Abstract
A mouse model with a Sertoli cell (SC)-selective ablation of the androgen receptor (AR)—the SCARKO mouse—demonstrated that the effects of androgens on spermatogenesis depend on the presence of an active AR in SC. This model has been extremely valuable in the study of the effects of androgens on the initiation of spermatogenesis. However, due to the early (prenatal) inactivation of the AR SCARKO mice develop a complete block in meiosis, making it impossible to study the effects of androgens on postmeiotic steps of germ cell development. It would therefore be of interest to develop a model in which the AR can be ablated at any chosen time point. Here we used a mouse line ubiquitously expressing a tamoxifen (TM)-inducible Cre recombinase to develop an inducible AR knockout model (iARKO). It is shown that treatment with TM (3 mg/day) for five consecutive days efficiently inactivates the AR in testicular tissue and decreases the expression of known AR-target genes in SC (Rhox5, Spinlw1) without markedly affecting testicular cell composition one day after the final injection. TM treatment did, however, decrease serum gonadotropin levels and the expression of several Leydig cell genes (StAR, Cyp17a1, Insl3), resulting in decreased testosterone levels. Nevertheless, the intratesticular testosterone concentration still far exceeds the estimated concentrations required to saturate the AR. It may be concluded that the study of androgen-responsive postmeiotic genes in SC may be feasible using a TM-inducible AR knockout model provided that appropriate controls are included correcting for off-target effects of TM.
Key words: spermatogenesis, tamoxifen, androgen action, transgenesis, inducible knockout
Introduction
Androgens play a key role in the control of spermatogenesis but the mechanisms underlying their effects remain poorly understood.1–4 Various strategies have been used to identify the steps in germ cell development that depend most on androgens. The most classical approach has been to study the effects of a drastic reduction in the intratesticular concentration of testosterone as well as the result of subsequent treatment by exogenous androgens. Drastic decreases in intratesticular testosterone (or testosterone action) were obtained by a variety of techniques including: surgical or chemical hypophysectomy, administration of exogenous androgens and/or estrogens, administration of anti-androgens, immunoneutralization of LH and administration of the Leydig cell (LC) toxicant ethane dimethane sulphonate (EDS).2 These approaches have greatly contributed to our understanding of the specific role of androgens in the control of spermatogenesis but they show a number of inherent limitations. First, all these techniques reduce the intratesticular level of testosterone to only a few percent of the control value.5 Given the high intratesticular level of testosterone under control conditions, however, some residual androgen action cannot be excluded. Second, it may take a considerable amount of time before a maximal reduction in intratesticular testosterone is observed. Third, none of these techniques offers any information on the exact target cell(s) responsible for the observed effects of androgens. More recently, genetic models including mice with an inherent defect in the production of gonadotropins (hpg mice) and transgenic mice with a defect in the LH receptor (LuRKO) have been used to study the effects of exogenous androgens on germ cell development.6–8 Also in these models however, identification of the testicular target cells responsible for the observed effects remains impossible.
The development of the conditional knockout technology has opened novel perspectives for the study of androgen action in the testis and has created the possibility to avoid or reduce some of the limitations imposed by previous approaches. Various mouse models have now been developed in which the AR gene has been selectively inactivated in distinct testicular target cells. The main conclusion from these models is that the Sertoli cell (SC) plays a central role in the effects of androgens on spermatogenesis9–12 although androgen action on peritubular myoid cells (PTMs) is also essential to support SC development and function.13 Most of the available models of SC-selective AR inactivation (SCARKO models) have used a Cre (Cyclization recombination) recombinase driven by the Anti-Müllerian Hormone (AMH) promoter to inactivate the AR gene.9–12 In these models the AR gene in SCs is inactivated around day 15 of embryonic development, more than a week before the encoded AR protein becomes normally expressed in these cells. These SCARKO mice typically develop a block in meiosis,9,10,12 supporting the contention that meiotic progression is one of the most important steps controlled by androgens. However, from the experimental approaches mentioned in the previous paragraph and a model with a less complete knockout of the AR in SC which also suffers from a general decrease in AR expression,11 it is known that androgens also have effects on postmeiotic events in the spermatogenic cascade: they are essential for adhesion of round and elongated spermatids to SCs14–18 and they also play a crucial role in the control of spermiation.4,16,17,19–21 Given the complete block at the level of meiosis in SCARKO mice, the cellular and molecular mediators of the latter processes cannot be studied in the SCARKO model. In order to overcome this limitation it would be of interest to develop an inducible SCARKO (iSCARKO) model in which the expression of the Cre recombinase is controlled not only in space but also in time. This could be done, for instance, by placing the Cre recombinase under the control of a promoter that drives Cre expression in adult SCs and by replacing the classical Cre recombinase by an inducible variant. Tamoxifen (TM)-inducible Cre recombinases (produced by fusing the native Cre to a modified estrogen receptor ligand binding domain that selectively recognizes TM and its active metabolite 4-hydroxy(OH)-TM) have been described in reference 22. Since mice expressing a TM-inducible Cre selectively in SC are not yet available, we explored the feasibility of this type of approach by generating an inducible global AR knockout model (iARKO). iARKO mice were produced by crossing a commercially available mouse strain that ubiquitously expresses a TM-inducible Cre recombinase (Cre-ER™) 23 with a mouse strain carrying a floxed exon 2 of the AR (ARflox mice) generated in our laboratory. Special attention was given to the extent and time course of TM-induced AR inactivation in the testis and to potential off-target effects of TM treatment on testicular function.
Results
TM treatment reduces accessory sex organ weight and AR expression in iARKO mice.
To distinguish between effects due to TM-induced AR ablation and effects directly related to TM itself, both ARflox/y mice ubiquitously expressing a TM-inducible Cre recombinase (iARKO) and control mice carrying a mutated AR with a floxed exon 2 were treated with either TM or vehicle (Fig. 1). Routinely TM treatment (3 mg/day intraperitoneally for five consecutive days) was initiated on day 50 and animals were killed on day 55. In some experiments mice received a lower dose of TM (1 mg/day) or were killed on later time points (day 61 or 104).
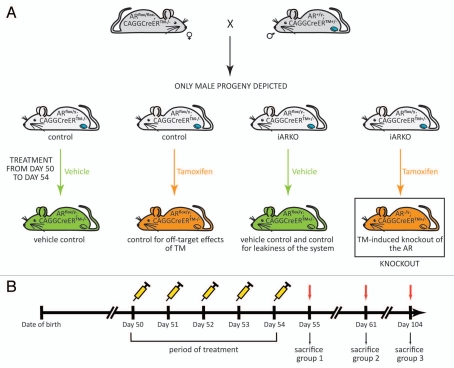
Figure 1.
Generation of iARKO mice and the tamoxifen (TM) induction protocol. (A) Mice with a ubiquitous knockout of the AR (iARKO) were generated by crossing female mice homozygous for a floxed AR allele (ARflox/flox) with male mice carrying a TM-inducible Cre recombinase (heterozygous; CAGGCreERTM+/−) as described in the Material and Methods section. As a result of this breeding scheme 50% of the male progeny carried the floxed AR allele as well as the ubiquitously expressed TM-inducible Cre recombinase (ARflox/y;CAGGCreERTM+/−: further referred to as iARKO mice) and 50% of the male progeny carried only the floxed AR allele (ARflox/y;CAGGCreERTM−/−: further referred to as control). Both control and iARKO mice were divided in a TM-treated (orange) and a control-treated (green) group. Knockout of the AR occurs only in TM-treated animals carrying a floxed AR allele and the TM-inducible Cre recombinase (AR−/y; CAGGCreERTM+/−; boxed mouse). Other treatment groups were included as a vehicle control, as a control for the effects of TM or as a control for the leakiness of the system (as indicated under each mouse). To derive a SC-specific knockout the promoter driving Cre expression has to be replaced by a promoter expressed specifically in adult SC. (B) A scheme of the TM induction protocol. From day 50 on male mice (iARKO and control) were injected intraperitoneally with TM (1 mg or 3 mg) or vehicle for five consecutive days. Animals were sacrificed and organs were collected one, seven or 50 d after the final treatment (day 55, 61 and 104 respectively).
