Abstract
Objective:
The purpose of this study was to assess the prevalence of and to identify epidemiologic, genetic, electrophysiologic, and neuroanatomic risk factors for autism spectrum disorders (ASD) in a cohort of patients with tuberous sclerosis complex (TSC).
Methods:
A total of 103 patients with TSC were evaluated for ASD. A retrospective review of patients' records was performed, including mutational analysis. EEG reports were analyzed for the presence of ictal and interictal epileptiform features. Brain MRI scans were evaluated for TSC neuropathology, including tuber burden.
Results:
Of the 103 patients with TSC, 40%were diagnosed with an ASD. On univariate analysis, patients with ASD were less likely to have mutations in the TSC1 gene. Patients with ASD also had an earlier age at seizure onset and more frequent seizures. On EEG, those with ASD had a significantly greater amount of interictal epileptiform features in the left temporal lobe only. On MRI, there were no differences in the regional distribution of tuber burden, although those with TSC2 and ASD had a higher prevalence of cyst-like tubers.
Conclusions:
The development of ASD in TSC is not well understood. Given our findings, ASD may be associated with persistent seizure activity early in development in particular brain regions, such as those responsible for social perception and communication in the left temporal lobe. The presence of cyst-like tubers on MRI could provide a structural basis or marker for ASD pathology in TSC, although studies assessing their effect on cortical function are needed.
Tuberous sclerosis complex (TSC) is an autosomal dominant disorder resulting from mutations in the TSC1 or the TSC2 gene.1,2 Neurologic involvement occurs in more than 90% of individuals and comprises several distinct lesions.3 Seizure disorders are present in 70%–90% of patients and often develop within the first year of life.4 Developmental and behavioral disorders, including autism spectrum disorders (ASD), are also frequently diagnosed in TSC.
ASD are characterized by impaired social interaction, restricted interests, and repetitive behaviors. ASD affects between 17% and 63% of patients with TSC, a prevalence dramatically higher than that of the general population.5,6 Studies suggest that mental retardation and early onset of epilepsy in TSC, in particular infantile spasms, are associated with the development of ASD in this group.7,8 In addition, there is evidence of an association between temporal lobe epileptiform foci with ASD in TSC.9 However, investigations seeking to implicate TSC genetics10–12 or neuropathology13–19 in ASD have yielded inconclusive results. Discrepancies between investigations may result, in part, from varying methods used to diagnose ASD. To date, no aspect of brain pathology in TSC has been shown to be necessary and sufficient for the development of ASD.
The present study investigates the relationship between ASD and TSC in an attempt to identify genetic, electrophysiologic, and neuroanatomic risk factors. To control for variability in the diagnostic criteria of ASD, the cohort was evaluated by a single neuropsychologist using standardized and validated measures. Our cohort size was selected to allow for appropriately powered analyses of genetic, clinical history, and electrophysiologic data.
METHODS
Participants.
We reviewed the clinical records of patients meeting the published clinical diagnostic criteria for TSC followed at the Herscot Center for Tuberous Sclerosis Complex at Massachusetts General Hospital.3 A total of 103 patients with ages ranging from 3 to 55 years had undergone comprehensive neuropsychologic (NP) testing with a single neuropsychologist from 2002 to 2009. NP testing included a standard battery to measure intelligence and adaptive functioning. The diagnosis of an ASD was based on DSM-IV criteria, using a clinical interview and completion of standardized questionnaires including the Child Symptom Inventory–4 Parent Checklist, Behavioral Assessment System for Children–2, and Gilliam Asperger's Disorder Scale.20–22 On review of patients' neurologic records, mean seizure frequency at the time of NP evaluation and maximum seizure frequency were coded as nonparametric variables as follows: 0, none; 1, fewer than one per month; 2, at least one per month; 3, at least one per week; and 4, daily.
Standard protocol approvals, registrations, and patient consents.
We received approval from the institutional review board of the Massachusetts General Hospital.
TSC mutational analysis.
All patients were offered genetic testing as part of their comprehensive evaluation, including mutational analysis of the TSC1 and TSC2 genes and detection for large DNA deletions and rearrangements of the TSC2 gene. Testing was performed at Athena Diagnostics (Worcester, MA) or the Massachusetts General Hospital Neurogenetic Diagnostic Laboratory (Boston, MA). Patients in whom genetic testing results were negative are classified herein as no mutation identified (NMI).
