Abstract
Adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide (formerly called AF-2364), is a potent analog of lonidamine [1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid] known to disrupt germ cell adhesion, most notably elongating and elongated spermatids, in the seminiferous epithelium of adult rat testes and thus, leads to infertility in rats. Since the population of spermatogonia and spermatogonial stem cells (SSCs) in the seminiferous tubules is not significantly reduced by the treatment of rats with adjudin, adjudin-induced infertility is highly reversible, which enables reinitiation of spermatogenesis and germ cell re-population of the voided seminiferous epithelium. Furthermore, adjudin appears to exert its effects at the testis-specific atypical adherens junction (AJ) type known as ectoplasmic specialization (ES), most notably the apical ES at the Sertoli cell-spermatid interface. Thus, the hypothalamic-pituitary-gonadal axis is not unaffected and systemic side-effects are minimal. This also makes adjudin a potential candidate for male contraceptive development. Herein, we critically evaluate recent findings in the field and provide an updated model regarding the mechanism underlying adjudin-induced apical ES disruption. In short, adjudin targets actin filament bundles at the apical ES, the hallmark ultrastructure of this testis-specific junction type not found in any other epithelia/endothelia in mammals, by suppressing the expression of Eps8 (epidermal growth factor receptor pathway substrate 8), an actin capping protein that also plays a role in actin bundling, so that actin filament bundles can no longer be maintained at the apical ES. This is concomitant with a mis-localization of Arp3 (actin-related protein 3, a component of the Arp2/3 complex that induces actin nucleation/branching) recruited by drebrin E, causing “unwanted” actin branching, further destabilizing actin filament bundles at the apical ES. Additionally, adjudin blocks the expression of PAR6 (partitioning defective protein 6) and 14-3-3 (also known as PAR5) considerably at the apical ES, disrupting the homeostasis of endocytic vesicle-mediated protein trafficking, which in turn leads to an increase in protein endocytosis. The net result of these changes destabilizes cell adhesion and induces degeneration of the apical ES, causing premature release of spermatids, mimicking spermiation.
Key words: adjudin, AF-2364, testis, spermatogenesis, apical ectoplasmic specialization, Eps8, Arp2/3 complex, drebrin E, seminiferous epithelial cycle, male contraception
Introduction
Lonidamine was first developed as an anti-cancer drug in the late 1960s, and it is a member of a new class of indazole-based compounds.1,2 Besides suppressing glycolysis in cancer cells by inhibiting hexokinase in mitochondria,3 most notably following radiation that sensitizes the cancer cells,4,5 studies have shown that lonidamine displays potent anti-spermatogenic effects by depleting germ cells from the seminiferous epithelium in rodents and primates.6–9 Lonidamine exerts its anti-spermatogenic effects, at least in part, by perturbing the specialized cell adhesion site at the Sertoli cell-elongating/elongated spermatid interface,10 most notably the actin-based cytoskeleton at the apical ES.11 These findings thus spark the interest among investigators, hoping to develop lonidamine into a potential male contraceptive.5,12 This attempt, however, was subsequently abandoned due to the toxicity of lonidamine found in early stage cancer patients,13,14 making it unsuitable for healthy men. However, lonidamine is still being used as an anti-cancer drug in Europe and Japan for advanced/late stage cancer patients, and it is also being actively investigated for late stage metastatic cancer treatment,15,16 including prostatic disorders,17 with some promising results.18–20 After more than a decade of intensive investigations in our laboratory by screening dozens of new analogs and derivatives of lonidamine, we first reported findings of a potential analog known as AF-2364, subsequently called adjudin because of its action to induce apical ES disruption,21 in 2001.22 In short, adjudin is a potent male contraceptive that induces germ cell exfoliation by exerting its effects primarily at the apical ES, causing transient male infertility (see Fig. 1).24 Since the adhesion of spermatogonia and SSCs at the stem cell niche is not affected, fertility rebounds over time when germ cells repopulate the voided seminiferous tubules via spermatogenesis.30 While adjudin is less toxic when compared with lonidamine based on acute toxicity test, chromosomal aberration test and mutagenicity study,31 the margin between its efficacy and safety still remains too narrow based on a sub-chronic toxicity study in rats.31 A new formulation of adjudin has been developed in our laboratory which maintains its potency but requires >10-fold less adjudin to exhibit its reversible anti-fertility effects (Mruk DD, Bonanomi M, Silvestrini B and Cheng CY, unpublished findings). However, these latest efficacy findings are outside the scope of this Opinion article, which will be reported in a forthcoming study.