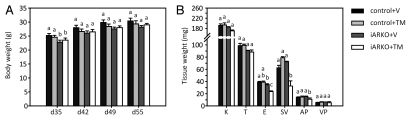
As shown in Figure 2A TM treatment (3 mg/day) does not affect body weight of control or iARKO animals as measured on day 55. It may be noted that control and iARKO mice also have identical body weights in the pretreatment period except on day 35 where—for unknown reasons—iARKO animals display a slight decrease in body weight. Figure 2B shows that under the same conditions TM treatment does not affect the weight of any of the studied androgen target organs (kidney, testis, epididymis, seminal vesicles, anterior prostate and ventral prostate) in control mice whereas in iARKO mice significant reductions in weight are observed for the epididymis, the seminal vesicles and the anterior prostate. At later time points after administration of TM (3 mg/day) the decrease in epididymis, seminal vesicle and anterior prostate weight in iARKO mice becomes more pronounced and a significant reduction in weight is also observed for testis, ventral prostate and kidney (Table 1). A similar decrease in androgen target organ weight is not observed in control mice (except for epididymis at day 61 and prostate at day 104) (Table 2). Comparable but somewhat less pronounced selective effects on target organ weight in iARKO animals are observed at day 55 after treatment with TM 1 mg/day (compare Tables 1 and 2). The observed effects on target organ weight are compatible with the hypothesis that TM selectively induces AR ablation in iARKO animals.
Figure 2.
Body and tissue weights of iARKO and control mice treated with vehicle (V) or tamoxifen (TM). (A) Body weights (mean ± SEM) of iARKO and control mice (ARflox/y) were measured before treatment (day 35, 42 and 49) and one day after treatment (day 55) with V or TM (3 mg/day for five consecutive days). (B) Tissue weights (mean + SEM) for kidney (K), testis (T), epididymis (E), seminal vesicle (SV), anterior prostate (AP) and ventral prostate (VP) measured one day after treatment (day 55). Control + V: control mice treated with vehicle (n = 7); control + TM: control mice treated with TM (n = 8); iARKO+V: iARKO mice treated with vehicle (n = 5); iARKO+TM: iARKO mice treated with TM (n = 5). ANOVA supplemented with a Fisher LSD test was used to compare body and tissue weights of different treatment groups. Values that differ significantly (p ≤ 0.05) are indicated by different lowercase letters (a–c).
Table 1.
Effect of tamoxifen (TM) treatment on tissue weights, testicular transcript levels and serum hormone levels in iARKO animals
| dosage TM/day | 1 mg | 3 mg | 3 mg | 3 mg | |
| sacrifice on day | day 55 | day 55 | day 61 | day 104 | |
| tissue weights | kidney | 95.9 ± 4.4 | 93.2 ± 1.9 | 83.2 ± 2.9* | 77.2 ± 6.2 |
| testis | 98.6 ± 5.5 | 95.9 ± 3.7 | 84.4 ± 5.0* | 31.9 ± 3.1* | |
| epididymis | 80.5 ± 3.5* | 67.9 ± 3.6* | 42.7 ± 2.8* | 35.1 ± 2.5* | |
| seminal vesicle | 54.1 ± 5.4* | 52.0 ± 6.2* | 13.2 ± 1.5* | 9.1 ± 1.4* | |
| anterior prostate | 65.3 ± 7.1* | 58.7 ± 7.3* | 37.7 ± 7.4* | 20.7 ± 3.9* | |
| ventral prostate | 82.4 ± 9.2 | 69.4 ± 19.8 | 52.9 ± 9.9* | 30.8 ± 6.5* | |
| q-RT-PCR on testis extracts | AR | 42.6 ± 5.9* | 13.7 ± 2.2* | 4.3 ±} 1.2* | 3.7 ± 1.4* |
| Rhox5 | 78.4 ± 6.3* | 51.1 ± 5.1* | 12.7 ± 1.6* | 4.8 ± 1.5* | |
| Spinlw1 | 112.2 ± 19. | 65.5 ± 4.9* | 40.4 ± 7.7* | 9.0 ± 2.9* | |
| StAR | 72.3 ± 10.1 | 117.1 ± 9.9* | 38.5 ± 6.0* | 137.2 ± 22.2 | |
| Cyp17a1 | 68.8 ± 9.5 | 68.7 ± 6.0* | 54.3 ± 9.2* | 69.7 ± 3.0* | |
| Insl3 | 44.6 ± 8.6* | 54.1 ± 6.8 | 5.1 ± 1.3* | 1.2 ± 0.2* | |
| serum level | FSH | ND | 63.2 ± 5.5* | ND | 65.0 ± 12.9 |
| LH | ND | 128.6 ± 32.5 | ND | 434.5 ± 63.0* | |
| Basal testosterone | ND | 57.3 ± 24.3 | ND | 41.6 ± 8.7 | |
| tubular diameter | ND | 94.9 ± 1.6 | 85.4 ± 1.3* | 68.5 ± 1.7* |
iARKO mice were treated with vehicle (V) or tamoxifen (TM; 1 or 3 mg/day) from day 50 on for five consecutive days. Tissue weights, testicular transcript levels (measured by qPCR) and serum hormone levels were measured one day after treatment (day 55), seven days after treatment (day 61) and 50 days after treatment (day 104). The data shown (mean ± SEM) represent values in iARKO mice treated with TM expressed relative to values observed in iARKO mice treated with V (arbitrarily assigned a value of 100). Statistical analysis was performed by ANOVA supplemented with a Fisher LSD test. Values that differ significantly (p ≤ 0.05) are indicated by an asterisk. ND = not determined.
Table 2.
