Abstract
Background:
Poststroke depression (PSD) is common after stroke; however, the relationship to poststroke function is inconclusive. Our objectives were to 1) determine the relationship between PSD at baseline (1 month poststroke) and function (12 weeks later) and 2) assess the impact of depression improvement on 12-week function among those with depression at baseline.
Methods:
We completed a secondary analysis of data from a cohort study of participants with and without PSD. We used logistic regression to identify factors associated with 12-week functional dependence for 1) all 367 participants and 2) the 174 participants with PSD.
Results:
In the PSD cohort, 3 characteristics were found to be independently associated with 12-week dependence: increased medical comorbidity (odds ratio [OR] 1.10, 95%confidence interval [CI] 1.02–1.22), increased stroke severity (OR 1.42, 95% CI 1.19–1.69), and increased baseline depression severity (OR 1.13, 95% CI 1.03–1.23). Depression severity was significantly different between those considered dependent and independent at 12 weeks (entire cohort, PHQ-9 7.31 vs 5.18, p = 0.008; depressed cohort, PHQ-9 9.94 vs 7.27, p = 0.019).
Conclusion:
Among study participants with PSD, the severity of depression symptoms at baseline was associated with dependence; however, our results are inconclusive as to whether improvement of depression is independently associated with functional recovery at 12 weeks. Even if the treatment and improvement of PSD does not directly influence functional recovery poststroke, it is essential for PSD to be identified and treated due to its high symptom burden and association with other negative health and social outcomes.
Each year approximately 795,000 people in the United States sustain a stroke1 and approximately one-third of survivors develop poststroke depression (PSD).2 PSD is common and associated with many negative consequences, including lengthened hospital stays, prolonged recovery time, increased mortality, and impaired functional outcomes.3–7
Several studies have reported that stroke survivors with PSD or depressive symptoms may demonstrate worse functional outcomes, impairment, and recovery compared to those without depression.3,4,8,9 Although many studies have linked PSD to worse functional outcomes, there is little known about the impact of PSD treatment and improvement on function after stroke. A recent Cochrane review of interventions for preventing PSD included only 14 PSD intervention trials.10 Results indicated no evidence that PSD treatment had a positive impact on cognition, function, or reduction of poststroke disabilities.
Care management of PSD, including guideline-adherent use of antidepressants, has been shown to significantly improve rates of depression remission, compared to usual poststroke care.11 Whether this improvement in depression is associated with concomitant improvement in function is not well-understood. The objectives of this study were to 1) determine the relationship between PSD at baseline (1 month after stroke) and functional outcomes (12 weeks after baseline enrollment) and 2) assess the impact of depression improvement on 12-week postenrollment functional recovery among those who screened positive for depression at baseline.
METHODS
Design.
This was a secondary analysis of data collected as part of the Activate-Initiate-Monitor (AIM) study.11 The AIM study included a randomized clinical intervention trial nested within a cohort study. Stroke survivors were identified in the inpatient setting. Those with depression were randomized at 1–2 months poststroke to either a depression care management intervention group or usual care group. Nondepressed stroke survivors were matched by site and time of enrollment (1–2 months poststroke) to depressed subjects. All subjects were prospectively assessed with depression, stroke severity, function, and other assessments at baseline (1–2 months poststroke) and at 12 weeks postenrollment. Thus all study participants were assessed at approximately the same time points.
We utilized data from both the depressed and nondepressed subjects to evaluate the impact of PSD on functional outcomes poststroke. To assess the impact of depression treatment on functional outcome, we analyzed data from the subjects with depression only.
Participants.
All study participants survived an ischemic stroke, were more than 18 years old, were able to speak and understand English, had a telephone, had no severe language or cognitive impairments, and were expected to live at least 6 months. Subjects with prior or current depression treatment were not excluded. Potential study subjects were identified at the time of their stroke hospitalization. During recruitment, 1,175 consecutive patients from 4 hospitals were potentially eligible, 783 were excluded due to absence of depression, refusal to be in the study, or a lack of follow-up for depression screening. While still in the hospital, all potential subjects were invited to participate in depression screening 1 to 2 months poststroke for possible study enrollment. Patients who screened positive for depression were further evaluated and were invited to enroll in AIM if a diagnosis of major or minor depression was determined. See the AIM article for further details and recruitment diagram.11
Standard protocol approvals, registrations, and patient consents.