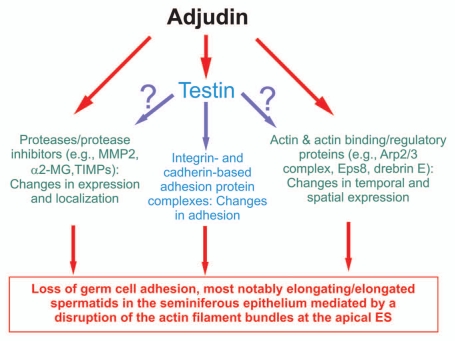
Figure 1.
A summary of effects of adjudin that impede spermatogenesis based on studies in the rat. This figure summarizes recent findings in the field regarding the effects of adjudin on different proteins, protein complexes or cellular structures in the seminiferous epithelium of rat testes. While testin and actin are the molecular targets of adjudin,23 it is not known at present if testin is also involved in the adjudin-mediated effects on the homeostasis of proteases/protease inhibitors and the actin-based cytoskeletal at the apical ES. This figure was prepared based on recent reviews in references 12 and 24–29. MMP2, matrix metalloproteinase 2; α2-MG, α2-macroglobulin; TIMPs, tissue metalloproteinases.
Herein, we focus on recent studies that delineate the mechanism of action of adjudin at the ultrastructures residing at the Sertoli cell-spermatid interface known as the apical ectoplasmic specialization (apical ES). Adjudin perturbs the restricted temporal and spatial expression of several actin-binding and regulatory proteins: Eps8,32 Arp3 33 and drebrin E.34 Furthermore, adjudin also disrupts the expression of polarity proteins, such as PAR6 (partitioning defective protein 6)35 and 14-3-3 (also known as PAR5),36 whose considerable loss at the apical ES leads to an increase in protein endocytosis. The net result of these changes alters the tightly packed actin filament bundles at the apical ES, causing their de-bundling and degeneration,35 and the loss and/or re-distribution of adhesion protein complexes at the apical ES via an increase in protein endocytosis further destabilizes the apical ES function, thereby disrupting adhesion and leading to spermatid loss from the epithelium. This is analogous to the events during spermiation that lead to germ cell loss from the epithelium. Since other epithelial/endothelial cells lack the unique features of the apical ES, namely the tightly packed bundles of actin filaments that lie perpendicular to the plasma membrane in Sertoli cells at the apical ES, the systemic toxicity of adjudin is thus minimal when compared with lonidamine. It is also noted that adjudin does not appear to exert its effects by affecting Sertoli and/or germ cell cells in the testis per se except the apical ES. Furthermore, neither the sperm reserve nor cell adhesion of the epithelial cells in the epididymis are affected since fertility in adjudin-treated rats can be maintained for up to ∼2–3-wk (without gross morphological/anatomical changes in new born pups) even though tubules are devoid of all germ cells in the seminiferous epithelium except spermatogonia/spermatogonial stem cells.24,37
Adjudin and Apical ES
Ectoplasmic specialization (ES), a testis-specific actin-based AJ type, is restricted to the: (1) Sertoli-spermatid interface from step 8–19 spermatids during spermiogenesis in the rat testis (known as apical ES). It is noted that in step 1–7 spermatids, cell adhesion is conferred by desmosome and gap junction without ES. Once apical ES appears, it is the only anchoring device that anchors elongating spermatids onto the Sertoli cell in the seminiferous epithelium, and it maintains both cell adhesion and spermatid polarity (orientation) until spermiation; (2) Sertoli-Sertoli cell interface at the blood-testis barrier (BTB), known as basal ES, which coexists with both TJ and gap junction to constitute the BTB. Thus, as opposed to apical ES, basal ES never exists on its own.12,38–41 This thus explains a recent report illustrating that the basal ES at the BTB is less susceptible to adjudin treatment versus the apical ES,30 because other coexisting junctions (e.g., TJ, gap junction, desmosome) at the BTB can maintain its integrity even if the basal ES is being disrupted by adjudin. Unlike actin-based AJ found in all other epithelia/endothelia, ES is typified by the presence of actin filament bundles that lie perpendicular to the plasma membrane and sandwiched in between cisternae of endoplasmic reticulum and the apposing plasma membrane of either Sertoli cell and spermatid in the apical ES or two Sertoli cells in the basal ES.12,39,40 However, these unique actin filament bundles are restricted only to the Sertoli cell at the ES, and no obvious ultrastructures can be detected in the elongating/elongated spermatid at the apical ES by electron microscopy, but they are found in both sides of the