Abstract
Choroid plexus carcinoma is a very rare tumor in adults. Here we report a rare case of choroid plexus carcinoma in an adult patient. A 24-year-old male presented with a right temporal intraventricular tumor with a cystic component also extending up to the cortex. Histological examination revealed complex papillary structures and glandular spaces showing stratification and multilayering of cells with nuclear crowding and numerous mitotic figures and large areas of necrosis. The patient went through a complete search for a possible primary keeping in mind the differential diagnosis of metastatic carcinoma that is more common in adults but there was no evidence of any other tumor. Finally a diagnosis of choroid plexus carcinoma was rendered. Immunohistochemical analysis for p53 protein showed positivity. Choroid plexus carcinoma is exceptionally rare in adults but cases do occur.
Keywords: Adult, choroid plexus carcinoma, histopathology
Introduction
Choroid plexus tumors are rare and among these choroid plexus papillomas are far more common than carcinomas. They arise from the ventricles with the origin being more common from lateral ventricles in children and fourth ventricle in adults. Distinction between choroid plexus papillomas and choroid plexus carcinomas is made histologically. Choroid plexus tumors comprise 0.4–0.6% of all brain tumors.[1] Among these, choroid plexus papillomas are twice as common as choroid plexus carcinomas.[2] About 20–40% of all choroid plexus tumors in children are carcinomas[1] that are exceptionally rare in adults.[3,4]
Here we report a rare case of choroid plexus carcinoma in an adult patient.
Case Report
A 24-year-old Indian male was admitted in neurosurgery department with complaints of headache, nausea, and vomiting for 2 months. Examination and routine investigations did not reveal any significant findings. CT and MRI scans revealed a right temporal brightly contrast enhancing intraventricular tumor with a cystic component also extending up to the cortex. It was difficult to say whether the tumor was primarily intraventricular or cortical. Right parietal craniotomy was done along with tumor removal as the tumor was showing mass effect. The patient also received radiotherapy.
Squash smears revealed papillaroid structures lined by columnar cells that looked uniform with no evidence of pleomorphism, necrosis, or mitotic figures. Only some cells showed mild atypia. Thus, a possibility of choroid plexus papilloma was given on squash smears. The tissue pieces received for histopathology were irregular with small cystic and nodular areas. Histological examination revealed complex papillary structures and glandular spaces. The papillary structures showed a central vascular core and the cells lining them were columnar. They showed stratification and multilayering of cells with nuclear crowding [Figure 1]. The cells had large oval to irregular hyperchromatic nuclei, numerous mitotic figures and large areas of necrosis were also seen [Figure 2]. However few areas showed a more well-differentiated tumor with papillae lined by cells showing minimal atypia, loss of polarity and occasional mitotic figures. Immunohistochemical analysis for S-100, Cytokeratin, GFAP, and p53 protein showed positivity [Figures 3–5]. Based on the morphology a diagnosis of choroid plexus carcinoma was rendered.
Figure 1.
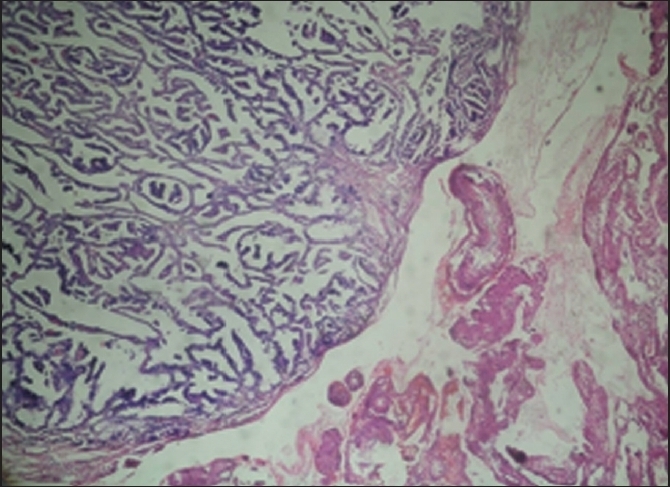
Complex papillary structures having a central vascular core with large areas of necrosis
Figure 2.