The clinical trial identifier number for AIM is NCT 00029172. The AIM study article contains the flow chart of participant recruitment for the trial.11 Human subjects approval was received for the initial study and this secondary analysis. All study participants consented.
Intervention.
Those in the depression intervention group received a care-management intervention including activation to increase understanding and acceptance of the diagnosis of depression, initiation of antidepressant medication, and monitoring of the effectiveness of the medication for 12 weeks.11 Those in the depression usual care group could receive antidepressant treatment at the discretion of their provider. Any antidepressant use during the 12 weeks was recorded during in-person assessments at 12 weeks postenrollment.
Assessments.
We collected demographic and clinical data at baseline. Clinical data included cognition (validated 6-item version of the Mini-Mental State Examination [MMSE]12) and medical comorbidities (assessed with the Cumulative Illness Rating Scale [CIRS]13). The CIRS rates 13 body systems on a 4-point scale and was scored by trained chart abstractors using a standardized tool.
Depression.
Depression diagnosis at study entry was assessed using the Patient Health Questionnaire (PHQ-9) as a screening tool, followed by the Structured Clinical Interview for Depression for diagnosis.14,15 The PHQ-9 is validated for depression screening in the poststroke population and includes 9 items assessing 9 symptoms of depression as defined by the DSM.16,17 Depression improvement was defined as a decrease in PHQ-9 scores from baseline to 12 weeks of at least 50% or a 12-week PHQ-9 score <10.
Functional status.
We measured functional status at baseline and 12 weeks after baseline with the modified Rankin scale (mRS), a categorical variable.18 It is a global overall measure of functional status and does not focus solely on general or on instrumental activities of daily living. The mRS is scored 0–5: 0 = no symptoms at all; 1 = no significant disability despite symptoms: able to carry out usual duties and activities; 2 = slight disability: unable to carry out all previous activities but able to look after own affairs without assistance; 3 = moderate disability: requiring some help but able to walk without assistance; 4 = moderately severe disability: unable to walk without assistance, and unable to attend to own bodily needs without assistance; and 5 = severe disability: bedridden, incontinent, and requiring constant nursing care and attention. Other studies have defined a poor outcome (dependence) as a mRS >2.19 Therefore, we specified the mRS as a dichotomous variable with 0–2 indicating functional independence and 3–5 representing dependence after stroke.19
Stroke severity.
The NIH Stroke Scale (NIHSS) was used to assess stroke severity.20 The NIHSS is an 11-item scale that includes consciousness, vision, language, sensory, and motor function. The NIHSS exhibits evidence of reliability and validity with increasing scores representing increasing stroke severity. We used the validated retrospective method of reviewing admission physical examination records to assign a baseline NIHSS for each subject.21
Statistical analysis.
To determine the relationship between PSD and functional outcome (12-week mRS score) after stroke, we analyzed data from depressed and nondepressed study participants (n = 392); subjects were excluded from analysis if the 12-week mRS score was missing (n = 25). There were no significant differences between those with and without a 12-week mRS score. We used independent t tests and χ2 analyses as appropriate to compare baseline demographics between those who were and were not independent at 12 weeks postbaseline (mRS ≤2 vs >2). We examined the bivariate association between the following variables and functional outcomes at 12 weeks: baseline depression scores (PHQ-9), depression history, CIRS comorbidity index, stroke severity, age, gender, race, and cognition. Variables associated with 12-week functional outcomes in the bivariate analyses and those that were identified via a priori clinical judgment (i.e., depression) were included in the multivariate logistic regression model. Variables with a p < 0.05 were kept in the final logistic regression model based on backward selection of variables.
