Abstract
Drebrin E, an actin-binding protein lacking intrinsic activity in the regulation of actin dynamics (e.g., polymerization, capping, nucleation, branching, cross-linking, bundling and severing), is known to recruit actin regulatory proteins to a specific cellular site. Herein, we critically evaluate recent findings in the field which illustrate that drebrin E works together with two other actin-binding proteins, namely Arp3 (actin-related protein 3, a component of the Arp2/3 complex that simultaneously controls actin nucleation for polymerization and branching of actin filaments) and Eps8 (epidermal growth factor receptor pathway substrate 8 that controls capping of the barbed ends of actin filaments, as well as actin filament bundling) to regulate the homeostasis of F-actin filament bundles at the ectoplasmic specialization (ES), a testis-specific atypical adherens junction (AJ) in the seminiferous epithelium. This is mediated by the strict temporal and spatial expression of these three actin-binding proteins at the apical and basal ES at the Sertoli cell-spermatid (step 8–19) and Sertoli-Sertoli cell interface, respectively, during the seminiferous epithelial cycle of spermatogenesis. In this Commentary, we put forth a possible model by which drebrin E may be acting as a platform upon which proteins (e.g., Arp3) that are needed to alter the conformation of actin filament bundles at the ES can be recruited to the site, thus facilitating changes in cell shape and cell position in the epithelium during spermiogenesis and spermiation. In short, drebrin E may be acting as a “logistic” distribution center to manage different regulatory proteins at the apical ES, thereby regulating the dynamics of actin filament bundles and modulating the plasticity of the apical ES. This would allow adhesion to be altered continuously throughout the epithelial cycle to accommodate spermatid movement in the seminiferous epithelium during spermiogenesis and spermiation. We also describe a hypothetical model, upon which functional studies can be designed in the future.
Key words: testis, actin binding protein, drebrin, drebrin E, Eps8, Arp2/3 complex, spermatogenesis, seminiferous epithelial cycle, spermiogenesis, blood-testis barrier, ectoplasmic specialization, atypical adherens junction, actin branching, actin nucleation
Introduction
Spermiogenesis is marked by the most obvious morphological changes in spermatids that take place in the seminiferous epithelium during spermatogenesis.1–5 The onset of spermiogenesis begins right after meiosis II in the apical (adluminal) compartment of the seminiferous epithelium, and it ends just prior to spermiation when sperm are released from the epithelium.6–8 During spermiogenesis, spermatids undergo a series of morphological changes which are categorized into steps. These are manifested by the condensation of genetic material in the spermatid head, formation of the acrosome over the nucleus, packaging of mitochondria into the mid-piece and elongation of the tail, and they can be classified into 19, 16 and 12 steps in rats, mice and humans, respectively.6,8–12 In fact, earlier studies using periodic acid-Schiff (PAS) staining of the mammalian testis to visualize changes in the Golgi region of spermatids, namely the development of the acrosome during spermiogenesis, have divided the seminiferous epithelium into I–XIV, I–XII and I–VI stages in rats, mice and humans, respectively. These stages depicted changes in cellular associations in cross-sections of seminiferous tubules, and thus generated the concept of the seminiferous epithelial cycle of spermatogenesis.13–15 Subsequent studies in the rat testis by electron microscopy have shown that adhesion sites surrounding the head of step 8–19 spermatids to be encircled entirely with a unique adherens junction (AJ) known as the ectoplasmic specialization (ES), which is typified by the presence of highly organized actin filament bundles sandwiched in between cisternae of endoplasmic reticulum and the apposing plasma membranes of the spermatid and the Sertoli cell but with the exception that these unique actin filament bundles are limited only to the Sertoli cell side (see Fig. 1).16–18 Once the ES appears at the interface of the step 8 spermatid and the Sertoli cell, it is the only anchoring device to confer spermatid adhesion, orientation and polarity, and it persists in the epithelium until