Effect of tamoxifen (TM) treatment on tissue weights, testicular transcript levels and serum hormone levels in ARflox/y control animals
| dosage TM / day | 1 mg | 3 mg | 3 mg | 3 mg | |
| sacrifice on day | day 55 | day 55 | day 61 | day 104 | |
| tissue weights | kidney | 105.2 ± 4.9 | 99.2 ± 3.1 | 94.9 ± 4.8 | 117.1 ± 13.1 |
| testis | 104.9 ± 3.3 | 95.7 ± 2.9 | 102.8 ± 1.6 | 94.1 ± 6.6 | |
| epididymis | 104.9 ± 4.5 | 90.8 ± 4.5 | 83.2 ± 5.8* | 95.3 ± 4.0 | |
| seminal vesicle | 108.0 ± 8.0 | 118.6 ± 5.9 | 82.6 ± 12.2 | 89.6 ± 3.4 | |
| anterior prostate | 109.4 ± 7.7 | 105.2 ± 7.6 | 105.9 ± 16.6 | 73.9 ± 7.8 | |
| ventral prostate | 95.2 ± 10.7 | 114.0 ± 12.7 | 104.3 ± 7.3 | 60.6 ± 5.0* | |
| q-RT-PCR on testis extracts | AR | 82.6 ± 8.5 | 112.3 ± 7.8 | 82.4 ± 15.2 | 111.4 ± 21.8 |
| Rhox5 | 103.6 ± 11.6 | 109.0 ± 6.1 | 108.2 ± 20.1 | 111.5 ± 9.4 | |
| Spinlw1 | 137.4 ± 18.2 | 115.9 ± 6.1* | 91.3 ± 11.0 | 138.0 ± 11.0* | |
| StAR | 14.1 ± 1.7* | 26.7 ± 3.7* | 7.5 ± 0.9* | 82.9 ± 8.7 | |
| Cyp17a1 | 59.0 ± 8.7* | 70.9 ± 4.9* | 59.4 ± 4.1* | 99.2 ± 10.0 | |
| Insl3 | 65.8 ± 8.8 | 58.9 ± 5.3* | 11.9 ± 2.0* | 29.4 ± 5.9* | |
| serum level | FSH | ND | 76.7 ± 4.1* | ND | 96.7 ± 15.0 |
| LH | ND | 35.4 ± 3.4* | ND | 242.6 ± 74.8 | |
| Basal testosterone | ND | 11.3 ± 6.6* | ND | 25.4 ± 10.4 | |
| tubular diameter | ND | 104.3 ± 2.0 | 101.3 ± 3.9 | 95.4 ± 1.2* |
ARflox/y control mice were treated with vehicle (V) or tamoxifen (TM; 1 or 3 mg/day) from day 50 on for five consecutive days. Tissue weights, testicular transcript levels (measured by qPCR) and serum hormone levels were measured one day after treatment (day 55), seven days after treatment (day 61) and 50 days after treatment (day 104). The data shown (mean ± SEM) represent values in ARflox/y mice treated with TM expressed relative to values observed in ARflox/y mice treated with V (arbitrarily assigned a value of 100). Statistical analysis was performed by ANOVA supplemented with a Fisher LSD test. Values that differ significantly (p ≤ 0.05) are indicated by an asterisk. ND = not determined.
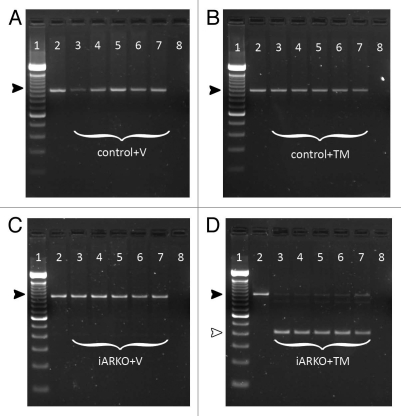
To examine whether TM treatment in fact results in AR inactivation (by excision of exon 2) in iARKO mice genomic DNA was prepared from a variety of tissues (tail tip, testis, epididymis, kidney and brain). PCR analysis (Fig. 3) confirms that no excision of exon 2 takes place in control mice treated with vehicle or TM or in iARKO mice treated with vehicle. In iARKO mice treated with TM, however, a smaller (404 bp) PCR fragment, representative for the inactivated AR, is observed. A very weak band corresponding to the PCR fragment of the intact floxed AR (952 bp) is also seen in all the organs studied suggesting that excision of exon 2 is not 100% efficient.
Figure 3.
The effect of tamoxifen (TM) and vehicle (V) treatment on AR inactivation in control and iARKO mice. Control (ARflox/y) or iARKO mice were treated with V or TM (3 mg/day) as indicated. Excision of the floxed exon 2 of the AR was monitored by PCR on genomic DNA obtained from a tail tip before treatment (lane 2) and genomic DNA obtained from a tail tip (lane 3), testis (lane 4), epididymis (lane 5), kidney (lane 6) and brain (lane 7) one day after treatment (day 55). Lane 1 shows a TrackItT™ 100 bp DNA ladder (Invitrogen). Lane 8 shows a negative control sample without genomic DNA. The 952 bp fragment (black arrowhead) corresponds to the intact floxed AR allele. Excision of the floxed exon 2 results in a 404 bp fragment (white arrowhead). Excision is only observed in iARKO mice treated with TM (D: lane 3–7). (A–D) Data derived from one representative animal from the indicated genotype.
TM treatment diminishes AR expression in iARKO mice testes.
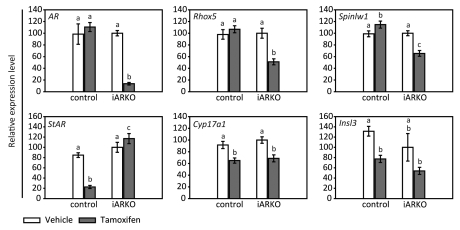
To evaluate more quantitatively the effect of TM administration on testicular AR expression in iARKO and control mice, transcript levels were measured by a qPCR assay specifically designed to detect only AR with an intact exon 2. As shown in Figure 4 TM (3 mg/day for five days) does not reduce AR transcript levels (as measured on day 55) in control mice. In the testes of iARKO mice, however, transcript levels are reduced to 14% of the vehicle treated control. Table 1 shows that in iARKO mice treated with 3 mg TM/day there is a further decrease in AR transcript levels (down to 4%) at day 61 and day 104. Moreover, a dose of 1 mg TM is considerably less effective (measured on day 55 only). Under none of the conditions studied TM affects AR transcript levels in control mice (Table 2).
Figure 4.
Transcript levels for the AR and representative SC and LC genes in testes of iARKO and control mice treated with vehicle (V) or tamoxifen (TM). Testicular transcript levels for AR, Rhox5, Spinlw1, StAR, Cyp17a1 and Insl3 in iARKO and control mice treated with V or TM (3 mg/day for five consecutive days) were measured by qPCR on day 55. Measurements were corrected for exogenously added luciferase mRNA as described in the Methods section. Values are expressed relative to the mean transcript level of iARKO mice treated with V (arbitrarily assigned a value of 100). Data represent the mean ± SEM of 5–7 independent measurements. Statistical analysis was performed as described in Figure 2. Values that differ significantly (p ≤ 0.05) are indicated by different lowercase letters (a–c).