To assess the impact of depression improvement on poststroke functional recovery, we analyzed only subjects with depression (n = 174 of the 188 with PSD and with no missing data). The above bivariate and multivariate analyses were repeated. Depression improvement was included in this second logistic regression. The 6-item MMSE score was excluded from both multivariate analyses as individuals required at least 4/6 to be included in the study. NIHSS was included in the modeling, regardless of the bivariate p value, in order to control for stroke recovery.
The level of significance for terms to be kept in the final model was set at p < 0.05. No imputations were made for missing data; those without a 12-week mRS or PHQ-9 were excluded from the analysis. All analyses were completed using SPSS statistical software, version 15.
Role of the funding source.
The funding source had no involvement or role in the collection, analysis, or interpretation of the data, or in the writing of this manuscript or decision to submit this manuscript for publication.
RESULTS
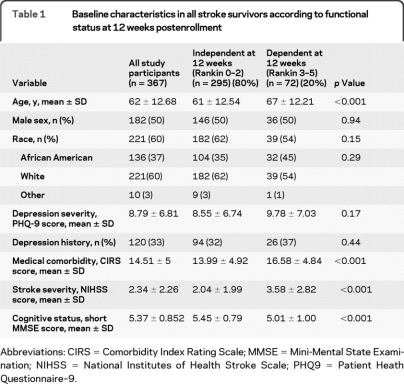
The characteristics of the 367 included individuals are found in table 1. The average age was 62 (±12.7) years, 50% were male (n = 182), and the majority of participants were white (60%).
Table 1.
Baseline characteristics in all stroke survivors according to functional status at 12 weeks postenrollment
Abbreviations: CIRS = Comorbidity Index Rating Scale; MMSE = Mini-Mental State Examination; NIHSS = National Institutes of Health Stroke Scale; PHQ9 = Patient Heath Questionnaire–9.
At 12 weeks postenrollment (4–5 months poststroke), 72 (20%) of the study participants were dependent and 295 (80%) were independent. Individuals dependent at 12 weeks were older (67 vs 61, p < 0.001); had increased medical comorbidities (CIRS, 16.6 vs 14.0, p < 0.001); had increased stroke severity (NIHSS, 3.58 vs 2.04, p < 0.001); and demonstrated decreased cognition (MMSE, 5.01 vs 5.45, p < 0.001). There were no differences in gender or race between those independent or dependent at 12 weeks (p = 0.94 and p = 0.29, respectively). Depression symptoms were not significantly different by functional group at 12 weeks postbaseline for the entire cohort.
The adjusted odds ratios (OR) and 95% confidence intervals (95% CI) from the multivariable analyses assessing factors associated with 12-week functional outcomes are presented in table 2. Four variables were included in the logistic regression: age, comorbidity index, stroke severity, and baseline depression. Three characteristics were independently associated with decreased functional outcome (dependence) at 12 weeks: increased baseline medical comorbidity (OR 1.10, 95% CI 1.04–1.17), increased NIHSS stroke severity (OR 1.36, 95% CI, 1.21–1.53), and increased age (OR 1.04, 95% CI, 1.02–1.07).
Table 2.
Independent baseline predictors of dependency, full cohort
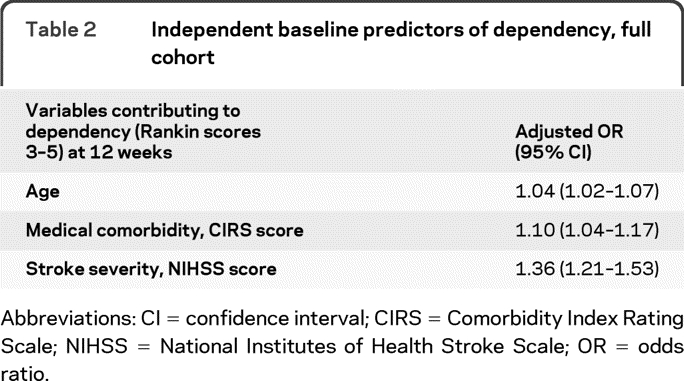
Abbreviations: CI = confidence interval; CIRS = Comorbidity Index Rating Scale; NIHSS = National Institutes of Health Stroke Scale; OR = odds ratio.