adjacent Sertoli cells at the basal ES. The actin filament bundles at the ES also confer the unusual adhesive strength of the apical ES. This is demonstrated by the observation that the mechanical force required to “pull” spermatids (step 8–19) away from Sertoli cells is almost 3 time stronger than that sufficient for detaching pre-step 8 spermatids, which are mainly anchored by desmosome.42 Yet desmosome is being considered to be one of the strongest adhesion junction types which uses intermediate filament for its attachment.43,44 Despite the remarkable adhesive force, apical ES is highly susceptible to adjudin since the presence of adjudin makes the apical ES more vulnerable than desmosome to be disrupted.45 While basal ES is structurally similar to the apical ES, adjudin was found to have minimal effects on the basal ES,46 even when adjudin was used at a dose 5-fold higher than its effective dose (250 vs. 50 mg/kg b.w.), it still took at least 2-wk before basal ES at the BTB was disrupted when apical ES was found to be disrupted within ∼6-h.30 This is likely due to the presence of co-existing junctions at the basal ES, namely TJ and/or gap junction, which constitute the BTB. Collectively, these findings illustrate that apical ES is one of the primary cellular targets of adjudin in the rat testis.23
Eps8, Arp2/3 Complex and Drebrin E in the Seminiferous Epithelium of Rat Testes during the Seminiferous Epithelial Cycle of Spermatogenesis
Eps8 is a multifunctional actin regulatory protein known to regulate actin bundling,47,48 thereby maintaining the unique actin filament bundles at the apical ES. On the other hand, Arp3 is a component of the Arp2/3 complex. In the presence of nucleation promoting factors such as cortactin and WASP (Wiskott-Aldrich syndrome protein) family proteins, including N-WASP (neuronal WASP) and SCAR/WAVE (suppressor of cAMP receptor/WASP family verprolin homologous), activated Arp2/3 complex induces actin nucleation/branching,47,48 perturbing the actin filament bundles at the apical ES. Interestingly, drebrin E (developmentally regulated brain protein E), is an actin-binding protein without any intrinsic catalytic function on the actin filament network per se.49 However, recent studies have shown that the pattern of stage-specific expression of drebrin E and its localization in the seminiferous epithelium mimic closely to that of Arp3.32,34 More important, drebrin E was found to have high affinity for Arp3, but not Eps8, and it structurally interacted with Arp3 and co-localized with Arp3 at the apical ES.34
In the normal testis, Eps8 was found to be highly expressed at the apical ES in stage V to early stage VIII, which appears to play a role in maintaining the actin filament bundles, but it became considerably diminished to a level virtually undetectable by late stage VIII, perhaps preparing the apical ES for spermiation.32 For Arp3, its expression is low at the apical ES in stage V tubules, apparently it is being used to allow restricted temporal and spatial actin branching to occur at the apical ES to facilitate the transit of elongating spermatids across the epithelium.33 However, Arp3 became highly expressed at the apical ES at stage VII, but it was restricted exclusively to the concave side of the spermatid head,33 at the site where endocytic vesicle-mediated protein trafficking is known to occur, and this ultrastructure was formerly designated tubulobulbar complex (apical TBC),48,50,51 and its expression dropped to an undetectable level at stage VIII of the epithelial cycle.33 Interestingly, the pattern of stage-specific expression and localization of drebrin E was found to closely mimic Arp3, and since drebrin E was found to structurally interact with Arp3,34 it seems that drebrin E recruits Arp3 to the apical ES site to execute its actin branching activity.
The highly restricted temporal and spatial expression of these three actin regulatory and/or binding proteins in the seminiferous epithelium during the epithelial cycle appear to regulate the actin filament bundles at the apical ES, from their bundling to their branched conformation and vice versa. This thus confers “plasticity” to the apical ES to facilitate the transit of elongating spermatids across the seminiferous epithelium during spermiogenesis and to prepare fully developed spermatids (i.e., spermatozoa) to be released from the epithelium at spermiation.