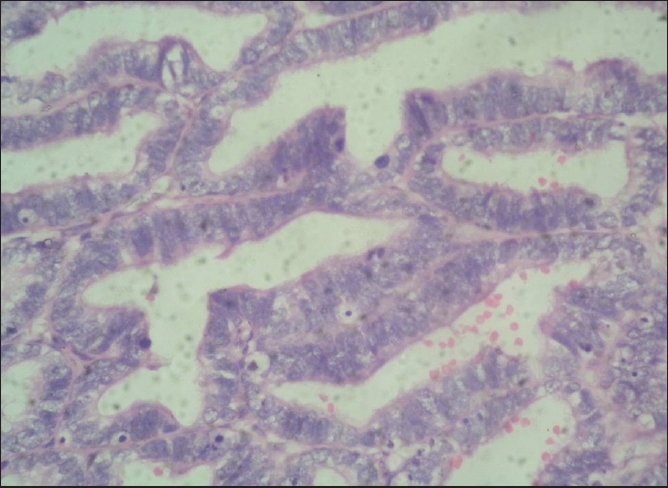
Lining cells show stratification and multilayering with large oval nuclei and show numerous mitotic figures
Figure 3.
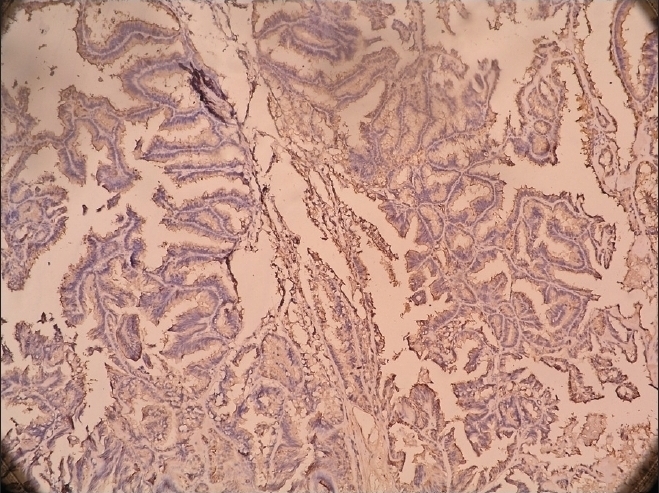
S-100 positivity
Figure 5.
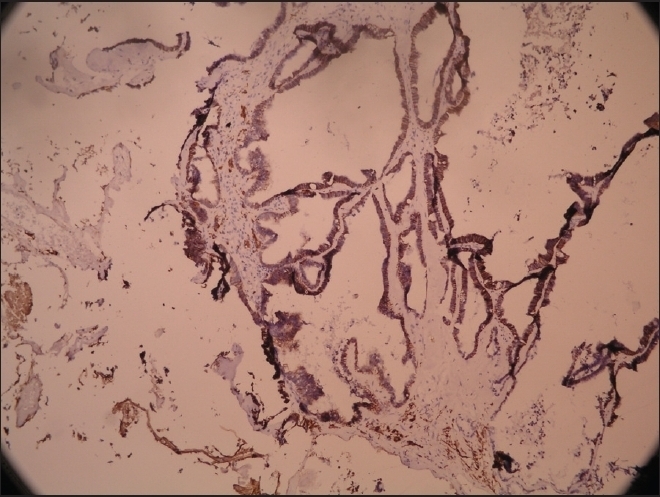
GFAP positivity
Figure 4.