EEG.
EEG records were analyzed for patients who had undergone at least 25 minutes of surface monitoring with a 10–20 system of electrode placement. In all cases, patients had been referred for EEG to further characterize and assess their seizure disorder. EEG reports were included in analysis only if wave amplitude and frequency were reported. When more than one EEG record was available, the EEG record closest in date to the last NP examination was analyzed. The EEG field was divided into lobes as follows: frontal, Fp1–2 and F3–4; temporal, F7–8 and T3–4; parietal, C3–4 and P3–4; and occipital, T5–6 and O1–2. The EEG record was assessed for the presence of ictal activity, slowing, and regional epileptiform features including spikes, sharp waves, and spikes and waves. When epileptiform discharges were reported as broadly distributed, spatial analysis of amplitudes was used to help in further localization.
MRI.
All patients were referred for brain MRI at the time of diagnosis with TSC, annually to screen for growth of subependymal nodules (SENs) to subependymal giant cell tumors, or as symptoms necessitated. MRI was performed on a 1.5- or 3.0-Tesla system (GE Signa, Madison, WI). Sequence details can be found in appendix e-1 on the Neurology® Web site at www.neurology.org. MRI scans acquired closest in time to the formal NP assessment were retrospectively reviewed by 2 study investigators (A.L.N. and P.M.) after consultation with a pediatric neuroradiologist. In patients who had a history of resective epilepsy surgery, preoperative imaging was used for analysis. Each cerebral lobe was evaluated for the presence of cortical tubers, cyst-like tubers (figure e-1, A and B), and cortical tubers with calcifications (figure e-1, C and D).15,23,24 Tubers demonstrating central or peripheral fluid-attenuation inversion recovery (FLAIR) suppression and concomitant increases in fast spin-echo T2 signal intensity were classified as cyst-like. Tubers with regions containing concomitant suppression of FLAIR and T2 signal intensity were classified as tubers with calcification. All lesions identified were verified on at least 2 sequences. The cortical region with the largest cortical tuber burden (by volume) and the cortical region with the largest sized cortical tuber were recorded. Cerebellar tubers, white matter radial glial bands, and number of SENs were noted. Discordant findings between the evaluators were resolved by consensus.
Statistical analysis.
Statistical analyses were performed using SPSS version 15.0 (SPSS, Inc., Chicago, IL). Individuals with TSC and ASD were considered case patients, and patients with TSC and without ASD were considered control patients. Continuous variables were analyzed by a 2-sample t test and are presented as means ± SE. Categorical variables were assessed by the Fisher exact test or χ2 test. Nonparametric variables were assessed by the Mann-Whitney U test. The Spearman rank order correlation was used to assess the relationship between cyst-like tubers and age. Forced entry binary logistic regression was performed to assess the impact of a number of factors on the likelihood of having ASD in our cohort. The exponentiation of the B coefficient values were used to determine odds ratios. All reported p values used 2-tailed tests of significance with α set at 0.05.
RESULTS
Sample characteristics.
Of 103 patients with TSC who met the inclusion criteria, 41 (40%) had ASD. Patients with TSC and ASD (TSC/ASD) were younger than those without ASD (9.9 ± 4.5 vs 16.2 ± 10.2 years, p < 0.001). Patients with TSC/ASD also had lower IQs than those without ASD (51 ± 28 vs 81 ± 30, p < 0.001). There was no significant difference in male gender between those with TSC/ASD (49%) and those without ASD (42%). Those with TSC/ASD did not differ significantly from those without ASD with regard to dermatologic, renal, cardiac, pulmonary, or ophthalmologic manifestations of TSC (table e-1).
Genetic analysis.
Genetic analyses of the TSC1 and TSC2 genes were available for 92 (89%) patients in this cohort. There was a difference in mutation type (TSC1, TSC2, or NMI) among patients with TSC/ASD and without ASD (main effect p = 0.006) (table 1); on subgroup analysis, patients with TSC/ASD had fewer TSC1 mutations (p = 0.002). There was no significant difference between the groups regarding the mutation type (missense, nonsense/frameshift, splice site, or deletion). Patients with TSC/ASD had more disease-causing mutations inactivating the hamartin interaction domain of the TSC2 gene than patients without ASD (28% vs 5%; p = 0.004) (figure e-2). There was no significant difference between the groups when the guanosine triphosphatase- activating protein (GAP) domain of TSC2 was examined, with 21 (58%) patients with TSC/ASD and 24 (43%) patients without ASD having loss of GAP function.