PAR6 and 14-3-3 in the Seminiferous Epithelium of Rat Testes during the Seminiferous Epithelial Cycle of Spermatogenesis
PAR6 and 14-3-3 (also known as PAR5) are two polarity proteins initially identified in C. elegans, which work together with the Crumbs and the Scribble polarity protein complexes to confer apico-basal cell polarity in non-mammalian and mammalian tissues, including the seminiferous epithelium of rat testes.52–54 Subsequent studies have shown that 14-3-3 is a multi-protein binding protein with at least 7 isoforms in mammals, and each 14-3-3 protein has as many as 200 partner proteins.55 Thus, 14-3-3 serves as a critical adaptor to recruit multiple proteins to a specific cellular site and/or domain in an epithelial cell, such as a Sertoli cell, to exert important cellular functions. In the seminiferous epithelium of rat testes, the expression of PAR6 is stage-specific.35 PAR6 was prominently localized to the apical ES, surrounding the entire heads of elongating spermatids in stage V to early VIII tubules, but diminished rapidly thereafter and it was virtually undetectable at the apical ES by late stage VIII.35 It was shown that in rats treated with adjudin, the induced premature loss of spermatid was associated with a significant loss of PAR6 at the apical ES, and these PAR6-depleted spermatids also lost: (i) their polarity/orientation with their heads no longer pointing toward the basement membrane and (ii) their adhesion,35 indicating that besides conferring polarity to spermatids, PAR6 may also confer cell adhesion at the apical ES. Interestingly, the expression and localization of 14-3-3 was found to closely mimic the pattern of PAR6 in the rat testis.36 Studies have shown that both proteins are important to maintain the homeostasis of endocytic vesicle-mediated protein trafficking in the Sertoli cell blood-testis barrier (BTB), since a knockdown of either PAR3 or 14-3-3 was found to accelerate the kinetics of integral membrane protein internalization at the BTB, such as JAM-A and N-cadherin.35,36 Collectively, these findings illustrate the possibility that PAR6 may work in concert with 14-3-3 to regulate apical ES function via their effects on the kinetics of protein endocytosis.
Adjudin Alters the Highly Restricted Temporal and Spatial Expression of Eps8, Arp3, Drebrin E, PAR6 and 14-3-3 in the Seminiferous Epithelium
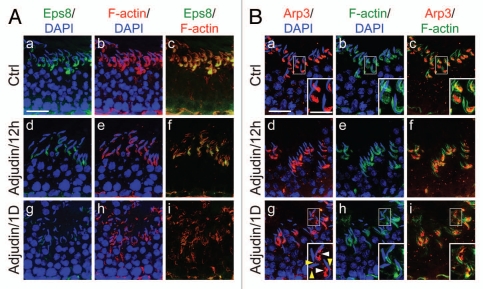
Following treatment of rats with adjudin, this male contraceptive was found to modulate the restricted temporal and spatial expression of Eps8,32 Arp3 33 and drebrin E34 in the seminiferous epithelium. For instance, as shown in Figure 2A, Eps8 was found to be restricted to the apical ES, co-localizing with F-actin at the site in normal rat testes. However, the expression of Eps8 considerably diminished within 12-h following adjudin treatment; by 1-d, Eps8 was almost undetectable at the apical ES, which was associated with truncation and mis-localization of F-actin, with apparent degeneration of actin filament bundles (Fig. 2A). For Arp3, it was also found to be restricted to the apical ES, most notably the concave side of the spermatid heads in stage VII tubules in normal rat testes as earlier reported (Fig. 2B).33 By 12-h after adjudin treatment, Arp3 was found to become truncated and fragmented; and by 1-d, Arp3 was no longer highly restricted to the concave side of the spermatid heads and became even more truncated and fragmented (Fig. 2B). Interestingly, this pattern, again, is analogous to drebrin E as recently reported,34 suggesting drebrin E recruited Arp3 to these sites so that it become mis-localized, and to induce actin branching, facilitating degeneration of the actin filament bundles at the apical ES.
Figure 2.