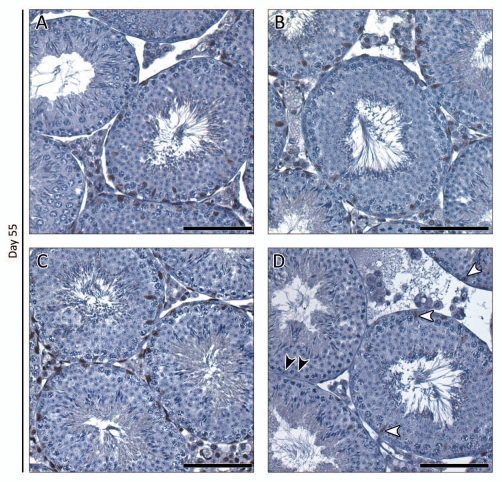
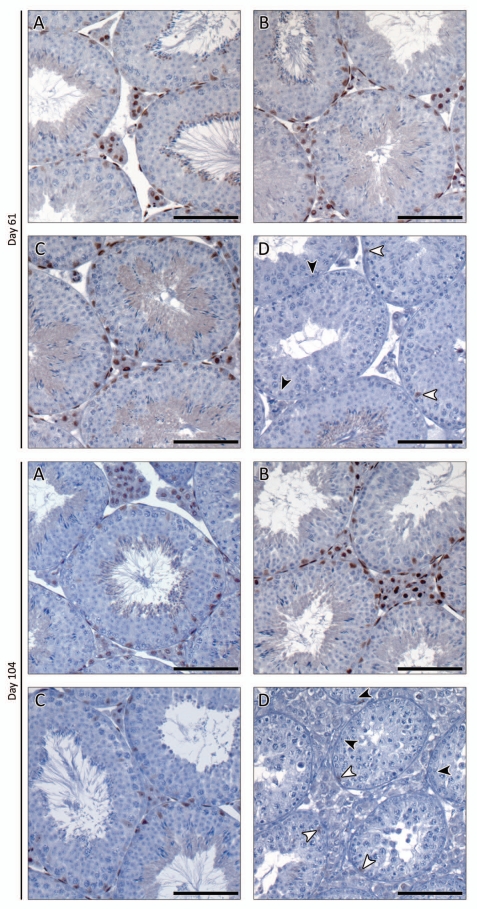
Immunohistochemical evaluation of AR protein expression immediately after (day 55), one week after (day 61) and 50 d after TM treatment reveals that AR expression is comparable in the testes of control mice treated with vehicle or TM and iARKO mice treated with vehicle (Figs. 5 and 6A–C). AR expression in TM-treated iARKO mice, however, is clearly diminished: staining is virtually absent in LC and PTM. Weak AR staining may be noted in some SC (Figs. 5D and 6D, white arrowheads). Interestingly, no noticeable effects on testicular morphology are seen in any of the treatment groups as evaluated by H&E staining on day 55 (data not shown). The latter observation is in line with the lack of effect on tubular diameter one day after final treatment (Tables 1 and 2). Seven and 50 d after treatment, however, impaired spermatogenesis was evident (compare Figs. 5D and 6D) as would be expected by lack of androgen signaling. This was further confirmed by a decrease in tubular diameters in iARKO mice treated with TM (Table 1).
Figure 5.
Immunohistochemical study of AR expression in testes of iARKO and control mice treated with vehicle (V) or tamoxifen (TM) and evaluated one day after treatment. AR expression was evaluated on day 55 in testes of iARKO and control mice treated with V or TM (3 mg/day for five consecutive days). At least five animals were studied for each experimental group. The intensity of AR staining was comparable in control mice treated with V or TM (A and B respectively) and iARKO animals treated with V (C). AR staining was markedly diminished, however, in iARKO mice treated with TM (D). In the latter animals virtually no AR staining was observed in LCs, PT Ms and testicular arterioles. Weak AR staining could be detected in a number of SCs (white arrowheads) whereas others were negative (black arrowheads). Scale bar = 100 µm.
Figure 6.
Immunohistochemical study of AR expression in testes of iARKO and control mice treated with vehicle (V) or tamoxifen (TM) and evaluated seven and 50 d after treatment. Animals were treated as explained in Figure 5 and AR expression was evaluated on day 61 and day 104 (as indicated on the left side of the concerning parts). At least five animals were studied for each experimental group. Again the intensity of AR staining was comparable in control mice treated with V or TM (A and B respectively) and iARKO animals treated with V (C). AR staining was markedly diminished in iARKO mice treated with TM (D). In the latter animals virtually no AR staining was observed in LCs, PT Ms and testicular arterioles. AR staining could be detected in a number of SCs (white arrowheads) whereas others were negative (black arrowheads). Scale bar = 100 µm.
Effects of TM treatment and AR ablation on Sertoli and Leydig cell function.
To study the effect of TM treatment and AR ablation on the expression of androgen-regulated genes in SC, we compared the effect of TM on the expression of Rhox5 and Spinlw1 in iARKO and control mice. Both Rhox5 and Spinlw1 have previously been shown to be under the direct control of the AR in SC.24–26 As shown in Figure 4 TM treatment does not decrease the transcript levels of the studied genes in control mice on day 55 whereas in iARKO mice expression of Rhox5 and Spinlw1 is reduced to 51% and 66% of the vehicle treated iARKO mice respectively. In iARKO mice further decreases in Rhox5 and Spinlw1 expression may be noted on day 61 and 104 (Table 1). The 1 mg TM dose is clearly less effective. In control mice TM has no effects on Rhox5 expression under any of the conditions studied and increases rather than decreases Spinlw1 expression (Table 2).
The effect of TM treatment and AR ablation on LC function was evaluated by measuring the transcript levels of three LC genes: StAR, Cyp 17a1 and Insl 3. In control mice TM treatment significantly reduces the expression of the studied genes under nearly all the conditions examined indicating that TM affects LC function via mechanisms that do not depend on AR inactivation (Fig. 4 and Table 2). In iARKO mice TM treatment also reduces the expression of Cyp17a1 and Insl3. The TM effect on StAR expression, however, seems to be neutralized by TM-induced AR inactivation (particularly at day 55 and 104) a finding that is compatible with the well-known inhibitory effects of androgens on StAR expression.27
Effects of TM treatment on the endocrine system.
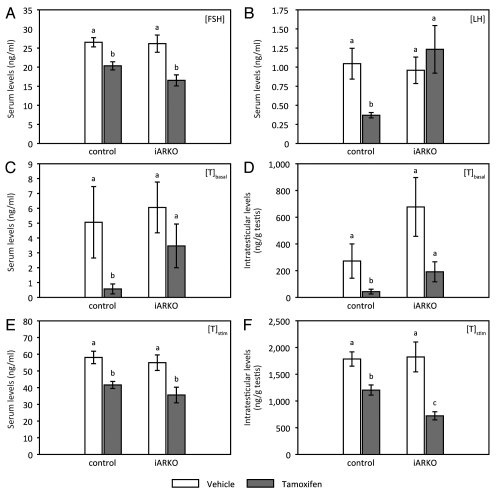
To further evaluate the effects of TM on endocrine function and particularly on androgen production, gonadotropin levels, serum testosterone levels and intratesticular testosterone levels were determined in vehicle and TM-treated control and iARKO mice. The data observed on day 55 are summarized in Figure 5. TM reduces FSH levels to a similar degree (by approximately 30%) in control and iARKO mice (Fig. 7A). LH levels (Fig. 7B) are significantly lowered (by approximately 60%) in TM-treated control mice but are unaffected in TM-treated iARKO mice, conceivably by reduced hypophyseo-hypothalamic feedback. In control mice serum and intratesticular testosterone levels (Fig. 7C and D respectively) are markedly reduced upon treatment with TM. In TM-treated iARKO mice a tendency to lower serum and intratesticular testosterone levels may also be noted, but—given the wide variation between individual animals—the difference is not statistically significant. Nonetheless, testosterone measurements after stimulation with human Chorionic Gonadotropin (hCG; Fig. 7E and F) indicate that the capacity of LC to produce androgens is impaired both in control and in iARKO mice treated with TM. Fifty days after TM treatment (day 104) LH levels tend to be increased in control mice (Table 2). A more pronounced and significant increase is observed in iARKO animals (Table 1).
Figure 7.
Evaluation of endocrine parameters in iARKO and control mice one day after the final injection with vehicle (V) or tamoxifen (TM). Serum levels of FSH, LH and testosterone and intratesticular levels of testosterone were measured in control and iARKO mice treated for five days with V or TM (n = 4−10). Measurements were performed on day 55, one day after the final injection. Assays were performed as described in the Methods section. Testosterone levels in serum (C and E) and testes (D and F) were measured under basal conditions (C and D; [T]basal) and 2 h after stimulation with hCG (E and F; [T]stim). Values represent the mean ± SEM. Statistical analysis was performed as described in Figure 2. Values that differ significantly (p ≤ 0.05) are indicated by different lowercase letters (a–c).