Table 1.
Characteristics of genetics, epilepsy, and MRI features in TSC
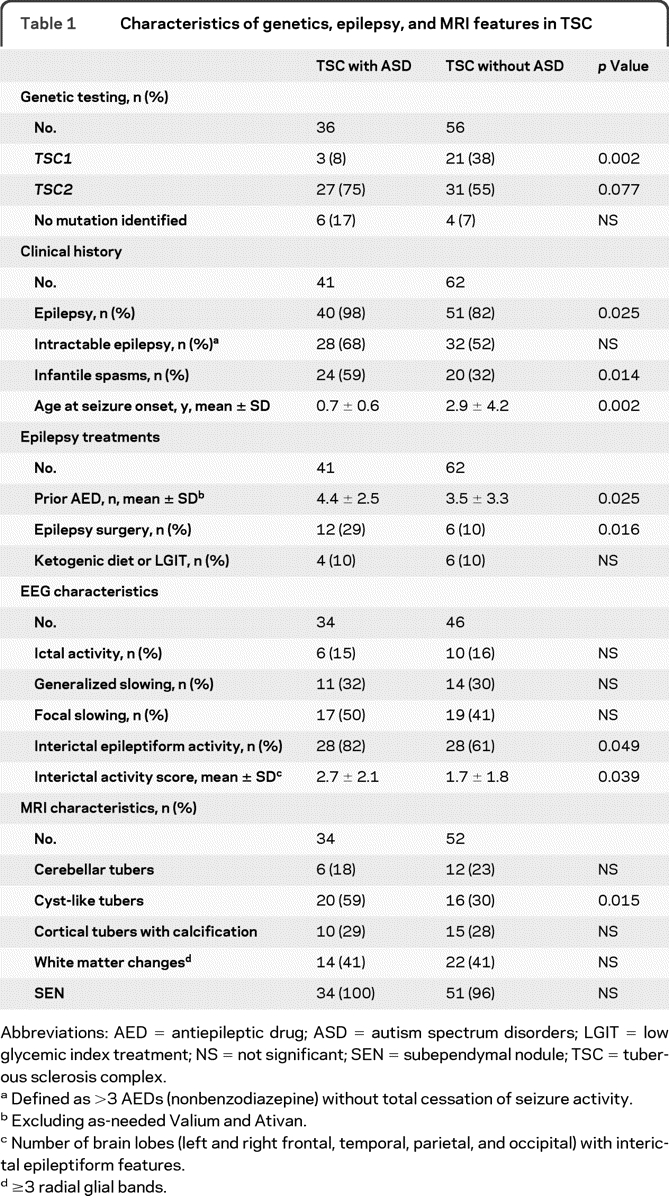
Abbreviations: AED = antiepileptic drug; ASD = autism spectrum disorders; LGIT = low glycemic index treatment; NS = not significant; SEN = subependymal nodule; TSC = tuberous sclerosis complex.
Defined as >3 AEDs (nonbenzodiazepine) without total cessation of seizure activity.
Excluding as-needed Valium and Ativan.
Number of brain lobes (left and right frontal, temporal, parietal, and occipital) with interictal epileptiform features.
≥3 radial glial bands.
Seizure history.
Several significant relationships between seizure history and ASD were found on analysis (table 1). Patients with TSC/ASD had an earlier age at seizure onset than those without ASD, which remained true when correcting for the increased presence of infantile spasms in patients with ASD (p = 0.002). At the time of NP evaluation, patients with ASD had a greater mean seizure frequency (2.1 ± 1.7) than those without ASD (1.4 ± 1.4; p = 0.047). In addition, patients with ASD had a greater prior maximum seizure frequency (3.5 ± 1.0; between once per week and daily) than those without ASD (2.8 ± 1.6; p = 0.034).
EEG data.