spermiation.8,18,19 Since it is restricted to the apical compartment, it is defined as the apical ES.20 Moreover, the ES is also found at the Sertoli-Sertoli cell interface at the BTB, and it is known as basal ES.21,22 It shares identical ultrastructural features with the apical ES, except that its typical features, namely the actin filament bundles and cisternae of endoplasmic reticulum, are found on both sides of the Sertoli cell.23,24 The unique arrangement of actin filament bundles at the ES, which is not found in any other anchoring junction type in the mammalian body, also confers remarkable adhesive strength to the ES. For instance, the apical ES was found to be significantly stronger than the desmosome which is restricted to the interface of pre-step 8 spermatids and the Sertoli cell.25 Interestingly, the apical ES undergoes extensive restructuring during spermiogenesis because of changes in cell shape and the relative location of developing spermatids within the seminiferous epithelium. For instance, elongating spermatids move toward the tubule lumen during stages XIV-III, but downward and toward the basement membrane during stages IV–V, followed once again by upward and toward to the luminal edge during stages VI–VIII. Thus, a unique mechanism must be in place to rapidly change the arrangement and distribution of actin filament bundles at the ES to facilitate cell movement and changes in cell shape of elongating spermatids during spermiogenesis. In this Commentary, we critically evaluate the role of actin-binding proteins (ABP or microfilament-associated proteins) in actin dynamics during spermiogenesis. While there are more than 100 actin-binding proteins found in eukaryotic cells, until recently there have been few studies conducted to assess the role of these proteins in spermiogenesis. Thus, this is a rapidly evolving area of research that deserves attention.
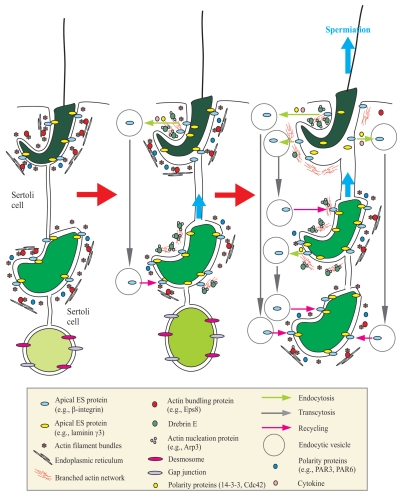
Figure 1.
A schematic drawing that illustrates an emerging concept regarding the role of actin-binding protein drebrin E in regulating spermiogenesis in the rat testis via its effects to recruit the actin nucleation protein Arp3 to the apical ES to facilitate junction restructuring during spermatogenesis. The left part in this figure illustrates intact apical ES (maintained by adhesion protein complexes such as integrin-laminin at the Sertoli cell-step 8–19 spermatid interface), gap junction and desmosome [at the Sertoli cell-step 1–7 spermatid interface] that confers proper adhesion of developing spermatids to the Sertoli cell in the seminiferous epithelium. Apical ES adhesion is conferred and strengthened by actin filament bundles sandwiched in between the cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, and this likely involves the presence of polarity proteins, such as PAR3 (partitioning-defective protein 3), PAR6.72 Highly organized F-actin filament bundles uniquely found at the apical ES are maintained by actin-bundling proteins, such as Eps8. During spermiogenesis, the transit of developing spermatids is facilitated by a surge in the expression of drebrin E, which recruits actin nucleation proteins (e.g., Arp3 in the Arp2/3 protein complex) to the apical ES to convert actin filament bundles into a branched network, causing the loss of “rigidity” of, but conferring “plasticity” to, the apical ES (see middle part). This thus destabilizes the apical ES, facilitating protein endocytosis, which is regulated by cytokines (e.g., TGFβ3 and TNFα) 8 and assisted by polarity proteins (e.g., 14-3-3, Cdc42).73,74 As spermiogenesis progresses, the elevated expression of drebrin E recruits more Arp3 to the apical ES, surrounding the head of elongated spermatids to further destabilize adhesion at the apical ES to facilitate the release of sperm at spermiation (i.e., degeneration of the apical ES at stage VIII of the epithelial cycle), and internalized apical ES proteins can be transcytosed and recycled to assemble “new” apical ES to anchor newly differentiated step 8 spermatids onto the epithelium (see right part). This thus provides an efficient physiological system to “re-use” many of the component proteins from the “old” apical ES site surrounding the head of step 19 spermatids to assemble the “new” apical ES in step 8 spermatids that arises during spermiogenesis. This emerging new concept is the basis for many functional studies in the future.