Discussion
The data presented show that, in the currently developed iARKO model, intraperitoneal treatment with TM (3 mg/day for five consecutive days) successfully inactivates the AR in all target tissues studied including the testis. One day after the final injection the concentration of AR transcripts in the testis, the main focus of our research, was reduced to 14% of the control. This reduction was accompanied by a significant decline in the mRNA concentrations of Rhox5 and Spinlw1 (to 51% and 66% of the control respectively), two SC genes that are directly controlled by the AR via androgen responsive elements (ARE) located in their promoter regions.24–26 Interestingly, at this early time point TM treatment did not affect testicular cell composition, a feature that may considerably facilitate future studies on changes in gene expression caused by AR inactivation. Moreover, identical treatment of control mice that do not carry the TM-inducible Cre recombinase did not show changes in the expression levels of Rhox5 and Spinlw1, indicating that the observed effects in iARKO animals (that carry the TM-inducible Cre) are caused by AR inactivation and not by any other direct or indirect effect of TM. Fifty days after treatment AR, Rhox5 and Spinlw1 transcript levels are further decreased and tubular diameter in TM treated iARKO mice is reduced to 68% of that observed in vehicle treated controls (Table 1). At this time point sperm production (as measured by histological evaluation of testicular sections and epididymal sperm counts) is virtually absent (Fig. 6D and Day 104; data not shown). In TM treated control mice none of the mentioned transcript levels is decreased and only a limited (5%; not statistically significant) decrease in tubular diameter is observed.
Inactivation of the testicular AR via activation of the TM-inducible Cre requires a high dose of TM. Administration of a 1 mg dose of TM had only limited effects on AR and Rhox5 expression (a decrease to 43% and 78% respectively) and did not affect Spinlw1 transcript levels. Moreover, even at the 3 mg/day dose of TM AR inactivation occurred more slowly than anticipated. AR mRNA concentration was decreased to 14% of the control one day after the final injection and decreased further down to 4% on day 7 and 50 after treatment. Even slower declines were observed for the expression of the androgen-controlled SC genes Rhox5 and Spinlw1 (down to 5% and 9% respectively 50 d after treatment). The slow decline in these SC genes controlled by androgens probably reflects that low levels of AR may still be able to maintain relatively high levels of expression. Alternatively, it may be speculated that the expression of these genes does not depend exclusively on direct effects of androgens but is also regulated indirectly via androgen-dependent processes such as the presence of particular stages of developing germ cells. Moreover, immunohistochemical studies both after treatment with a 1 mg dose of TM (data not shown) and a 3 mg dose of TM (Figs. 5 and 6) suggest that AR inactivation in SCs may be slower or less efficient than in other testicular cells. In fact, one day after treatment the AR is undetectable in LCs, interstitial cells and PTMs whereas some SCs still stain positive. Whether this less efficient AR inactivation in SCs is due to a lower expression of the Cre transgene in SCs or to a less efficient penetration of TM in SCs, conceivably due to the expression of drug transporters involved in the protective function of SCs during germ cell development,28,29 remains to be investigated.
Estrogens are known to affect testicular function by a variety of pathways.30,31 Accordingly, when high doses of TM are used to activate the inducible Cre in iARKO mice, the possibility should be considered that some of the observed effects are not due to AR inactivation but to confounding (off-target) effects of TM. TM is a non-steroidal anti-estrogen commonly used for the treatment of hormone-dependent early breast cancer in pre-menopausal women.32 Depending on the concentration used, and the target organ studied, however, its action may range from that of a pure estrogen antagonist to that of an estrogen agonist.32 In the present study there are obvious effects of TM treatment on the endocrine system. In control animals treated with TM, FSH and LH concentrations in serum, one day after treatment, are diminished by 23% and 65% respectively indicating that TM acts as an ER agonist at the pituitary-hypothalamic level.33 Interestingly, in identically treated iARKO mice the effect of TM on LH concentration is neutralized, most likely by inactivation of the AR in the central feedback system and by the subsequent elimination of the suppressive effects of androgens on LH secretion. Furthermore, TM treatment of control mice markedly reduces serum as well as intratesticular levels of testosterone. The observation that this reduction is (partially) reversed in iARKO mice suggests that this effect may be related at least in part to the effects of TM on LH. This is obviously not the only explanation, however. In fact, stimulation with hCG reveals that treatment with TM decreases the capacity of LCs to produce testosterone. This decrease is at least as severe in iARKO animals as in control mice. LCs are known to express the estrogen receptor (ER; both ERα and ERβ) 34,35 and estrogens have been proven to be important regulators of LC steroidogenesis and proliferation.36–39 Studies in ERα knockout mice indicate that the effects of estrogens (or estrogenic endocrine disruptors) on LC function are mainly mediated through the ERα.37,40,41 To evaluate potential direct effects of TM on LC function we measured transcript levels of three genes known to be downregulated by estrogens: StAR (encoding a protein involved in the mitochondrial transport of cholesterol, a rate-limiting step in steroidogenesis42), Cyp17a1 (encoding the 17α-hydroxylase/17,20-lyase, a key enzyme in the steroidogenic pathway) and Insl3 (a regulator of testicular descent and a marker of differentiated LCs43). Expression of all three marker genes was decreased in testes of TM-treated control animals immediately after treatment confirming earlier in vitro and in vivo studies showing that TM acts as an ER agonist in LCs.33,44 In iARKO animals the inhibitory effect of TM on StAR expression was completely abolished, an observation compatible with TM-induced AR inactivation in LCs and with the known inhibitory effects of androgens on StAR expression.27 Despite these inhibitory effects of TM on LC steroidogenesis it should be stressed that the decrease in intratesticular testosterone remains relatively mild in control animals and even non-significant in iARKO mice. Residual levels still exceed 10 nM, a concentration that should be sufficient to saturate the AR2,45,46 as also reflected by the lack of effect of TM on the expression of Rhox5 and Spinlw1 in control animals. An important implication is that the effects observed on SC gene expression are the result of AR inactivation and not of diminished testosterone concentrations in the testis.
The use of other inducible knockout strategies (not dependent on TM administration) was certainly contemplated. One option is the combined application of a tetracycline-controlled transactivator and Cre/loxP technology. For the development of a time-controlled cell-specific knockout of the AR the “Tet-On”-system, which will switch on gene expression when tetracycline/doxycycline is administered, could be used.47 This system relies on the existence/generation of three distinct mouse lines: (1) a mouse line that expresses a mutant/reverse tetracycline-controlled transactivator (rtTA) in a cell-specific manner, (2) a mouse line expressing Cre under the control of the minimal CMV promoter fused to seven Tet-operator (tetO) sequences and (3) the ARflox mouse line. The mating scheme of these cell-specific tetracycline-inducible knockout mice is therefore more complicated and increases animal housing costs considerably. Moreover, also in this system, off-target effects of the inducer, doxycycline, will have to be considered. Doxycycline is an antibiotic that can inhibit protein synthesis48 and that acts as an inhibitor of matrix metalloproteinases, which are known to be essential in the dynamic regulation of the SC barrier.49 Moreover, experience with doxycycline-inducible systems in our laboratory indicates that these systems often display relatively high levels of basal (uninduced) activity. Another option might be the use of an interferon-inducible Cre recombinase. In this system Cre is controlled by a promoter that is strongly activated by the administration of interferon-α or -β.50 Again off-target effects of interferon treatment should be carefully considered. In fact, interferon is not only an important cytokine in the control of the immune system but also a paracrine regulator of normal testicular function with effects on both germ cell development and steroidogenesis.51,52 Moreover, endogenous interferon has been reported to induce leaky Cre expression in absence of the inducer.50
It may be concluded that TM treatment in iARKO mice largely inactivates AR expression in testicular cells and significantly affects transcript levels of androgen-regulated genes in SCs as well as in LCs. Immediately after the five-day TM treatment period testicular cell composition is not noticeable affected, making the iARKO a promising experimental tool to identify androgen-regulated genes in testicular target cells. High doses of TM are needed to obtain efficient AR ablation in the testis and these doses have effects on the testis that are not directly related to the ability of TM to induce Cre activation and AR ablation. These effects can largely be accounted for, however, by including control animals receiving identical TM treatment. The TM-induced decrease in testosterone production and FSH concentration observed in the present experiments, for instance, is not important enough to confound interpretation of the results. Even if this would turn out to be a problem under certain conditions, the effects can probably be neutralized by exogenous administration of testosterone and/or FSH. The iARKO model has the advantage that AR inactivation can be induced at a chosen time point, an interesting feature that is lacking in SCARKO mice and that precludes studies on postmeiotic effects of androgens on germ cell development in the latter model. The disadvantage of the iARKO is that AR ablation is not cell-specific. The data shown here, however, prove that it should be feasible and worthwhile to develop an iSCARKO model in which the TM-inducible Cre recombinase is placed under the control of a SC-selective promoter making Cre expression controllable both in space and in time. Attempts to develop such an iSCARKO model are underway.