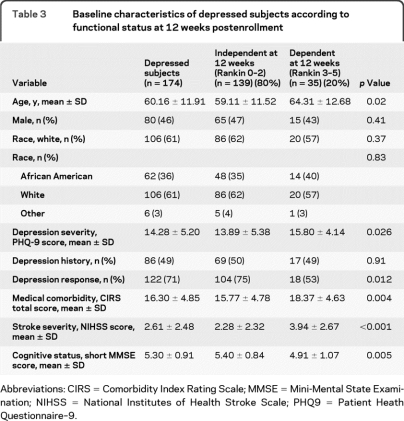
Table 3 shows the baseline characteristics for the depressed only cohort (n = 174). Those dependent at 12 weeks postenrollment (n = 35) had increased baseline depression scores (15.8 vs 13.9, p = 0.026) than those independent at 12 weeks. Additionally, we found 71% (122/173) of subjects with depression met criteria for depression improvement (regardless of treatment group). They were more likely to be independent at 12 weeks when compared to those who did not demonstrate depression improvement (75% vs 53%, p = 0.012).
Table 3.
Baseline characteristics of depressed subjects according to functional status at 12 weeks postenrollment
Abbreviations: CIRS = Comorbidity Index Rating Scale; MMSE = Mini-Mental State Examination; NIHSS = National Institutes of Health Stroke Scale; PHQ9 = Patient Heath Questionnaire–9.
Additionally, subjects in the depressed group who were dependent at 12 weeks postenrollment were older (64 vs 59, p = 0.020), and demonstrated more medical comorbidities (CIRS, 18.4 vs 15.8, p = 0.004), increased stroke severity (NIHSS, 3.94 vs 2.28, p < 0.0001), and decreased cognition (MMSE, 4.91 vs 5.40, p = 0.005).
The results from the second multivariable analysis with the depressed only group are found in table 4. We included age, CIRS, stroke severity, baseline depression, and depression improvement into the model. Three characteristics were found to be independently associated with 12-week function in the depressed group: increased baseline medical comorbidity (OR 1.10, 95% CI 1.02–1.22), increased NIHSS stroke severity (OR 1.42, 95% CI 1.19–1.69), and increased baseline PHQ-9 depression severity (OR 1.13, 95% CI 1.03–1.23). Depression response was significantly associated with dependence at 12 weeks in the bivariate analysis, and there was also a nonsignificant trend for this association in the multivariate model (OR 0.60, 95% CI 0.25–1.46).
Table 4.
Independent predictors of dependency, depressed cohort
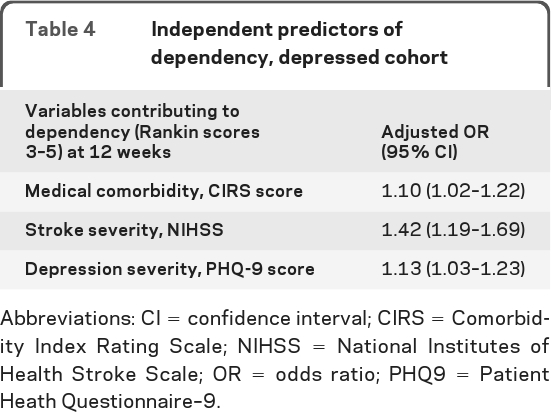
Abbreviations: CI = confidence interval; CIRS = Comorbidity Index Rating Scale; NIHSS = National Institutes of Health Stroke Scale; OR = odds ratio; PHQ9 = Patient Heath Questionnaire–9.
