The first evidence of a potential relationship between the cyclooxygenase 2 (COX-2) enzyme and human cancers was reported in 1994, when COX-2 mRNA levels were found to be markedly elevated in colorectal carcinomas (1). Several subsequent reports verified and extended these findings (2). COX-2 (prostaglandin endoperoxide synthase) converts arachidonic acid to bioactive lipids, including prostaglandins (PGs) and thromboxanes. Considerable evidence suggests that COX-2 may contribute to the development of colorectal cancer as well as other human cancers. For example, in a mouse model of familial adenomatous polyposis, knocking out COX-2 protects against the formation of intestinal polyps, suggesting that COX-2 is involved in adenoma formation (3). Recently, COX-2 transgenic mice have been created to further explore the role of COX-2 in cancer. Transgenic mice with COX-2 expression driven by the keratin-5 promoter did not develop skin cancer spontaneously but were much more sensitive to carcinogen-induced tumor formation, indicating that COX-2 overexpression alone was not sufficient to induce skin cancer (4). However, Hla and colleagues (5) generated COX-2 transgenic mice in which expression was driven by the murine mammary tumor virus (MMTV) promoter. Interestingly, breast carcinomas develop spontaneously in multiparous female MMTV–COX-2 mice. These studies have extended our understanding of the role of cyclooxygenase in carcinogenesis by demonstrating that forced expression of COX-2 alone is sufficient to induce mammary gland cancer. In this issue of PNAS, Chang et al. (6) report studies that studies delineate the molecular mechanism(s) by which COX-2-derived prostaglandin E2 (PGE2) induces tumor-associated angiogenesis, which is required for the initiation and/or progression of mammary cancer in MMTV–COX-2 mice. They observed that PGE2 induced angiogenesis at the earliest stage of tumor development, even before PGE2-induced mammary gland hyperplasia, providing a new understanding of the role of angiogenesis in this process. They also found that the nonselective nonsteroidal antiinflammatory drug (NSAID) indomethacin inhibited both PGE2-induced angiogenesis and tumor progression. Additionally, they confirmed the role of COX-2 by using the COX-2 selective inhibitor celecoxib.
Epidemiological, experimental, and clinical studies have demonstrated that aspirin and other NSAIDs reduce the risk of colorectal cancer (7). Because many other human cancers reportedly have elevated levels of COX-2 and overproduce prostaglandins, intense research efforts are underway to evaluate the role of NSAIDs in both the prevention and possible treatment of a number of human malignancies, including breast, stomach, pancreas, urinary tract, lung, and prostate cancer. For example, there are significant elevations of COX-2 protein levels in 43% of human invasive breast cancers and 63% of ductal carcinomas in situ (8). The limited observational studies published reveal conflicting results regarding a correlation between NSAID use and reduction in the risk for breast cancer. However, the current report by Chang et al. provides a better understanding of the molecular effects of NSAIDs in breast cancer and supports the concept that COX-2 may provide an “early” target for breast cancer prevention.
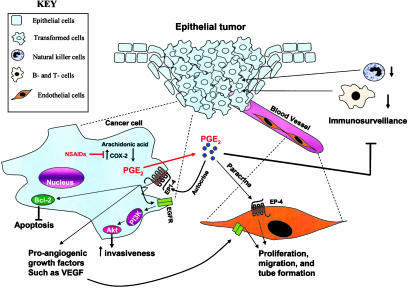
Prostaglandin E2 induces angiogenesis at the earliest stage of tumor development.
Angiogenesis, the sprouting of capillaries from preexisting vasculature, occurs during embryonic development, wound repair, and tumor growth. Because it is thought to play a central role in human tumor development, inhibition of tumor-associated angiogenesis has been touted as a promising therapeutic strategy. In fact, it is generally felt that neovascularization is required for tumors to grow >2–3 mm in size. COX-2 modulates angiogenesis by increasing the production of angiogenic factors, such as vascular endothelial growth factor (9). The role of COX-2 in angiogenesis during tumor development has been refined by Chang et al. (6). The authors used a MMTV–COX-2 transgenic mouse model to examine whether induction of angiogenesis correlates with progression of mammary cancer. Surprisingly, they report that microvessel density increased, even in very early stages of tumor development (hyperplasia at terminal ductal lobular unit, a precursor lesion for mammary tumorigenesis). Previously, angiogenesis was thought to play a major role in later stages of tumor development. Moreover, Chang et al. (6) found that indomethacin inhibited both angiogenesis and tumor growth, suggesting that NSAIDs suppress tumor development by blocking angiogenesis at a much earlier stage than was previously appreciated. Work by Leahy et al. (10) supports this idea; they found that celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells by inhibiting COX-2. Clearly, it will be of great interest to determine the mechanism by which indomethacin inhibits angiogenesis in their model system.