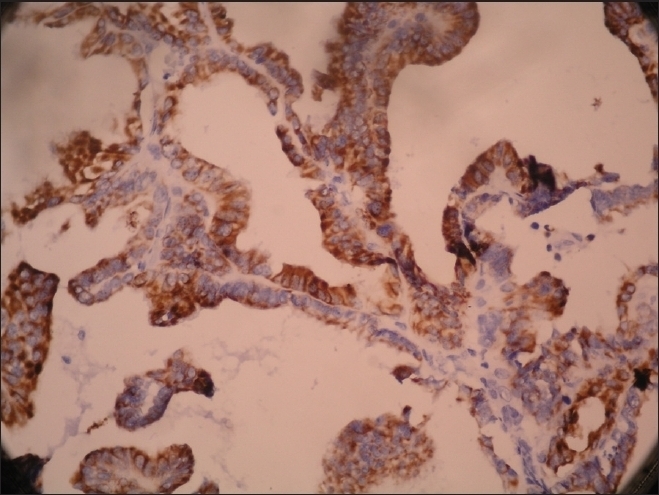
Pan cytokeratin positivity
Discussion
Choroid plexus papillomas comprise 1% of all brain tumors and malignant progression to carcinoma is very uncommon though it has been reported a few times.[5–8] On microscopic examination an array of branching fibrovascular fronds is seen lined by single layer of uniform cuboidal or columnar cells. The atypical choroid papillomas show a complex architecture, cytologic atypia, and mitotic activity. These have an increased likelihood of recurrence and progression to carcinoma.
Choroid plexus carcinoma is an aggressive tumor that must be distinguished from choroid plexus papilloma and distinction between these entities can sometimes be difficult. The main distinguishing factors are presence of necrosis, mitotic activity, and growth pattern.
Choroid plexus carcinoma is a highly aggressive malignant tumor WHO grade-III[1] that usually presents with CSF obstruction commonly in the lateral ventricles (50%) followed by IV ventricle (40%), third ventricle (5%), and multiple ventricles (5%).[1] Almost all choroid plexus carcinomas are seen in children[9,10] and are extremely rare in adults.[3,4]
On cytologic squash smear preparations, the tumor is seen to have an irregular papillary architecture and comprising of pleomorphic cells with foci of necrosis and calcification. Grossly these tumors show a papillary or cauliflower-like appearance. Histologically the tumor shows a papillary pattern and pleomorphic lining cells. There is marked necrosis and mitotic activity that differentiates it from choroid plexus papilloma. A rare variant known as rhabdoid choroid plexus carcinoma has been described that shows solid sheets of undifferentiated cells and papillary features along with rhabdoid cells.[8]
The differential diagnosis includes choroid plexus papilloma, villous hypertrophy of choroid plexus, papillary variant of ependymoma or meningioma and metastatic papillary neoplasms. Differentiation is usually based on associated clinical, cytologic, morphologic, and immunohistochemical features. There is currently no established protocol for the treatment of choroid plexus carcinoma. The main goal is complete resection of the tumor that improves the prognosis. Chemotherapy and postsurgical radiotherapy may be considered if the patient is an adult.[11]
Based on the clinical and histopathological findings we diagnosed our case as a classical choroid plexus carcinoma except for the fact that this patient was an adult which is extremely rare. We also advised thorough investigation of the patient for a possible primary elsewhere with metastasis to the choroid plexus keeping in mind the age of patient. The tumor must be differentiated from metastatic carcinoma which is especially important in case of choroid plexus carcinoma in adults as it is an extremely rare tumor in adults.[9] None of the investigations revealed any primary tumor elsewhere in the body. Metastasis has been reported in the literature from renal cell carcinoma,[10] esophagus,[12] and carcinoma of thyroid.[4]
On immunohistochemical staining, it was found that the tumor was positive for S-100, cytokeratin and GFAP. This combination also favors the diagnosis of choroid plexus carcinoma as also suggested in reviews.[11] Immunohistochemistry for p53 also revealed positivity in this case. Many cases of choroid plexus carcinoma have been seen to be associated with Li-Fraumeni syndrome that is an autosomal dominant disorder involving a mutation in germ cell line of p53 tumor suppressor gene on chromosome 17p13.1.[12,13] It is even thought that the presence of choroid plexus carcinoma could be taken as an indicator of a p53 mutation specially in the setting of a family history of cancer.[11]