Materials and Methods
Ethics statement.
All animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the “Ethical Committee Animal Tests” of the Catholic University of Leuven.
Generation of transgenic mice.
Mice with an inducible ubiquitous knockout of the AR (iARKO) were generated by crossing female mice (98% CD1) homozygous for a floxed AR allele (ARflox/flox) 9 with male mice (B6.Cg-Tg(CAG-cre/Esr1)5Amc/J from The Jackson Laboratory) carrying a tamoxifen (TM)-inducible Cre recombinase. The expression of this Cre is controlled by a chimeric promoter of the cytomegalovirus immediate-early enhancer and the chicken β-actin promoter/enhancer (CAGGCreER™).23 As a result of this breeding scheme 50% of the male progeny carried the floxed AR allele as well as the ubiquitously expressed TM-inducible Cre recombinase (ARflox/y; CAGGCreERTM+/−: further referred to as iARKO mice) and 50% of the male progeny carried only the floxed AR allele (ARflox/y;CAGGCreERTM−/−: further referred to as control) (Fig. 1A).
Handling of mice and induction of AR knockout by treatment with TM.
Routinely TM (T5648, Sigma-Aldrich) was dissolved in ethanol (150 mg/ml), by sonication (5 min) and warming up to 40°C. The ethanol solution was diluted 1/10 in arachis oil (Fagron) to a final concentration of 15 mg TM/ml.22 To induce AR knockout, iARKO and control mice were injected intraperitoneally with TM (1 mg or 3 mg) or with vehicle (ethanol in arachis oil: 1/10) for five consecutive days starting from day 50 (Fig. 1B). TM- and vehicle-treated animals were housed in separate cages.
Mice were weighed before treatment (on day 35, 42 and 49), one day after the final injection (day 55) and thereafter once a week until sacrifice. One, seven and 50 d after TM or vehicle treatment transgenic animals were sacrificed by CO2 asphyxiation followed by cervical dislocation. Blood was collected by cardiac puncture and serum was isolated by centrifugation. Serum samples were stored at −20°C until use for hormone analysis. Organs (liver, brain, kidney, testes, epididymides, seminal vesicles, anterior and ventral prostates) were fixed in Bouin's fixative for 6 h or snap-frozen in liquid nitrogen immediately after removal and stored at −80°C until further processing.
PCR genotyping.
The genotype of iARKO and control animals was determined by PCR on genomic DNA extracted from tail tips. The presence of the Cre transgene was probed by use of the primer pair: 5′-GCG ATT ATC TTC TAT ATC TTC AGG-3′ (forward primer) and 5′-GCC AAT ATG GAT TAA CAT TCT CCC-3′ (reverse primer), revealing the presence or absence of a 380 bp band. The floxed AR allele was detected by use of the primer pair: 5′-AGC CTG TAT ACT CAG TTG GGG-3′ (forward primer) and 5′-AAT GCA TCA CAT TAA GTT GAT ACC-3′ (reverse primer). The floxed AR and the AR with an excised exon 2 could be recognized by a 952 bp and a 404 bp band respectively. Effective excision of exon 2 was also studied on genomic DNA extracted from testis, epididymis, kidney and brain.
RNA extraction and quantitative RT-PCR.
RNA was prepared from testes derived from iARKO and control mice, treated with TM or vehicle, on day one, seven and 50 after the final injection. Testes were weighed and homogenized in a Dounce homogenizer (Kontes Co.) and RNA was isolated with the RNeasy® Mini kit (Qiagen) according to the manufacturer's instructions, encompassing an on-column deoxyribonuclease I (DNase I) treatment. Luciferase mRNA (10 ng; Promega) was added to the whole testis sample at the start of the RNA extraction procedure to control for the efficiency of RNA extraction, RNA degradation and the reverse transcription step and to allow specific mRNA levels to be expressed per testis.53
cDNA was synthesized from 1 µg RNA using Superscript II RT, RNaseOUT®™ and random hexamer primers (Invitrogen Life Technologies, Inc.) according to the manufacturer's protocol. For quantification of gene expression, the 7500 Fast Real-Time PCR system (Applied Biosystems) was used running the ‘Fast RT-PCR’ protocol (2 min at 50°C, 2 min at 95°C and 40 cycles of 3 sec at 95°C and 30 sec at 60°C). For the quantification of AR, Spinlw1 (serine protease inhibitor-like, with Kunitz and WAP domains 1; formerly known as Eppin), StAR (steroidogenic acute regulatory protein), Cyp17a1 (steroid 17-α-hydroxylase/17,20-lyase) and luciferase transcripts by quantitative real-time PCR (qPCR), each 10 µl qPCR reaction mix contained 1x Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen), 150 nM of each primer and 0.05 µM ROX reference Dye (Invitrogen). For the AR an assay was designed that specifically recognizes transcripts containing exon 2. The uniqueness of the amplified bands was verified by performing melting curves after the real-time PCR amplifications. For the Rhox5 qPCR assay, each 10 µl qPCR contained 1x TaqMan® Universal PCR Master Mix (Applied Biosystems), 1 µM of each primer and 4 µM probe. All samples were run in triplicate. The quantity of target mRNA was normalized to an external luciferase mRNA standard, added before RNA extraction as described above. For comparison of transcript levels, values were expressed relative to the average value of iARKO animals treated with vehicle (arbitrarily assigned a value of 100). Sequences of primers and probes are described in Table 3.
Table 3.