EEG reports that met the inclusion criteria for analysis were available for 80 of the 103 patients evaluated for ASD, including 34 of 41 (83%) patients with ASD and 46 of 62 (74%) patients without ASD. There was no significant difference when the time latency of EEG acquisition to NP evaluation between the 2 groups was compared (1.6 ± 1.9 years for TSC/ASD and 2.1 ± 3.5 years for TSC without ASD). Nearly all EEG records had been recorded in both sleep and awake states in the TSC/ASD group (94%) and the TSC without ASD group (87%). Furthermore, the groups did not differ significantly in the number of EEG records that contained more than 24 hours of data recordings (47% of patients with TSC/ASD and 35% of patients with TSC without ASD).
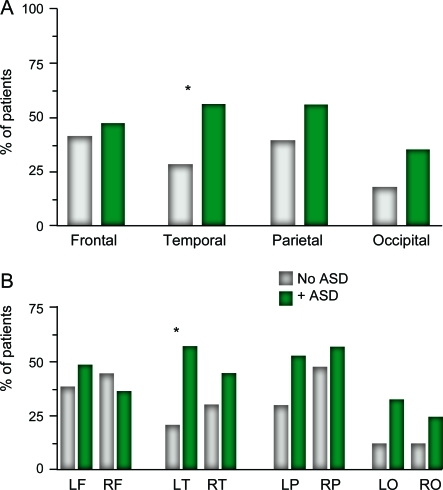
Analysis of EEG reports demonstrated no difference regarding the presence of ictal events, focal slowing, generalized slowing, or the degree of slowing between the groups (table 1). Those with TSC/ASD did have a significant increase in the presence of interictal epileptiform features on EEG and had more brain lobes affected with such features than those without ASD (table 1). Epileptiform features were significantly increased in the temporal lobe of patients with TSC/ASD (figure 1A), specifically the left temporal lobe (figure 1B). No such difference was observed between the groups when epileptiform features in the right temporal lobe or any other brain region were compared. There was a trend for increased interictal epileptiform activity in the occipital lobes of patients with TSC/ASD compared with that in patients without ASD, specifically the left occipital lobe.
Figure 1. Localization of interictal epileptiform features in tuberous sclerosis complex (TSC).
(A, B) Results are presented as the percentage of patients with TSC with interictal epileptiform activity (including spikes, sharp waves, and spikes and waves) on EEG, in a given anatomic brain region. *p < 0.05. ASD = autism spectrum disorders; F = frontal; L = left; O = occipital; P = parietal; R = right; T = temporal.
MRI data.
Of the 103 patients, 87 (84%) had brain MRI data meeting the inclusion criteria. Of these 87, 34 (39%) met the criteria for diagnosis of ASD. The average age at MRI for patients with TSC/ASD was 8.6 ± 0.8 years and for those with TSC without ASD was 13.1 ± 1.3 years (p = 0.004). There was no significant difference between the groups when the time from NP assessment to MRI was compared (TSC/ASD 0.6 ± 0.1 year and TSC without ASD 0.7 ± 0.1 year). Of the 87 patients, 13 (15%) had undergone resective epilepsy surgery (10 with ASD). Six patients without preoperative imaging who met the inclusion criteria were excluded from analyses of the largest regional tuber burden and size.
MRI features.
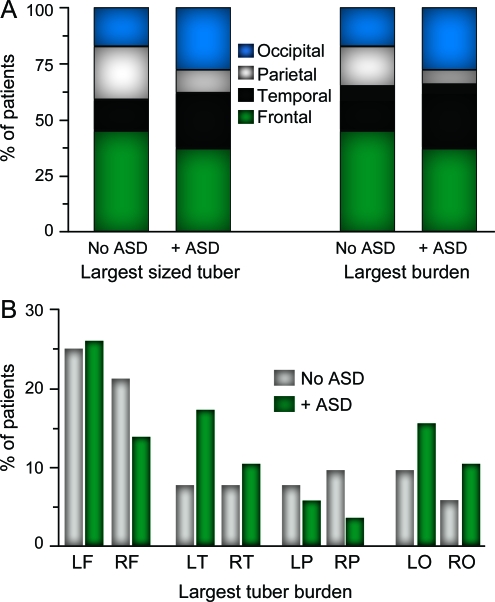
Cortical tubers were present on MRI in 84 of 87 (97%) patients. The 3 patients without evidence of tubers did not have the diagnosis of ASD; 2 carried TSC1 gene mutations, and 1 had NMI. Cortical tubers were present in all brain lobes in 72 of 87 (83%) patients evaluated. The largest sized tuber and tuber burden were identified most frequently in the left or right frontal lobes (41% of patients). There was no significant difference in the regional distribution of the largest sized tuber or tuber burden among patients with TSC/ASD and without ASD (figure 2 A). However, there was a trend for patients with ASD to have their largest tuber burden but not the largest sized tuber in the left temporal lobe (p = 0.09 for burden; p = 0.29 for size) (figure 2B). Comparisons of additional MRI stigmata of TSC between these groups are found in table 1.