Summary
An adult patient presented with a right temporal intraventricular tumor with a cystic component also extending up to the cortex. Histological examination revealed features of a choroid plexus carcinoma. The patient went through a complete search for a possible primary keeping in mind the differential diagnosis of metastatic carcinoma that is more common in adults but there was no evidence of any other tumor. Finally a diagnosis of choroid plexus carcinoma was rendered although it a rare entity among adults.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tomiyama A, Nakayama H, Aoki K, Ueda M. Solitary metastasis of renal cell carcinoma to the third ventricular choroid plexus with rapid clinical manifestation by intratumoral hemorrhage. Neurol India. 2008;56:179–81. doi: 10.4103/0028-3886.41997. [DOI] [PubMed] [Google Scholar]
- 2.Sung WS, Dubey A, Erasmus A, Hunn A. Solitary choroid plexus metastasis from carcinoma of the oesophagus. J Clin Neurosci. 2008;15:594–7. doi: 10.1016/j.jocn.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bucerius J, Meyka S, Michael B, Biersack HJ, Eter N. Papillary thyroid carcinoma with an uncommon spread of hematogenous metastases to the choroid and the skin. J Natl Med Assoc. 2008;100:104–7. doi: 10.1016/s0027-9684(15)31183-4. [DOI] [PubMed] [Google Scholar]
- 4.Jeibmann A, Wrede B, Peters O, Wolff JE, Paulus W, Hasselblatt M. Malignant progression in choroid plexus papillomas. J Neurosurg. 2007;107:199–202. doi: 10.3171/PED-07/09/199. [DOI] [PubMed] [Google Scholar]
- 5.Tena-Suck ML, Gómez-Amador JL, Ortiz-Plata A, Salina-Lara C, Rembao-Bojórquez D, Vega-Orozco R. Rhabdoid choroid plexus carcinoma: A rare histological type. Arq Neuropsiquiatr. 2007;65:705–9. doi: 10.1590/s0004-282x2007000400032. [DOI] [PubMed] [Google Scholar]
- 6.Tena Sanabria ME, Herrera Sánchez D, Hernández López J, Huicochea Montiel JC, Rodríguez A. Li-Fraumeni familial cancer syndrome: Case report and review of the literature. Acta Ortop Mex. 2007;21:99–104. [PubMed] [Google Scholar]
- 7.Berger C, Thiesse P, Lellouch-Tubiana A, Kalifa C, Pierre-Kahn A, Bouffet E. Choroid plexus carcinomas in childhood: Clinical features and prognostic factors. Neurosurgery. 1998;42:470–5. doi: 10.1097/00006123-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Cornford ME. Coincident choroid plexus carcinoma and adrenocortical carcinoma with elevated p53 expression. A case report of an 18-month-old boy with no family history of cancer. Arch Pathol Lab Med. 2001;126:70–2. doi: 10.5858/2002-126-0070-CCPCAA. [DOI] [PubMed] [Google Scholar]
- 9.Dani RD, Gandhi V, Thakkar G, Patel P, Kiran P. Choroid plexus carcinoma: A rare case. Indian J Radiol Imaging. 2004;14:419–22. [Google Scholar]
- 10.Wyatt SS, Price R, Holthouse D, Elsaleh H. Choroid plexus carcinoma in an adult. Australas Radiol. 2001;45:369–71. doi: 10.1046/j.1440-1673.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- 11.Gopal P, Parker JR, Debski R, Parker JC., Jr Choroid plexus carcinoma. Arch Pathol Lab Med. 2008;132:1350–4. doi: 10.5858/2008-132-1350-CPC. [DOI] [PubMed] [Google Scholar]
- 12.Krutilkova V, Trkova M, Fleitz J, Gregor V, Novotna K, Krepelova A, et al. Identification of five new families strengthens the link between childhood choroid plexus carcinoma and germline TP53 mutations. Eur J Cancer. 2005;41:1597–603. doi: 10.1016/j.ejca.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Chow E, Jenkins JJ, Burger PC, Reardon DA, Langston JW, Sanford RA, et al. Malignant evolution of choroid plexus papilloma. Pediatr Neurosurg. 1999;31:127–30. doi: 10.1159/000028847. [DOI] [PubMed] [Google Scholar]


