protective protein. Treatment with Salacia chinensis root extract (500 mg/kg significantly decreased the level of cholesterol, triglycerides, phospholipids, VLDL and LDL as compared to hyperlipidemic control. There was significant increase in HDL as compared to control. This effect may be due to the increased activity of lecithin: cholesterol acetyl transferase which incorporates free cholesterol, free LDL into HDL and transferred back to VLDL and intermediate density lipoprotein.
Decrease in the triglyceride level may be due to the increase in activity of the endothelium bound lipoprotein lipase which hydrolyzes the triglyceride into fatty acid or due to inhibition of lipolysis so that fatty acids do not get converted to triglyceride. Lipoproteinlipase releases fatty acids from chylomicrons and very low-density lipoproteins (VLDL) in the circulation. Half of those are taken up for storage. Catecholamines can suppress lipolysis via their action on 2-adrenoceptors, but they mostly have the opposite effect of insulin and, by acting on /3-adrenoreceptors, increase cyclic AMP and the phosphorylation of hormone-sensitive lipase, stimulating lipolysis and the release of fatty acids in the circulation.
With the aid of lipoprotein lipase found in the endothelial walls of vessels, VLDL are then converted into LDL, where the cholesterol can either be used as substrates for steroid synthesis or for bile acids. The rest is deposited in extra hepatic tissue or becomes oxidized in the arterial walls, becoming the precursors of plaque formation. In hypercholesterolemia, the formation of bile acids needs to be supported, while simultaneously inhibiting the deposition and oxidation of LDL.
Hepatic cholesterol synthesis is accelerated by triton WR 1339. Moreover, triton physically alters very low density lipoproteins rendering them refractive to the action of lipolytic enzymes of blood and tissues, preventing or delaying their removal from blood.[18] Hence the hypolipidemic effect of extracts could be due to an increased catabolism of cholesterol into bile acids.
Conclusion
The results obtained from the pharmacological screening have led to the conclusions that, chloroform extract of Salacia chinensis roots have significant antihyperlipidemic activity. Hence it can be exploited as an antihyperlipidemic therapeutic agent or adjuvant in existing therapy for the treatment of hyperlipidemia. Further study by measurement of heparin-releasable plasma LpL activity and LCAT activity is significant and can be undertaken.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Ansarullah, Jadeja RN, Thounaojam MC, Patel V, Devkar RV, Ramachandran AV. Antihyperlipidemic potential of a polyherbal preparation on triton WR 1339 (Tyloxapol) induced hyperlipidemia: A comparison with lovastatin. Int J Green Pharm. 2009;3:119–24. [Google Scholar]
- 2.Ghule BV, Ghante MH, Saoji AN, Yeole PG. Hypolipidemic and antihyperlipidemic effects of Lagenariasiceraria (Mol.) fruit extracts. Indian J Exp Biol. 2006;44:905–9. [PubMed] [Google Scholar]
- 3.Nomura H, Kimura Y, Okamoto O, Shiraishi G. Effects of antihyperlipidemic drugs and diet plus exercise therapy in the treatment of patients with moderate Hypercholesterolemia. Clin Ther. 1996;18:196. doi: 10.1016/s0149-2918(96)80028-2. [DOI] [PubMed] [Google Scholar]
- 4.Inman WD, Reed MJ. In-ventors; Shaman Pharmaceuticals, assignee Triterpenoid compound for the treatment of diabetes. US Patent 5, 691, 386. 1997 Nov 25; [Google Scholar]
- 5.Gopalakrishnan S, Ismail ST, Begum HV, Elango V. Anti-inflammatory activity of Salaciaoblonga Wall.And Azimatetracantha Lam. J Ethnopharmacol. 1997;56:145–52. doi: 10.1016/s0378-8741(96)01523-1. [DOI] [PubMed] [Google Scholar]
- 6.Naveen A. Hepatoprotective activity of ethanolic extract of root bark of Salacia chinensis. J Pharm Res. 2010;3:833–4. [Google Scholar]
- 7.Sellamuthu PS, Muniappan BP, Perumal SM, Kandasamy M. Antihyperglycemic effect of mangiferin in streptozotocin induced diabetic rats. J Health Sci. 2009;55:206–14. [Google Scholar]
- 8.Yoshikawa M, Zhang Y, Wang T, Nakamura S, Matsuda H. New triterpene constituents, foliasalacins A1 -A4, B1-B3, and C, from the leaves of Salacia chinensis. Chem Pharm Bull (Tokyo) 2008;56:915–20. doi: 10.1248/cpb.56.915. [DOI] [PubMed] [Google Scholar]
- 9.Jansakul C, Jusapalo N, Mahattanadul S. Hypotensive effect of n-butanol extract from stem of Salacia chinensis in rats, ISHS Acta Horticulturae 678: III WOCMAP Congress on Medicinal and Aromatic Plants. Targeted Screening of Medicinal and Aromatic Plants, Economics and Law. Vol. 4 [Google Scholar]
- 10.Ministry of Health (India) (948).Pharmacopoeia of India. Government of India. 1982:650. [Google Scholar]
- 11.Khandewal KR. 14th ed. Pune, India: Nirali Prakashan; 2005. Practical Pharmacognosy; pp. 146–57. [Google Scholar]
- 12.Alam AM, Ahuja A, Baboota S, Gidwani SK, Ali J. Formulation and evaluation of Pharmaceutically equivalent parental depot suspension of methyl prednisolone acetate. Indian J Pharm Sci. 2009;71:30–3. doi: 10.4103/0250-474X.51949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.India: Ministry of Social Justice and Empowerment; 2000. Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), OECD Guidelines for the testing of chemicals, revised draft guidelines 423: Acute Oral toxicity- Acute toxic class method, revised document. [Google Scholar]
- 14.Pande VV, Dubey S. Antihyperlipidemic activity of Sphaeranthus indicus on atherogenic diet induced hyperlipidemia in rats. Int J Green Pharm. 2009;3:159–61. [Google Scholar]
- 15.Allian CC, Poon LS, Chan CS, Richmond W, Paul CF. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 16.Pari L, Latha M. Effect of Cassia auriculata flowers on blood sugar levels, serum and tissue lipids in streptozotocin diabetic rats. Singapore Med J. 2002;43:617–21. [PubMed] [Google Scholar]
- 17.Saravana K, Mazumder A, Saravanan VS. Antihyperlipidemic activity of Camellia sinensis leaves in Triton WR-1339 induced albino rats. Pharmacogn Mag. 2008;4:60–4. [Google Scholar]
- 18.Friedman M, Byers SO. Mechanism underlying hypercholesterolemia induced by triton WR-1339. Am J Physiol. 1957;190:439–45. doi: 10.1152/ajplegacy.1957.190.3.439. [DOI] [PubMed] [Google Scholar]