Figure 2. Regional distribution of the largest sized cortical tuber and largest tuber burden in patients with tuberous sclerosis complex (TSC)/autism spectrum disorders (ASD) and without ASD.
(A) Regional percentages represent the cumulative total of the left and right region cases. (B) Largest tuber burden by brain lobe. F = frontal; L = left; O = occipital; P = parietal; R = right; T = temporal.
Cyst-like tubers.
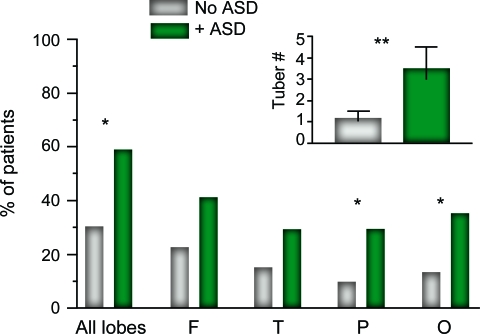
Cortical tubers meeting the criteria for cyst-like tubers were present in 36 of 87 (41%) patients. Patients with TSC/ASD were more likely to have these lesions than patients without ASD (p = 0.015) (table 1). Moreover, cyst-like tubers were far more numerous in patients with TSC/ASD (3.5 ± 0.4) than in patients without ASD (1.2 ± 0.4; p = 0.005) (figure 3, inset). The increase in cyst-like tubers did not localize to a particular brain region when patients with and without ASD were compared (figure 3). There was a correlation between a patient's age and the number of cyst-like tubers on MRI, with younger patients having more lesions (r = −0.45; p < 0.001), which remained when controlling for the diagnosis of ASD (partial coefficient = −0.26; p = 0.014). Of note, 34 of the 36 (94%) patients with cyst-like tubers had a TSC2 gene mutation; the remaining 2 patients (one with TSC/ASD and one without ASD) had TSC1 gene mutations.
Figure 3. Total and regional distributions of cyst-like tubers in patients with tuberous sclerosis complex (TSC)/autism spectrum disorders (ASD) and without ASD.
Inset: Number of cyst-like tubers in patients with TSC/ASD (3.5 ± 0.4) and without ASD (1.2 ± 0.4). Values presented are means ± SE. *p < 0.05; **p < 0.01. F = frontal; O = occipital; P = parietal; T = temporal.
Logistic regression.
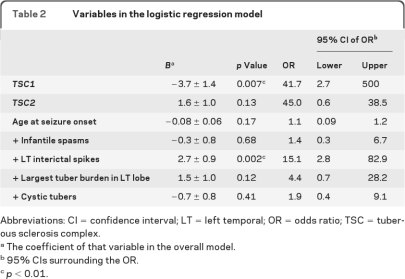
Logistic regression was performed with 7 independent variables, chosen from their statistical significance on univariate analysis of ASD in TSC (table 2). Complete datasets were available for 66 of 103 (64%) patients. On analysis, the model correctly classified 73% of cases (p = 0.001); the positive predictive value was 71%, and the negative predictive value was 74%. As shown in table 2, only 2 of the variables chosen had a unique and significant contribution: a TSC1 mutation decreased the chance of ASD more than 41 times, and the presence of interictal spikes in the left temporal lobe increased the chance of ASD by more than 15 times.
Table 2.
Variables in the logistic regression model
Abbreviations: CI = confidence interval; LT = left temporal; OR = odds ratio; TSC = tuberous sclerosis complex.
The coefficient of that variable in the overall model.
95% CIs surrounding the OR.
p < 0.01.
DISCUSSION
The rate of ASD in TSC varies widely in published reports from 17% to 63%.6,7,11 In our cohort of patients with TSC evaluated by a single neuropsychologist, the prevalence of ASD was 40%. We also report a lower prevalence of TSC1 mutations in those with TSC and ASD. Although we sampled from a tertiary clinic population, our findings correlate well with prior investigations examining larger groups of patients with TSC.25–27 Moreover, we feel that sampling bias has been minimized by implementing a policy at our TSC clinic to have all children and young adults with TSC undergo a formal NP evaluation to better serve our population.