Oligonucleotide primers and probes used for qPCR
| Gene name | Gene symbol | Accession No. | 5′ Primer (Fw) |
| 3′ Primer (Rv) | |||
| Probe | |||
| Androgen receptor | AR | NM_013476 | Fw: 5′-GAC ATG CGT TT G GAC AGT ACC A-3′ |
| Rv: 5′-TGA CAG CCA GAA GCT TCA TCT C-3′ | |||
| Reproductive homeobox 5 | Rhox5 | NM_008818 | Fw: 5′-TCA TCA TT G ATC CTA TT C AGG GTA TG-3′ |
| Rv: 5′-CTC TCC AGC CTG GAA GAA AGC-3′ | |||
| Probe: 5′-6-FAM-CTC GGA AGA ACA GCA TGA TGT GAA AGC A-TAMRA-3′ | |||
| Serine protease inhibitor-like, with Kunitz and WAP domains 1 (eppin) | Spinlw1 | NM_029325 | Fw: 5′-GCT TCT GCT CCA AGC TCT GTG-3′ |
| Rv: 5′-TT G CAG TGC TCA AAG TGC TCT C-3′ | |||
| Steroidogenic acute regulatory protein | StAR | NM_011485 | Fw: 5′-CCG GAG CAG AGT GGT GTC A-3′ |
| Rv: 5′-CAG TGG ATG AAG CAC CAT GC-3′ | |||
| Steroid 17-α-hydroxylase/17,20-lyase | Cyp17a1 | NM_007809 | Fw: 5′-GGG CAC TGC ATC ACG ATA AA-3′ |
| Rv: 5′-GAT CTA AGA AGC GCT CAG GCA-3′ | |||
| Luciferase | Luciferase | L4561 (from Promega) | Fw: 5′-TCG AAG TAT TCC GCG TAC GTG-3′ |
| Rv: 5′-GCC CTG GTT CCT GGA ACA A-3′ |
Histological evaluation and measurement of tubular diameters.
Testes derived from iARKO and control mice treated with vehicle or TM were immersion fixed in Bouin's fluid for 6 h at room temperature before being transferred into 70% ethanol until further processing. Fixed tissues were processed for 12 h in an automated Shandon Citadel™ 1,000 tissue processor (Nijmegem, The Netherlands) and embedded in paraffin wax (Paraplast plus Tissue embedding medium; McCormick Scientific). Five-micrometer sections were cut, floated onto Superfrost® Plus slides (Menzel-Gläser, Thermo Scientific) and dried at 37°C overnight before being used for (immuno)histochemistry as described below.
Immunohistochemical detection of AR protein was performed on dewaxed and rehydrated sections. Heat-induced antigen retrieval was performed for 10 min in 0.01 M citrate acid buffer (pH 6.0) using a pressure cooker. Thereafter slides were blocked for endogenous peroxidase activity by incubation in 3% (vol/vol) H2O2 (VWR International) in methanol for 30 min at room temperature. Between each of the following steps two washes (5 min) were performed using Tris [(Hydroxymethyl)methylamine-buffered saline (TBS; 0.05 M Tris at pH 7.4 and 0.9% (wt/vol) saline)]. Sections were blocked with TBS containing 5% bovine serum albumin (wt/vol; Sigma-Aldrich) and normal goat serum (1/4 dilution; Dako) at room temperature before incubation overnight at 4°C with a rabbit anti-AR antiserum (1/750 in blocking buffer; sc-816 lot D3010 from Santa Cruz Biotechnology). Negative control samples were incubated overnight with blocking buffer without primary antibody. Thereafter, the sections were incubated with Envision + System-HRP labeled Polymer Anti-Rabbit (K4003 from Dako) for 45 min at room temperature. Bound antibodies were visualized by color development with 3,3′-diaminobenzidine tetrahydrochloride chromogenic substrate (K3468, Liquid DAB+ kit, Dako), monitored microscopically. Subsequently, sections were washed in tap water, counterstained with Haematoxylin Gill3 (Prosan nv), dehydrated and mounted with DePex (VWR international). Images were captured using a Leica DMR microscope with a Sony DXC-9100P camera.
For morphological evaluation, 5 µm paraffin sections were prepared as described above. Subsequently, tissue sections were dewaxed, rehydrated and stained with Haematoxylin Gill3 (Prosan nv) and eosin (71304E; Richard-Allan Scientific®) for 2 min and 30 sec respectively. Finally, sections were dehydrated and mounted with DePex (VWR international).
Tubular diameters were measured at 20x magnification using an Axioplan 2 microscope (Carl Zeiss) and AxioVision Release 4.6 software (Carl Zeiss). Three non-overlapping fields containing round or nearly round tubular sections were measured for each animal analyzed (at least three animals for each treatment group at all different ages). An equivalent tubular diameter was calculated as the average of two perpendicular measurements of each tubule for further analysis.
Measurements of LH, FSH and testosterone.
Serum FSH levels were measured via a double-antibody RIA using reagents supplied by Dr. A.F. Parlow (Harbor-University of California-Los Angeles) and the National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Peptide Program. The standard preparation used was mFSH, RP (lot AFP5308D), the tracer was prepared from rFSH IOD (lot AFP5178B) and the antiserum was rabbit anti-rFSH-S-11 A.S. (lot AFPC0972881). Testosterone was measured both under basal conditions and after stimulation with human Chorionic Gonadotropin (hCG; Pregnyl, Organon) in serum samples and in testicular extracts using the Testo-RIA-CT kit (Bio-Source International; detection limit, 0.05 ng/ml). Stimulated testosterone levels were obtained from animals treated with hCG (0.4 IU hCG/g body weight) 2 h before sacrifice. Testicular extracts were obtained from testes snap-frozen in liquid nitrogen immediately after sacrifice of the animals and stored at −80°C until further processing. Collected testes were weighed and pulverized with a mortar cooled to −80°C. The pulverized testes were homogenized in 2 ml PBS (at 4°C) and were extracted twice with 4 ml of cyclohexane/ethylacetate (50/50 vol/vol; VWR International). Each time the mixture was shaken for 30 min and centrifuged at 1,200x g for 10 min at room temperature. The two organic phases from the extraction steps were pooled and were evaporated to dryness in a vacuum centrifuge (SAVANT SPD111V SpeedVac Concentration; Thermo Scientific). The final residue was dissolved in 200 µl charcoal stripped human serum. Serum LH levels were measured via an immunofluorometric assay (IFMA/Delfia) at the University of Turku, Institute of Biomedicine, Department of Physiology.54,55
Statistical analysis.
Comparison of body weights, organ weights, qPCR data, measurement of tubular diameters and hormone levels for iARKO and control animals treated with vehicle or TM at each age was made by ANOVA supplemented with a Fisher multiple comparison test using NCSS2000 software (NCSS Statistical Analysis and Data Analysis Software). A p-value ≤ 0.05 was considered statistically significant.
Acknowledgments
The technical assistance of Wendy De Keyzer is greatly appreciated. The work described here was supported by a research grant from the Research Fund of the Katholieke Universiteit Leuven (OT/07/068A; www.kuleuven.be), by a grant and a postdoctoral fellowship (to K. De Gendt; G.0458.05N) from the Fund for Scientific Research Flanders (FWO; www.fwo.be) and by a Ph.D. grant (to A. Willems; SB-73064) from the Agency for Innovation by Science and Technology in Flanders (IWT; www.iwt.be). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations
- 4-OH-TM
4-hydroxy-tamoxifen
- AMH
anti-müllerian hormone
- AR
androgen receptor
- ARE
androgen response element
- AR−
an androgen receptor allele with an excised exon 2
- ARflox
an androgen receptor allele with a floxed exon 2
- Cre
cyclization recombination
- Cre-ER™
a tamoxifen-inducible Cre recombinase
- EDS
ethane dimethane sulphonate
- ER
estrogen receptor
- FSH
follicle-stimulating hormone
- H&E
hematoxyline-eosine
- hCG
human chorionic gonadotropin
- hpg
hypogonadal
- iARKO
an inducible (general) AR knockout model
- iSCARKO
an inducible Sertoli cell-selective AR knockout model
- LC
Leydig cell
- LH
luteinizing hormone
- LuRKO
transgenic mice with a defect in the LH receptor
- PCR
polymerase chain reaction
- PTM
peritubular myoid cell
- SCARKO
a Sertoli cell-selective AR knockout model
- TM
tamoxifen
- V
vehicle
Disclosure of Potential Conflicts of Interest
The authors have declared that no competing interests exist.