Adjudin disrupts the restricted temporal and spatial expression of Eps8 and Arp3 in the rat testis. Dual-labeled immunofluorescence analysis was used to assess changes in the adjudin-mediated disruption of the highly restricted spatial and temporal expression of Eps8 (green) and F-actin (red) shown in (A) vs. Arp3 (green) and F-actin shown in (B) in the seminiferous epithelium of rat testes as earlier described in reference 32 and 33. In (A and B), the control (Ctrl) cross-sections illustrate a stage VII tubule from normal rat testes. By 12 h (hour) following adjudin treatment, a considerable loss of Eps8 was detected at the apical ES, and by 1D (day), Eps8 was virtually not detected, and this loss of Eps8, an actin bundling protein at the apical ES, was found to associate with a truncation of F-actin due to the loss of the organized actin filament bundles at the apical ES (see d–f and g–i vs. a–c in A). For Arp3, it displayed a distinctive different pattern vs. Eps8 following adjudin treatment. While both Eps8 and Arp3 were prominently expressed at the apical ES at stage VII in control rats (see a–c in B vs. a–c in A), however, Arp3 was highly restricted to the concave side of the spermatid head where endocytic vesicle-mediated protein trafficking is known to occur.40 By 12 h following adjudin treatment, Arp3 became mis-localized and this mis-localization was more obvious by 1D after adjudin treatment. For instance, by 1D, Arp3 was found to become truncated (see “white” arrowheads in the enlarged inset in B:g). Additionally, Arp3 was also found to be mis-localized, surrounding other parts of the spermatid head, instead of restricted to the concave side of the spermatid head (see “yellow” arrowheads in the enlarged inset in B:g). Thus, the actin filament bundles at the apical ES were transformed to a branched network because Arp3 induces actin nucleation/branching. The combined changes of these two actin regulatory proteins induced by adjudin thus led to the degeneration of the actin filament bundles at the apical ES. These changes coupled with changes in drebrin E, PAR6 and 14-3-3 as described in the main text destabilizes the apical ES, rendering a loss of adhesion at the apical ES which leads to spermatid loss from the epithelium. Bar in A:a or B:a = 40 µm, which applies to b–i in A or B; bar in inset in B:a = 20 µm, which applies to insets in b–c and g–i.
More interesting, the adjudin-induced disappearance of Eps8 and mis-localization and/or truncation of Arp3 and drebrin E were also found to associate with a loss of PAR6 35 and 14-3-3 36 proteins at the apical ES. Earlier studies have also demonstrated that PAR6 and 14-3-3 are necessary to maintain proper endocytic vesicle-mediated trafficking in Sertoli cells at the basal ES since their knockdown by RNAi was found to induce an acceleration of integral membrane protein internalization.35,36 Taken collectively, we speculate that the adjudin-induced loss of PAR6 and 14-3-3 at the apical ES also accelerates endocytic vesicle-mediated protein internalization, thereby further destabilizing cell adhesion at the apical ES. The net result of these changes leads to spermatid loss from the epithelium as depicted in Figure 3.
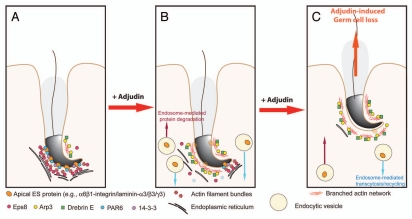
Figure 3.
A hypothetical model that summarizes the mechanism by which adjudin induces spermatid loss from the seminiferous epithelium of adult rat testes. In a normal rat testis (A), apical ES adhesion is maintained by the apical ES adhesion protein complexes (e.g., α6β1-integrin-laminin-α3β3γ3, N-cadherin-β-catenin, nectin-2/3-afadin) using the highly organized actin filament bundles for attachment. The actin filament bundles at the apical ES are maintained by the Eps8, PAR6 and 14-3-3. However, following exposure of rats to adjudin, this drug disrupts the highly restricted temporal and spatial expression of Eps8, Arp3, drebrin E, PAR6 and 14-3-3 in the seminiferous epithelium as detailed in the text. In short, Eps8 considerably diminishes at the apical ES with a concomitant mis-localization and truncation of Arp3, the actin filament bundles thus become disrupted and replaced with actin branched network. At the same time, PAR6 and 14-3-3 also become diminished, accelerating endocytic vesicle-mediated internalization of integral membrane proteins (e.g., integrins, cadherins, nectins), thereby destabilizing the apical ES (B), which eventually leads to the premature loss of spermatids from the epithelium, mimicking spermiation as shown in (C).