We completed a post hoc exploratory analysis of those who were dependent at baseline (n = 92). Comparing scores for the above variables, the following were significantly different between those who were independent (n = 40) and dependent (n = 52) at 12 weeks: age (61 vs 67, p = 0.01); medical comorbidities (CIRS, 14.67 vs 16.96, p = 0.02); and cognition (MMSE, 5.45 vs 5, p = 0.05). This is parallel to our above analyses where we included both those who were independent and dependent at baseline; only NIHSS was not significantly different, but this is due to everyone being dependent at baseline. None of the variables were maintained in the multivariate modeling. We completed similar exploratory analyses on the PSD-only cohort. For those with depression who were dependent at baseline (n = 56), we found only medical comorbidities to be maintained in the predictive model (OR 1.21, 95% CI 1.04–1.42). There was a nonsignificant trend of baseline depression being associated with 12-week dependence (OR 1.08, 95% CI 0.96–1.24). However, it should be noted that sample size decreased substantially to <30 per group, allowing for the possibility of increased error.
In a second set of exploratory analyses, we examined the data from the PSD-only cohort. We included treatment group (AIM intervention vs control) and antidepressant use (regardless of treatment group) to the logistic regression model (along with the above variables: CIRS, NIHSS, PHQ-9); neither variable independently predicted functional status at 12 weeks. We also examined the association of 12-week depression scores to functional outcome in bivariate and multivariate analyses. In both the entire cohort and the depressed cohort, there was a significant difference in 12-week PHQ-9 scores between those who were dependent and independent at 12 weeks postenrollment (entire cohort, 7.31 vs 5.18, p = 0.008; depressed cohort, 9.94 vs 7.27, p = 0.019). When forced into the logistic regression model, 12-week PHQ-9 scores, but not baseline scores, were independently associated with 12-week dependence for the entire cohort only (OR 1.06, 95% CI 1.01–1.11). The smaller sample size in these analyses increases the probability of a Type II error in this exploratory analysis.
DISCUSSION
This study demonstrates that among depressed and nondepressed stroke survivors, increased age, more medical comorbidities, and baseline stroke severity, but not baseline depression symptoms, are independently associated with dependence after stroke. Among those with PSD, baseline depression severity, along with increased medical comorbidities and stroke severity, is independently associated with dependence after stroke. Although depression improvement was associated with improved functional outcome in the bivariate analysis, this did not remain significant in the multivariable model.
Previous studies have been inconsistent in their findings regarding the association between poststroke depression and functional outcomes. Researchers found depression was related to functional impairment after stroke in people over the age of 65.22 Others have not found depression to be related to functional outcomes after stroke.23,24
Time since stroke may be important when examining the relationship between depression and functional recovery. Researchers have demonstrated that functional outcomes related to depression may change during the poststroke recovery time and that depression may negatively impact the timing of functional recovery.9,25 In this regard, we found 12-week depression severity to be associated with dependence. However, due to the cross-sectional nature of assessing depression severity at the same time point that dependence was ascertained, we cannot establish the directionality of the association: dependence may have been the consequence or the cause of depression at 12 weeks.
We also found cognition (MMSE) and neurologic impairment (NIHSS) to be significantly related to function in the bivariate analysis. Recently, it was demonstrated that antidepressant use was beneficial to cognitive recovery when initiated during the first 3 months poststroke.26 This was regardless of change in depression. Others have found that those with depression improvement demonstrated significantly better activities of daily living functional improvement then those without a remittance of depression.3,27 However, timing of functional outcomes changes are not well-understood.3 Our outcomes were assessed at 4–5 months poststroke; perhaps additional functional recovery would be found with continued depression treatment over a longer period of time.
Of additional note, this trend of lower depression scores with 12-week dependence is maintained when we included only those with baseline depression and dependence. Otherwise, only comorbidities were found to be predictive of 12-week dependence. It is likely that our small sample size of less than 30 is too small for predictive modeling. Further research regarding those considered dependent at baseline or directly after the stroke is necessary. Functional recovery and outcomes may be impacted by different variables for individuals functioning at a higher level during the earlier phases of poststroke recovery.