COX-2-derived PGE2 is the most abundant PG found in solid malignancies that express elevated enzyme levels. PGE2 binds to cell surface receptors that belong to the family of seven-transmembrane G protein-coupled rhodopsin-type receptors, designated as EP1, EP2, EP3, and EP4. Activation of EP receptors by ligand binding is associated with changes in the level of second messengers (for review, see ref. 11). For example, activation of EP2 and EP4 receptors leads to increased intracellular cAMP levels. However, EP1 activation increases cytosolic free calcium levels, and activation of EP3 receptors results in a decrease in intracellular cAMP levels. Genetic studies in mouse models indicate that EP1, EP2, and EP4 receptors are involved in small intestinal polyp formation, but EP3 is not (12–14). Supporting these observations, Hla's group showed that EP1, EP2, and EP4 receptors were elevated, whereas EP3 receptor levels were decreased, in mammary tumors in the MMTV–COX-2 mice. However, Amano et al. (15) recently reported that EP3 receptor activation is required for lung tumor-associated angiogenesis and tumor growth. In the future, it will be important to determine whether COX-2 can modulate PGE2 receptor expression and identify the precise role each receptor signaling pathway plays in regulating angiogenesis and tumor growth. Finally, it has been demonstrated that EP2 and EP4 are expressed in gut epithelial cells but not in endothelial cells (16). However, Hla's group reported that EP2 is expressed in mammary ductal and alveolar epithelial cells and that EP4 is primarily expressed in stromal cells but not in mammary epithelial cells. The fact that EP4 is expressed in endothelial cells in the MMTV–COX-2 transgenic mice may explain why COX-2 regulates angiogenesis at such an early stage of tumor development. It will be important to determine whether PGE2 can directly regulate endothelial cell proliferation, migration, and tube formation by binding to the EP4 receptor.
A large body of evidence indicates that long-term NSAID use decreases the risk for colorectal cancer. COX-2-derived PGE2 can contribute to tumor development through several mechanisms (see Fig. 1) including (i) promotion of angiogenesis, (ii) inhibition of apoptosis, (iii) increased invasiveness/motility, and (iv) modulation of inflammation and immune responses (17). The molecular mechanism responsible for PGE2-induced colorectal cancer cell migration and invasion is known to involve an epidermal growth factor receptor (EGFR)–phosphatidylinositol 3-kinase–Akt pathway (18). However, the mechanisms by which PGE2 modulates apoptosis are still largely unknown. One potential mechanism with regard to its regulation of programmed cell death is that PGE2 reduces the basal apoptotic rate by increasing the level of antiapoptotic proteins such as BCL-2 (19) or other members of the BCL gene family, such as MCL-1. In addition, COX-independent effects of NSAID-induced apoptosis have also been reported (20). In general, COX-2-derived PGE2 suppresses immunosurveillance by down-regulating T and B cell proliferation, cytotoxic activity of natural killer cells, and cytokines such as IL-12 and tumor necrosis factor α (20).
Fig. 1.
Possible mechanisms of COX-2-derived PGE2 contributions to tumor development. In epithelial tumors of the mammary gland, COX-2-derived PGE2 may stimulate proangiogenic factors such as vascular endothelial growth factor, which promotes tumor-associated angiogenesis. Solid malignancies are made up of multiple types of cells that produce signals that work in both a paracrine and autocrine manner as depicted.
Tumor initiation and progression are affected by multiple genetic alterations. For example, HER-2/neu gene amplification plays a significant role in a subset of breast cancers. A significant correlation between COX-2 and HER-2 expression has been reported in human breast cancer. Dannenberg's group (21) recently reported that high COX-2 expression was detected in 14 of 15 HER-2-positive breast cancer samples and in only 4 of 14 HER-2-negative specimens. Moreover, it has been demonstrated that the EGFR signaling pathway is involved in many different types of cancer, including colorectal, breast, and lung cancer (22). The recent evidence that combined treatment with a nonselective NSAID plus an EGFR-tyrosine kinase inhibitor significantly decreases polyp formation in MinAPC+/– mice supports the notion that combinations of different agents for cancer prevention and treatment may be more effective (23). Obviously, the strategy to target multiple pathways simultaneously may be critical to improving the efficacy of not only single-agent therapy, but also of combined modality therapy in the prevention and treatment of breast cancer and other human cancers.
See companion article on page 591.