References
- 1.Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. Androgens and spermatogenesis: lessons from transgenic mouse models. Philos Trans R Soc Lond B Biol Sci. 2010;365:1537–1556. doi: 10.1098/rstb.2009.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2. New York: Raven Press; 1994. pp. 1363–2434. [Google Scholar]
- 3.Sharpe RM. Sertoli Cell Biology and Signal Transduction: Androgen Regulation. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. 1. San Diego: Elsevier Academic Press; 2005. pp. 199–216. [Google Scholar]
- 4.O'Donnell L, Narula A, Balourdos G, Gu YQ, Wreford NG, Robertson DM, et al. Impairment of spermatogonial development and spermiation after testosterone-induced gonadotropin suppression in adult monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2001;86:1814–1822. doi: 10.1210/jc.86.4.1814. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan RI, Wreford NG, Robertson MD, De Kretser DM. Hormonal control of spermatogenesis. Trends Endocrinol Metab. 1995;6:95–101. doi: 10.1016/10432760(94)00215-P. [DOI] [PubMed] [Google Scholar]
- 6.Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/en.136.12.5311. [DOI] [PubMed] [Google Scholar]
- 7.Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology. 1999;140:3938–3946. doi: 10.1210/en.140.9.3938. [DOI] [PubMed] [Google Scholar]
- 8.Pakarainen T, Zhang FP, Makela S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146:596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 9.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C, Chen YT, Yeh SD, Xu QQ, Wang RS, Guillou F, et al. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 12.Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, et al. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology. 2009;150:4755–4765. doi: 10.1210/en.2009-0416. [DOI] [PubMed] [Google Scholar]
- 13.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23:4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Donnell L, McLachlan RI, Wreford NG, Robertson DM. Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology. 1994;135:2608–2614. doi: 10.1210/en.135.6.2608. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell L, McLachlan RI, Wreford NG, De Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- 16.Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec. 1977;187:347–366. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- 17.Kerr JB, Millar M, Maddocks S, Sharpe RM. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec. 1993;235:547–559. doi: 10.1002/ar.1092350407. [DOI] [PubMed] [Google Scholar]
- 18.Cameron DF, Muffly KE, Nazian SJ. Reduced testosterone during puberty results in a midspermiogenic lesion. Proc Soc Exp Biol Med. 1993;202:457–464. doi: 10.3181/00379727-202-43559. [DOI] [PubMed] [Google Scholar]
- 19.Beardsley A, O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, O'Donnell L, McLachlan RI, Robertson DM. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology. 2000;141:2779–2785. doi: 10.1210/en.141.8.2779. [DOI] [PubMed] [Google Scholar]
- 21.McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, De Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 22.Feil S, Valtcheva N, Feil R. Inducible Cre mice. Methods Mol Biol. 2009;530:343–363. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 24.Barbulescu K, Geserick C, Schuttke I, Schleuning WD, Haendler B. New androgen response elements in the murine Pem promoter mediate selective transactivation. Mol Endocrinol. 2001;15:1803–1816. doi: 10.1210/me.15.10.1803. [DOI] [PubMed] [Google Scholar]
- 25.Shanker S, Hu Z, Wilkinson MF. Epigenetic regulation and downstream targets of the Rhox5 homeobox gene. Int J Androl. 2008;31:462–470. doi: 10.1111/j.1365-2605.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 26.Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, et al. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci USA. 2007;104:4961–4966. doi: 10.1073/pnas.0610814104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houk CP, Pearson EJ, Martinelle N, Donahoe PK, Teixeira J. Feedback inhibition of steroidogenic acute regulatory protein expression in vitro and in vivo by androgens. Endocrinology. 2004;145:1269–1275. doi: 10.1210/en.20031046. [DOI] [PubMed] [Google Scholar]
- 28.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22:289–318. doi: 10.1210/er.22.3.289. [DOI] [PubMed] [Google Scholar]
- 31.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furr BJ, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/01637258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 33.Parte P, Balasinor N, Gill-Sharma MK, Maitra A, Juneja HS. Temporal effect of tamoxifen on cytochrome P450 side chain cleavage gene expression and steroid concentration in adult male rats. J Steroid Biochem Mol Biol. 2002;82:349–358. doi: 10.1016/S0960-0760(02)00193-0. [DOI] [PubMed] [Google Scholar]
- 34.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod. 2000;62:310–317. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]
- 36.Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, et al. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002220292. [DOI] [PubMed] [Google Scholar]
- 37.Delbè G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology. 2005;146:2454–2461. doi: 10.1210/en.20041540. [DOI] [PubMed] [Google Scholar]
- 38.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/en.137.11.4796. [DOI] [PubMed] [Google Scholar]
- 39.Martin LJ, Tremblay JJ. Nuclear receptors in Leydig cell gene expression and function. Biol Reprod. 2010;83:3–14. doi: 10.1095/biolreprod.110.083824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology. 2007;148:5507–5519. doi: 10.1210/en.2007-0689. [DOI] [PubMed] [Google Scholar]
- 41.Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, et al. Increased exposure to estrogens disturbs maturation, steroidogenesis and cholesterol homeostasis via estrogen receptor alpha in adult mouse Leydig cells. Endocrinology. 2009;150:2865–2872. doi: 10.1210/en.20081311. [DOI] [PubMed] [Google Scholar]
- 42.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 43.Ivell R, Anand-Ivell R. Biology of insulin-like factor 3 in human reproduction. Hum Reprod Update. 2009;15:463–476. doi: 10.1093/humupd/dmp011. [DOI] [PubMed] [Google Scholar]
- 44.Lin T, Murono EP. Tamoxifen inhibits Leydig cell steroidogenesis: in vivo and in vitro studies. Metabolism. 1982;31:543–547. doi: 10.1016/0026-0495(82)90092-0. [DOI] [PubMed] [Google Scholar]
- 45.Verhoeven G, Cailleau J. Follicle-stimulating hormone and androgens increase the concentration of the androgen receptor in Sertoli cells. Endocrinology. 1988;122:1541–1550. doi: 10.1210/endo-122-4-1541. [DOI] [PubMed] [Google Scholar]
- 46.Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989;124:3043–3049. doi: 10.1210/endo-124-6-3043. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13:121–128. doi: 10.1016/S10849521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- 48.Goldman RA, Hasan T, Hall CC, Strycharz WA, Cooperman BS. Photoincorporation of tetracycline into Escherichia coli ribosomes. Identification of the major proteins photolabeled by native tetracycline and tetracycline photoproducts and implications for the inhibitory action of tetracycline on protein synthesis. Biochemistry. 1983;22:359–368. doi: 10.1021/bi00271a020. [DOI] [PubMed] [Google Scholar]
- 49.Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 50.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 51.Hedger MP, Meinhardt A. Cytokines and the immunetesticular axis. J Reprod Immunol. 2003;58:1–26. doi: 10.1016/S01650378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 52.Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech. 2009;72:620–628. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- 53.Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145:318–329. doi: 10.1210/en.2003-1055. [DOI] [PubMed] [Google Scholar]
- 54.van Casteren JI, Schoonen WG, Kloosterboer HJ. Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod. 2000;62:886–894. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 55.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. doi: 10.1210/en.132.4.1687. [DOI] [PubMed] [Google Scholar]