We report that TSC mutations inactivating the hamartin domain of the TSC2 gene were associated with ASD in TSC. Although unlikely to be a causal relationship, mutations in these regions of the TSC genes may markedly increase risk of ASD, and in combination with mutations in other modifier genes of ASD, the phenotype can be observed. Here, our methods detected germline mutations in TSC genes in peripheral blood. The somatic, “second-hit” mutation in the remaining TSC allele may also contribute to phenotypic variability in TSC. It is possible that the timing and cellular location of the second hit mutation can have downstream effects on a developing brain, including learning, behavior, and epileptogenesis. Although the pathogenesis of some brain lesions in TSC may not be dependent on a second-hit mutation,28 further investigations interrogating the relationship between neurologic phenotype and somatic mutations in TSC pathology, including cyst-like tubers, are warranted.
Here, we found that interictal epileptiform features in the temporal lobe are associated with ASD, specifically, the left temporal lobe. This laterality has not been reported previously. Interestingly, the prevalence of interictal epileptiform features was markedly high in both groups, demonstrating the epileptogenicity of the brain in TSC. Localization of focal interictal epileptiform features in patients with TSC has been demonstrated to be stable for more than 10 years.29 Thus, although the dates of our EEG data and NP evaluations were often disparate in a given patient, our data may still accurately depict the electrographic nature of his or her brain at the time of NP evaluation.
Intriguingly, we report that timing of seizure onset and increased seizure frequency were associated with ASD. From this, it is plausible that early detection and treatment of both clinical seizures and epileptiform features in TSC could change the course of disease progress. Indeed, evidence from our group and recent evidence from a small cohort in Italy suggest that early and effective treatment may improve neurodevelopmental outcomes.30,31 Routine EEG in the first years of life may provide a useful screening technique in all patients with TSC to assess both subclinical seizures and epileptiform features. However, delineating the role that such abnormalities may have on the progression of ASD in TSC will pose substantial methodologic challenges.
Despite our use of the MRI FLAIR sequences, which have an increased sensitivity for tuber identification,23,24 we failed to find a significant relationship with tuber localization and ASD. A number of explanations exist for these findings, including methodologic flaws that may be inherent to assessment of cortical tubers by MRI in TSC. Histologic evidence suggests that brain pathology in TSC extend beyond the limits of cortical tubers visualized on MRI.32,33 Our inability to demonstrate a clear relationship between cortical tuber burden and ASD could represent our inability to accurately evaluate the widespread cortical disorganization in these patients. Indeed, a study assessing gray and white matter volumes, in conjunction with tuber burden, found that only abnormalities in volume and in not burden were predictive of memory deficits in patients with TSC.34 Thus, although cortical tuber burden evaluated by MRI may be a better marker of brain disease in TSC than cortical tuber size or number alone, it still may fall short of ascertaining the full extent of the neuropathology of TSC.19,25,35
Cyst-like cortical tubers are a neuroanatomic finding in TSC, the prognostic significance of which is under investigation.36 The mechanism by which cyst-like cortical tubers develop is unknown, as is their natural history. Here we find that cyst-like tubers are more common and more numerous and affect more brain regions in patients with TSC/ASD. Further investigations examining the relationship between cyst-like tubers and epileptogenic foci could reveal a pathophysiologic link between these lesions and ASD. Because interictal epileptiform activity has been hypothesized to alter cognition and behavior, persistent electrophysiologic abnormalities related to cyst-like tubers could contribute to the development of ASD in TSC.37,38 Epileptiform features, separate from or in concert with ictal events, could provide considerable dysfunction or “static” in the brain and contribute to the high prevalence of ASD in TSC.38,39 One cannot rule out the possibility that cyst-like tubers, EEG abnormalities, or the other risk factors for ASD identified herein are markers for more significant brain pathology in TSC, rather than the etiologic basis for ASD in TSC. Several investigations have demonstrated a more severe neurologic phenotype in patients with TSC2 mutations,10,12,26,27 including increased prevalence of cyst-like tubers.36 Here, results of our multiple regression analysis demonstrate that TSC mutation type as well as temporal lobe epileptiform activity were associated with ASD, providing evidence that these variables are independent predictors of ASD in TSC. However, this finding does not exclude that possibility that the pathogenesis of ASD in this population may result from a more global brain dysfunction.