References
- 1.Eberhart, C. E., Coffey, R. J., Radhika, A., Giardiello, F. M., Ferrenbach, S. & DuBois, R. N. (1994) Gastroenterology 107, 1183–1188. [DOI] [PubMed] [Google Scholar]
- 2.Marnett, L. J. & DuBois, R. N. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 55–80. [DOI] [PubMed] [Google Scholar]
- 3.Oshima, M., Dinchuk, J. E., Kargman, S. L., Oshima, H., Hancock, B., Kwong, E., Trzaskos, J. M., Evans, J. F. & Taketo, M. M. (1996) Cell 87, 803–809. [DOI] [PubMed] [Google Scholar]
- 4.Muller-Decker, K., Neufang, G., Berger, I., Neumann, M., Marks, F. & Furstenberger, G. (2002) Proc. Natl. Acad. Sci. USA 99, 12483–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu, C. H., Chang, S. H., Narko, K., Trifan, O. C., Wu, M. T., Smith, E., Haudenschild, C., Lane, T. F. & Hla, T. (2001) J. Biol. Chem. 276, 18563–18569. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S.-H., Lui, C. H., Conway, R., Han, D. K., Nithipatikom, K., Trifan, O. C., Lane, T. F. & Hla, T. (2004) Proc. Natl. Acad. Sci. USA 101, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta, R. A. & Dubois, R. N. (2001) Nat. Rev. Cancer 1, 11–21. [DOI] [PubMed] [Google Scholar]
- 8.Half, E., Tang, X. M., Gwyn, K., Sahin, A., Wathen, K. & Sinicrope, F. A. (2002) Cancer Res. 62, 1676–1681. [PubMed] [Google Scholar]
- 9.Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M. & DuBois, R. N. (1998) Cell 93, 705–716. [DOI] [PubMed] [Google Scholar]
- 10.Leahy, K. M., Ornberg, R. L., Wang, Y., Zweifel, B. S., Koki, A. T. & Masferrer, J. L. (2002) Cancer Res. 62, 625–631. [PubMed] [Google Scholar]
- 11.Breyer, R. M., Bagdassarian, C. K., Myers, S. A. & Breyer, M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 661–690. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe, K., Kawamori, T., Nakatsugi, S., Ohta, T., Ohuchida, S., Yamamoto, H., Maruyama, T., Kondo, K., Ushikubi, F., Narumiya, S., et al. (1999) Cancer Res. 59, 5093–5096. [PubMed] [Google Scholar]
- 13.Mutoh, M., Watanabe, K., Kitamura, T., Shoji, Y., Takahashi, M., Kawamori, T., Tani, K., Kobayashi, M., Maruyama, T., Kobayashi, K., et al. (2002) Cancer Res. 62, 28–32. [PubMed] [Google Scholar]
- 14.Sonoshita, M., Takaku, K., Sasaki, N., Sugimoto, Y., Ushikubi, F., Narumiya, S., Oshima, M. & Taketo, M. M. (2001) Nat. Med. 7, 1048–1051. [DOI] [PubMed] [Google Scholar]
- 15.Amano, H., Hayashi, I., Endo, H., Kitasato, H., Yamashina, S., Maruyama, T., Kobayashi, M., Satoh, K., Narita, M., Sugimoto, Y., et al. (2003) J. Exp. Med. 197, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugimoto, Y., Narumiya, S. & Ichikawa, A. (2000) Prog. Lipid Res. 39, 289–314. [DOI] [PubMed] [Google Scholar]
- 17.Dempke, W., Rie, C., Grothey, A. & Schmoll, H. J. (2001) J. Cancer Res. Clin. Oncol. 127, 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchanan, F. G., Wang, D., Bargiacchi, F. & DuBois, R. N. (2003) J. Biol. Chem. 278, 35451–34517. [DOI] [PubMed] [Google Scholar]
- 19.Sheng, H., Shao, J., Morrow, J. D., Beauchamp, R. D. & DuBois, R. N. (1998) Cancer Res. 58, 362–366. [PubMed] [Google Scholar]
- 20.Gasparini, G., Longo, R., Sarmiento, R. & Morabito, A. (2003) Lancet Oncol. 4, 605–615. [DOI] [PubMed] [Google Scholar]
- 21.Subbaramaiah, K., Norton, L., Gerald, W. & Dannenberg, A. J. (2002) J. Biol. Chem. 277, 18649–18657. [DOI] [PubMed] [Google Scholar]
- 22.Kelloff, G. J., Fay, J. R., Steele, V. E., Lubet, R. A., Boone, C. W., Crowell, J. A. & Sigman, C. C. (1996) Cancer Epidemiol. Biomarkers Prev. 5, 657–666. [PubMed] [Google Scholar]
- 23.Torrance, C. J., Jackson, P. E., Montgomery, E., Kinzler, K. W., Vogelstein, B., Wissner, A., Nunes, M., Frost, P. & Discafani, C. M. (2000) Nat. Med. 6, 1024–1028. [DOI] [PubMed] [Google Scholar]