Concluding Remarks and Future Perspectives
The model depicted in Figure 3 summarizes our latest findings regarding the likely mechanism of action by which adjudin induces spermatid loss from the seminiferous epithelium. Adjudin exerts its effects by disrupting the highly restricted temporal and spatial expression of actin regulatory proteins: Eps8, Arp3 and drebrin E; as well as polarity proteins: PAR6 and 14-3-3. While these two groups of proteins are not thought to be physiologically related, adjudin somehow exerts its effects via these two groups of proteins as partners to modulate the most critical component, namely the actin filament bundles, at the apical ES. It is our belief that these findings not only provide the mechanistic insights on the action of adjudin in the seminiferous epithelium, they will enhance our understanding in the intricate interactions of polarity proteins and actin binding/regulatory proteins, and how these two seemingly unrelated groups of proteins regulate spermatogenesis, in particular spermiogenesis and spermiation.52,56,57 Furthermore, multiple targets are revealed based on the information depicted in Figure 3, which can serve as candidates for male contraceptive development.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD, U54 HD029990, Project 5 to C.Y.C.; R01 HD056034 and R01 HD056034-02-S1 to C.Y.C.).
References
- 1.Silvestrini B, Palazzo G, De Gregorio M. Lonidamine and related compounds. Prog Med Chem. 1984;21:110–135. [PubMed] [Google Scholar]
- 2.Corsi G, Palazzo G, Germani C, Scorza Barcellona P, Silvestrini B. 1-Halobenzyl-1H-indazole-3-carboxylic acids. A new class of antispermatogenic agents. J Med Chem. 1976;19:778–783. doi: 10.1021/jm00228a008. [DOI] [PubMed] [Google Scholar]
- 3.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakara S, Kalia VK. Optimizing radiotherapy of brain tumours by a combination of temozolomide and lonidamine. Indian J Med Res. 2008;128:140–148. [PubMed] [Google Scholar]
- 5.Silvestrini B. Basic and applied research in the study of indazole carboxylic acids. Chemotherapy. 1981;27:9–20. doi: 10.1159/000238040. [DOI] [PubMed] [Google Scholar]
- 6.Lobl TJ, Bardin CW, Gunsalus GL, Musto NA. Effects of lonidamine (AF 1890) and its analogues on follicle-stimulating hormone, testosterone and rat androgen binding protein concentrations in the rat and rhesus monkey. Chemotherapy. 1981;27:61–76. doi: 10.1159/000238046. [DOI] [PubMed] [Google Scholar]
- 7.Lobl TJ. 1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid (DICA), an exfoliative antispermatogenic angent in the rat. Arch Androl. 1979;2:353–363. doi: 10.3109/01485017908987337. [DOI] [PubMed] [Google Scholar]
- 8.Traina ME, Guarino M, Urbani E, Saso L, Eleuteri P, Cordelli E, et al. Lonidamine transiently affects spermatogenesis in pubertal CD1 mice. Contraception. 2005;72:262–267. doi: 10.1016/j.contraception.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Maranghi F, Mantovani A, Macri C, Romeo A, Eleuteri P, Leter G, et al. Long-term effects of lonidamine on mouse testes. Contraception. 2005;72:268–272. doi: 10.1016/j.contraception.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 10.De Martino C, Malcorni W, Bellocci M, Floridi A, Marcante M. Effects of AF1312 TS and lonidamine on mammalian testis. A morphological study. Chemotherapy. 1981;27:27–42. doi: 10.1159/000238043. [DOI] [PubMed] [Google Scholar]
- 11.Malorni W, Meschini S, Matarrese P, Arancia G. The cytoskeleton as a subcellular target of the anti-neoplastic drug lonidamine. Anticancer Res. 1992;12:2037–2045. [PubMed] [Google Scholar]
- 12.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berruti A, Bitossi R, Gorzegno G, Bottini A, Alquati P, De Matteis A, et al. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of phase III study with a factorial design. J Clin Oncol. 2002;20:4150–4159. doi: 10.1200/JCO.2002.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Papaldo P, Lopez M, Cortesi E, Cammilluzzi E, Antimi M, Terzoli E, et al. Addition of either lonidamine or granulocyte colony-stimulating factor does not improve survival in early breast cancer patients treated with high-dose epirubicin and cyclophosphamide. J Clin Oncol. 2003;21:3462–3468. doi: 10.1200/JCO.2003.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Milane L, Duan Z, Amiji M. Therapeutic efficacy and safety of paclitaxel/lonidamine loaded EGFR-targeted nanoparticles for the treatment of multi-drug resistant cancer. PLoS ONE. 2011;6:24075. doi: 10.1371/journal. pone.0024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calviño E, Estañ MC, Simon GP, Sancho P, Boyano-Adanez MD, de Blas E, et al. Increased apoptotic efficacy of lonidamine plus arsenic trioxide combination in human leukemia cells. Reactive oxygen species generation and defensive protein kinase (MEK/ERK, AktmTOR) modulation. Biochem Pharmacol. 2011 doi: 10.1016/j.bcp.2011.08.017. In press. [DOI] [PubMed] [Google Scholar]