Taken together, our data lend support for an underlying role of genetic, physiologic, and structural abnormalities in the development of ASD in TSC, although none of these can fully explain the high prevalence of ASD in this population. Further investigations aiming to elucidate the relationship between neuropathology in TSC and ASD may be served well by using mouse models of TSC that exhibit some degree of neuronal disorganization, including the Tsc1 conditional knockout mouse.40
Supplementary Material
ACKNOWLEDGMENT
The authors thank P. Ellen Grant for consultation regarding the radiographic analysis of these data.
- ASD
- autism spectrum disorders
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FLAIR
- fluid attenuation inversion recovery
- GAP
- guanosine triphosphatase-activating protein
- NMI
- no mutation identified
- NP
- neurophysiologic
- SEN
- subependymal nodule
- TSC
- tuberous sclerosis complex
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Dr. Adam L. Numis with the guidance of Dr. Elizabeth A. Thiele.
DISCLOSURE
Dr. Numis, Dr. Major, Dr. Montenegro, D.A. Muzykewicz, and Dr. Pulsifer report no disclosures. Dr. Thiele serves on the Board of Directors of the Tuberous Sclerosis Alliance and on scientific advisory boards for the Angelman Syndrome Foundation and the Charlie Foundation; serves on the speakers' bureau for and has received funding for travel and speaker honoraria from UCB; serves as a consultant for Lundbeck Inc.; and receives research support from Lundbeck Inc., the NIH, and the Tuberous Sclerosis Alliance.
REFERENCES
- 1. European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305–1315 [DOI] [PubMed] [Google Scholar]
- 2. van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997;277:805–808 [DOI] [PubMed] [Google Scholar]
- 3. Roach ES, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol 2004;19:643–649 [DOI] [PubMed] [Google Scholar]
- 4. Gomez M. Natural history of cerebral tuberous sclerosis. In: Gomez MR, Sampson JR, Whittemore VH. eds. Tuberous Sclerosis Complex: Developmental Perspectives in Psychiatry, 3rd ed New York: Oxford University Press; 1999 [Google Scholar]
- 5. Wong V. Study of the relationship between tuberous sclerosis complex and autistic disorder. J Child Neurol 2006;21:199–204 [DOI] [PubMed] [Google Scholar]
- 6. de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. Eur Child Adolesc Psychiatry 2007;16:16–24 [DOI] [PubMed] [Google Scholar]
- 7. Gutierrez GC, Smalley SL, Tanguay PE. Autism in tuberous sclerosis complex. J Autism Dev Disord 1998;28:97–103 [DOI] [PubMed] [Google Scholar]
- 8. Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J Child Neurol 2008;23:520–525 [DOI] [PubMed] [Google Scholar]
- 9. Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 2002;125:1247–1255 [DOI] [PubMed] [Google Scholar]
- 10. Au KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 2007;9:88–100 [DOI] [PubMed] [Google Scholar]
- 11. Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav 2007;11:506–513 [DOI] [PubMed] [Google Scholar]
- 12. Lewis JC, Thomas HV, Murphy KC, Sampson JR. Genotype and psychological phenotype in tuberous sclerosis. J Med Genet 2004;41:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jambaque I, Cusmai R, Curatolo P, Cortesi F, Perrot C, Dulac O. Neuropsychological aspects of tuberous sclerosis in relation to epilepsy and MRI findings. Dev Med Child Neurol 1991;33:698–705 [DOI] [PubMed] [Google Scholar]
- 14. Bolton PF, Griffiths PD. Association of tuberous sclerosis of temporal lobes with autism and atypical autism. Lancet 1997;349:392–395 [DOI] [PubMed] [Google Scholar]
- 15. Doherty C, Goh S, Young Poussaint T, Erdag N, Thiele EA. Prognostic significance of tuber count and location in tuberous sclerosis complex. J Child Neurol 2005;20:837–841 [DOI] [PubMed] [Google Scholar]
- 16. Eluvathingal TJ, Behen ME, Chugani HT, et al. Cerebellar lesions in tuberous sclerosis complex: neurobehavioral and neuroimaging correlates. J Child Neurol 2006;21:846–851 [DOI] [PubMed] [Google Scholar]