Finally, our models in the PSD cohort that included treatment group (AIM intervention vs control) and antidepressant use (regardless of treatment group) found neither variable independently predicted function at 12 weeks postenrollment. Depressed subjects who demonstrated depression improvement had better functional outcomes at 12 weeks (approximately 4–5 months poststroke). There was a trend for this association between depression improvement and less dependence (OR 0.60, 95% CI 0.25–1.46) in the multivariate model, though the CIs were wide and included 1.0. The few participants in the PSD cohort who also were dependent at 12 weeks (n = 35) limits our power to detect this association. Other authors have found functional recovery to be related to remission of PSD23,28 but in general, there is little research regarding the impact of depression treatment on functional outcomes.
Possible explanations for this lack of association in our study may be because not all intervention patients had depression improvement and a number of control patients did have depression improvement. A second reason we may be limited in detecting this association is the overall high level of function in the participants; at 12 weeks, 80% of the stroke survivors were classified as independent.
Several other potential study limitations exist. First, since the study participants had relatively mild stroke, the mRS may not be sensitive enough to distinguish clinically important differences in functional status in this cohort. This also affects the generalizability of our findings to other older or more impaired stroke cohorts. Future research should focus on people considered to be dependent directly after stroke. We also excluded those with severe language or cognitive impairments. Additionally, we were not able to determine cause and effect between depression and function; function may predict depression and depression may predict function. Finally, only 35 participants were depressed and dependent in functioning, thus limiting the power of our trial to examine the independent effect of depression treatment on a secondary outcome such as functional recovery.
Our data demonstrated that among the subset of patients with PSD, the severity of depression symptoms is associated with functional outcome. Our study is inconclusive as to whether improvement of depression is independently associated with functional recovery at 12 weeks. Even if the treatment and improvement of PSD does not directly influence functional recovery in all stroke survivors, it is essential for PSD to be identified and treated due to its high symptom burden and association with other negative health and social outcomes. Further research exploring whether PSD treatment is related to functional recovery in a more heterogeneous and larger group of stroke survivors is necessary to help identify other mechanisms to improve stroke survivors' outcomes.
Footnotes
- AIM
- Activate-Initiate-Monitor
- CI
- confidence interval
- CIRS
- Cumulative Illness Rating Scale
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- MMSE
- Mini-Mental State Examination
- mRS
- modified Rankin scale
- NIHSS
- NIH Stroke Scale
- OR
- odds ratio
- PHQ-9
- Patient Health Questionnaire
- PSD
- poststroke depression
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Sutherland.
DISCLOSURE
Dr. Schmid receives research support from the US Veterans Administration. Dr. Kroenke serves on scientific advisory boards for Eli Lilly & Company and Forest Laboratories, Inc.; has received funding for travel and speaker honoraria from Eli Lilly & Company, Forest Laboratories, Inc., and Pfizer Inc.; serves on the editorial boards of Archives of Internal Medicine, General Hospital Psychiatry, Psychosomatics, and Journal of Clinical Psychiatry Primary Care Companion; receives research support from Eli Lilly & Company, the NIH (NIMH and NCI), and the US Veterans Administration; and has served as a consultant for Ohio State University, University of Illinois, and Cambridge Hospital. Dr. Hendrie reports no disclosures. Dr. Bakas serves on the advisory board of the American Stroke Association Advisory Board and receives research support from the NIH (NINDS, NINR). Dr. Sutherland reports no disclosures. Dr. Williams serves as Health Policy and Outcomes Section Co-chair for Stroke and receives research support from the US Veterans Administration.