Actin-Binding Proteins, Actin Dynamics and Spermiogenesis
Actin is a component of one of the three cytoskeletons in eukaryotic cells found in Sertoli and germ cells in the seminiferous epithelium, which exists either as globular actin (G-actin) or filamentous actin (F-actin).26 The formation and maintenance of F-actin filament bundles, such as those found at the ES, involves the assembly of actin monomers into filaments which are then bundled. This process is mediated by end-to-end and side-to-side protein contacts via the actions of formins [e.g., mDia1/2 (diaphanous-related formin proteins 1 and 2) are members of the formin family that are expressed by the rat testis],27 which initiate actin filament nucleation and elongation,28–30 and actin cross-linking proteins31,32 (note: cross-linkers that anchor the plasma membrane to the actin-based cytoskeleton, e.g., vinculin33) and actin-bundling proteins (i.e., crosslinking actin filaments into bundles),28,34 [e.g., espin,35 fimbrin,33 α-actinin,36 fascin,37 Eps8 (epidermal growth factor receptor pathway substrate 8, also an actin capping protein) 38], all of which have been shown to be putative components of the apical and basal ES. However, filament bundle plasticity is conferred by proteins that facilitate actin nucleation and actin filament branching [e.g., Arp3 (actin-related protein 3, a component of the Arp2/3 protein complex),39,40 N-WASP (neural or neuronal Wiskott-Aldrich syndrome protein),39,40 WAVE1 (WASP-family verprolin homologous protein 1),41 and cortactin40 (note: N-WASP, WAVE1 and cortactin are involved in Arp2/3 complex activation before the Arp2/3 complex can exert its actin nucleating and branching activity29,42)]. In essence, the Arp2/3 protein complex helps to create a branched actin network, thereby eliminating the “rigidity” associated with actin filament bundles at the apical ES. This thus destabilizes the ES, which in turn, facilitates spermatid movement across the epithelium via the action of endocytic vesicle-mediated protein trafficking events43,44 and likely involving polarity proteins as well45,46 (see Fig. 1). Furthermore, F-actin can be broken down (i.e., depolymerized) 47 by cofilin48 and/or gelsolin,49 both of which are found at the ES, converting F-actin into G-actin and facilitating spermatid movement. Additionally, actin reorganization is also maintained by GTPases, such as RhoB,50 Cdc4251 and Rac1.51 In short, these actin-binding proteins control the dynamics of the actin cytoskeleton via nucleation, elongation, capping, bundling, cross-linking, severing and depolymerization, thereby facilitating changes in cell shape and in the location of spermatids in the epithelium during spermiogenesis (Fig. 1). As noted above, virtually all the proteins that are involved in these processes have been identified in the testis during the past decade and localized to the apical and basal ES at sites where actin filament bundles are present,52,53 illustrating that they are involved in actin remodeling to facilitate spermiogenesis.
Does Drebrin E Serve as a Platform to Recruit Actin Regulatory Proteins to the ES in the Seminiferous Epithelium?
As reported in this issue of Spermatogenesis, we have identified drebrin E (developmentally regulated brain protein E) to be a component of the apical and basal ES in the rat testis. Drebrin was originally identified in avians as neuronal drebrin A (adult), along with two other embryonic isoforms known as E1 and E2.54,55 Initially, it was described as a brain protein that regulates cell shape and plasticity,56 in particular dendritic spine morphogenesis.57 Subsequent studies showed drebrins to be members of the ADF-H (actin depolymerizing factor homology) domain family of actin-binding proteins58 and to be involved in actin dynamics, including formation of actin bundles,59 recruitment of proteins (e.g., chemokine receptor CXCR4) via changes in actin polymerization,60 building of dendritic spines and stabilization of gap junctions,61 actin remodeling via its interaction with Ras GTPases,62 and formation of lamellipodia and filopodia.63 Thus, drebrins have numerous cellular functions via their role as actin-binding proteins. Interestingly, drebrins do not possess any F-actin severing, bundling, capping, nucleating or cross-linking activity per se, and they do not have any intrinsic biological activity.54,64–66 However, drebrins were found to compete with the binding of actin regulatory proteins, such as α-actinin, fascin and tropomyosin to F-actin,54,55,67,68 thereby regulating the actin network. In short, drebrins regulate actin dynamics largely via their ability to “maintain” the “proper” levels of actin regulatory proteins to specific cellular domains in response to changes in environment, growth and development, pathological conditions and toxicants.