- 17. Walz NC, Byars AW, Egelhoff JC, Franz DN. Supratentorial tuber location and autism in tuberous sclerosis complex. J Child Neurol 2002;17:830–832 [DOI] [PubMed] [Google Scholar]
- 18. Wong V, Khong PL. Tuberous sclerosis complex: correlation of magnetic resonance imaging (MRI) findings with comorbidities. J Child Neurol 2006;21:99–105 [DOI] [PubMed] [Google Scholar]
- 19. Asano E, Chugani DC, Muzik O, et al. Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology 2001;57:1269–1277 [DOI] [PubMed] [Google Scholar]
- 20. Gadow K, Sprafkin J. Child Symptom Inventory-4 Screening and Norms Manual. Stony Brook, NY: Checkmate Plus; 2002 [Google Scholar]
- 21. Gilliam J. Gilliam Asperger's Disorder Scale, Examiner's Manual. Austin, TX: Pro-Ed; 2001 [Google Scholar]
- 22. Reynolds C, Kamphaus R. Behavior Assessment System for Children, 2nd ed. Circle Pines, MN: American Guidance Service; 2004 [Google Scholar]
- 23. Pinto Gama HP, da Rocha AJ, Braga FT, et al. Comparative analysis of MR sequences to detect structural brain lesions in tuberous sclerosis. Pediatr Radiol 2006;36:119–125 [DOI] [PubMed] [Google Scholar]
- 24. Jurkiewicz E, Jozwiak S, Bekiesinska-Figatowska M, Pakula-Kosciesza I, Walecki J. Cyst-like cortical tubers in patients with tuberous sclerosis complex: MR imaging with the FLAIR sequence. Pediatr Radiol 2006;36:498–501 [DOI] [PubMed] [Google Scholar]
- 25. Jansen FE, Braams O, Vincken KL, et al. Overlapping neurologic and cognitive phenotypes in patients with TSC1 or TSC2 mutations. Neurology 2008;70:908–915 [DOI] [PubMed] [Google Scholar]
- 26. Jones AC, Shyamsundar MM, Thomas MW, et al. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 1999;64:1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology 2007;68:62–64 [DOI] [PubMed] [Google Scholar]
- 28. Niida Y, Stemmer-Rachamimov AO, Logrip M, et al. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet 2001;69:493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jansen FE, van Huffelen AC, Bourez-Swart M, van Nieuwenhuizen O. Consistent localization of interictal epileptiform activity on EEGs of patients with tuberous sclerosis complex. Epilepsia 2005;46:415–419 [DOI] [PubMed] [Google Scholar]
- 30. Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol 2010;14:146–149 [DOI] [PubMed] [Google Scholar]
- 31. Goh S, Kwiatkowski DJ, Dorer DJ, Thiele EA. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology 2005;65:235–238 [DOI] [PubMed] [Google Scholar]
- 32. Boer K, Troost D, Jansen F, et al. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008;28:577–590 [DOI] [PubMed] [Google Scholar]
- 33. Ridler K, Bullmore ET, De Vries PJ, et al. Widespread anatomical abnormalities of grey and white matter structure in tuberous sclerosis. Psychol Med 2001;31:1437–1446 [DOI] [PubMed] [Google Scholar]
- 34. Ridler K, Suckling J, Higgins NJ, et al. Neuroanatomical correlates of memory deficits in tuberous sclerosis complex. Cereb Cortex 2007;17:261–271 [DOI] [PubMed] [Google Scholar]
- 35. Ridler K, Suckling J, Higgins N, Bolton P, Bullmore E. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol 2004;19:658–665 [DOI] [PubMed] [Google Scholar]
- 36. Chu-Shore CJ, Major P, Montenegro M, Thiele E. Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology 2009;72:1165–1169 [DOI] [PubMed] [Google Scholar]
- 37. Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 2006;8:504–515 [DOI] [PubMed] [Google Scholar]
- 38. Zhou JL, Lenck-Santini PP, Zhao Q, Holmes GL. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia 2007;48:720–731 [DOI] [PubMed] [Google Scholar]
- 39. Zhou JL, Shatskikh TN, Liu X, Holmes GL. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci 2007;25:3667–3677 [DOI] [PubMed] [Google Scholar]
- 40. Meikle L, Talos DM, Onda H, et al. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci 2007;27:5546–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.