REFERENCES
- 1. Lloyd-Jones D, Adams RJ, Brown TM, et al. on behalf of the American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation 2010;121:e46–e215 [DOI] [PubMed] [Google Scholar]
- 2. Andersen G, Vestergaard K, Riis J, Lauritzen L. Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand 1994;90:190–195 [DOI] [PubMed] [Google Scholar]
- 3. Bilge C, Kocer E, Kocer A, Turk Boru U. Depression and functional outcome after stroke: The effect of antidepressant therapy on functional recovery. Eur J Phys Rehabil Med 2008;44:13–18 [PubMed] [Google Scholar]
- 4. Paolucci S, Antonucci G, Pratesi L, Traballesi M, Grasso MG, Lubich S. Poststroke depression and its role in rehabilitation of inpatients. Arch Phys Med Rehabil 1999;80:985–990 [DOI] [PubMed] [Google Scholar]
- 5. Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998;158:1133–1138 [DOI] [PubMed] [Google Scholar]
- 6. Schubert DS, Burns R, Paras W, Sioson E. Increase of medical hospital length of stay by depression in stroke and amputation patients: a pilot study. Psychother Psychosom 1992;57:61–66 [DOI] [PubMed] [Google Scholar]
- 7. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry 2004;161:1090–1095 [DOI] [PubMed] [Google Scholar]
- 8. Ramasubbu R, Robinson RG, Flint AJ, Kosier T, Price TR. Functional impairment associated with acute poststroke depression: the Stroke Data Bank Study. J Neuropsychiatry Clin Neurosci 1998;10:26–33 [DOI] [PubMed] [Google Scholar]
- 9. Lai SM, Duncan PW, Keighley J, Johnson D. Depressive symptoms and independence in BADL and IADL. J Rehabil Res Dev 2002;39:589–596 [PubMed] [Google Scholar]
- 10. Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database Syst Rev 2008;CD003437. [DOI] [PubMed] [Google Scholar]
- 11. Williams LS, Kroenke K, Bakas T, et al. Care management of poststroke depression: a randomized, controlled trial. Stroke 2007;38:998–1003 [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 13. Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626 [DOI] [PubMed] [Google Scholar]
- 14. Spitzer RL, Williams JB, Gibbon M. Instruction Manual for the Structured Clinical Interview for DSM-III-R. New York: Biometrics Research Development, New York State Psychiatric Institute; 1986 [Google Scholar]
- 15. Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID): I: history, rationale, and description. Arch Gen Psychiatry 1992;49:624–629 [DOI] [PubMed] [Google Scholar]
- 16. Williams LS, Brizendine EJ, Plue L, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke 2005;36:635–638 [DOI] [PubMed] [Google Scholar]
- 17. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rankin J. Cerebral vascular accidents in patients over the age of 60: II: prognosis. Scott Med J 1957;2:200–215 [DOI] [PubMed] [Google Scholar]
- 19. Sulter G, Steen C, Jacques De K. Use of the Barthel index and modified Rankin Scale in acute stroke trials. Stroke 1999;30:1538–1541 [DOI] [PubMed] [Google Scholar]
- 20. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870 [DOI] [PubMed] [Google Scholar]
- 21. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke 2000;31:858–862 [DOI] [PubMed] [Google Scholar]
- 22. Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc 2004;52:774–778 [DOI] [PubMed] [Google Scholar]
- 23. Saxena SK, Ng TP, Koh G, Yong D, Fong NP. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta Neurol Scand 2007;115:339–346 [DOI] [PubMed] [Google Scholar]
- 24. Hama S, Yamashita H, Shigenobu M, et al. Depression or apathy and functional recovery after stroke. Int J Geriatr Psychiatry 2007;22:1046–1051 [DOI] [PubMed] [Google Scholar]
- 25. Fruehwald S, Gatterbauer E, Rehak P, Baumhackl U. Early fluoxetine treatment of post-stroke depression: a three-month double-blind placebo-controlled study with an open-label long-term follow up. J Neurology 2003;250:347–351 [DOI] [PubMed] [Google Scholar]
- 26. Jorge RE, Acion L, Moser D, Adams HP, Jr, Robinson RG. Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry 2010;67:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chemerinski E, Robinson RG, Arndt S, Kosier JT. The effect of remission of poststroke depression on activities of daily living in a double-blind randomized treatment study. J Nerv Ment Dis 2001;189:421–425 [DOI] [PubMed] [Google Scholar]
- 28. Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke 2001;32:113–117 [DOI] [PubMed] [Google Scholar]