- 17.Brawer MK. Lonidamine: basic science and rationale for treatment of prostatic proliferative disorders. Rev Urol. 2005;7:21–26. [PMC free article] [PubMed] [Google Scholar]
- 18.De Marinis F, Rinaldi M, Ardizzoni A, Bruzzi P, Pennucci MC, Portalone L, et al. The role of vindesine and lonidamine in the treatment of elderly patients with advanced non-small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force. Tumori. 1999;85:177–182. doi: 10.1177/030089169908500306. [DOI] [PubMed] [Google Scholar]
- 19.Amadori D, Frassineti GL, De Matteis A, Mustacchi G, Santoro A, Cariello S, et al. Modulating effect of lonidamine on response to doxorubicin in metastatic breast cancer patients: rsults from a multicenter prospective randomized trial. Breast Cancer Res Treat. 1998;49:209–217. doi: 10.1023/A:1006063412726. [DOI] [PubMed] [Google Scholar]
- 20.Dogliotti L, Danese S, Berutti A, Zola P, Buniva T, Bottini A, et al. Ciplatin, epirubicin and lonidamine combination regimen as first-line chemotheraphy for metastatic breast cancer: a pilot study. Cancer Chemother Pharmacol. 1998;41:333–338. doi: 10.1007/s002800050747. [DOI] [PubMed] [Google Scholar]
- 21.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- 23.Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis. 2011;1:137–146. doi: 10.4161/spmg.1.2.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Mok KW, Lie PPY, Mruk DD, Mannu J, Mathur PP, Silverstini B, Cheng CY. The apical ectoplasmic specialization-blood-testis barrier functional axis is a novel target for male contraception. Biology and Regulation of Blood-Tissue Barriers. 2011 doi: 10.1007/978-1-4614-4711-5_17. http://www.landesbioscience.com/curie/chapter/5148/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok KW, Mruk DD, Lie PPY, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminifeorus epithelium of mammalian testes. Reproduction. 2011;141:571–580. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CY, Mruk DD. New frontiers in non-hormonal male contraception. Contraception. 2010;82:476–482. doi: 10.1016/j.contraception.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 30.Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012 doi: 10.1111/j.13652605.2011.01183.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 32.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li MWM, Xiao X, Mruk D, Lam YL, Lee WM, Lui WY, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- 38.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:A–AW. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogl AW, Vaid KS, Guttman JA. The Setoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 43.Green KJ, Simpson CL. Desmosomes: New perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 44.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by adjudin. J Androl. 2006;27:790–794. doi: 10.2164/jandrol.106.000422. [DOI] [PubMed] [Google Scholar]
- 46.Su L, Cheng CY, Mruk DD. Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo. Int J Biochem Cell Biol. 2010;42:1864–1875. doi: 10.1016/j.biocel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng CY, Mruk DD. Actin binding proteins and spermatogenesis. Some unexpected findings. Spermatogenesis. 2011;1:99–104. doi: 10.4161/spmg.1.2.16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact inter-cellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 51.Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL) and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 52.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assémat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 54.Cheng CY, Wong EWP, Lie PPY, Li MWM, Mruk DD, Yan HHN, et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: An unexpected turn of events. Spermatogenesis. 2011;1:105–115. doi: 10.4161/spmg.1.2.15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S, Wong EWP, Li MWM, Lee WM, Cheng CY. 14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis. J Endocrinol. 2009;202:327–336. doi: 10.1677/JOE-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: A versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]