Drebrin E was found to be an ES protein, displaying stage-specific expression at the apical and basal ES in the seminiferous epithelium during the epithelial cycle.67 The most striking observation is that the stage-specific expression of drebrin E closely resembles that reported for Arp3,39 a component of the actin branching nucleation regulatory protein Arp2/3 complex, particularly at the apical ES.39 More importantly, drebrin E was highly expressed at the apical ES at stage VII,67 co-localizing with Arp3 to the concave side of the elongating spermatid head where extensive endocytic vesicle-mediated protein trafficking events are known to take place, begining at stage VII to prepare for the release of sperm at spermiation at stage VIII.7,23 Recent studies have shown that proteins known to be involved in protein endocytosis, namely clathrin, cortactin and N-WASP, are also found at the same site.39,40,69 Additionally, drebrin E was found to structurally interact with Arp3, but not with occludin, FAK, β-catenin and Esp8 in the testis.67 Furthermore, the interaction of drebrin E and Arp3 was significantly induced following treatment of Sertoli cells with TGFβ3 and TNFα, which have been shown to induce spermatid loss from the epithelium when administered intratesticularly at concentrations that could be achieved under physiological conditions in the microenvironment, mimicking in many ways “spermiation.”70,71 Thus, these findings illustrate that drebrin E, although it has no intrinsic activity, can recruit Arp3, an actin branching nucleator, to the apical ES at stage VII of the epithelial cycle to induce actin remodeling via an increase in endocytic vesicle-mediated protein trafficking events, perhaps under the influence of cytokines (e.g., TGFβ3, TNFα) (Fig. 1). This, in turn, destabilizes the apical ES to prepare for spermiation because of changes in protein distribution at the apical ES (Fig. 1). Furthermore, endocytosed apical ES integral membrane proteins (e.g., N-cadherin, nectins, β1-integrin) at the “old” apical ES can be transcytosed and recycled to the “new” apical ES that arises as the result of spermiogenesis at the interface of the Sertoli cell and step 8 spermatid (Fig. 1).4 In short, this hypothesis is supported by the fact that apical ES degeneration at the luminal edge of the epithelium also marks the appearance of step 8 spermatids near the basal compartment, as well as the appearance of the “new” apical ES at the Sertoli cell-spermatid interface at stage VIII of the epithelial cycle.4 Thus, these events are analogous to restructuring of the BTB to facilitate the transit of preleptotene spermatocytes across the immunological barrier in which integral membranes at the “old” BTB are endocytosed, transcytosed and recycled to the “new” BTB to maintain the barrier integrity during spermatocyte transit.23 It is obvious that many other proteins are involved in these events, such as nonreceptor protein kinases, polarity proteins and endosomal proteins. For instance, drebrin E and Arp3 were virtually undetectable at the apical ES at the interface of step 8–17 spermatids-Sertoli cell, but expressed intensely only at the interface of step 18–19 spermatids-Sertoli cells at stages V–VII of the epithelial cycle.39,67 Thus, other actin bundling proteins, such as Eps8,38 are probably maintaining the integrity of actin filament bundles at the apical ES. Nonetheless, the model depicted in Figure 1 serves as a hypothesis upon which functional experiments can be designed. Future studies will help to understand the role of this critical actin-binding protein in spermiogenesis and spermiation.
Summary and Future Perspectives
As discussed above, drebrin E may serve as a platform to recruit necessary actin regulatory proteins to the apical ES to affect F-actin filament bundles which confer apical ES dynamics during spermiogenesis. Additional drebrin E binding partners, besides Arp3, at the apical ES must be identified. The mechanism(s) by which drebrin E recruits Arp3 to the apical ES at the interface of Sertoli cells and step 18–19 spermatids must also be delineated, which may involve nonreceptor protein kinases (e.g., c-Src, FAK) and polarity proteins (e.g., PAR3, Scribble). In short, drebrin E is likely working together with the Arp2/3 complex and Eps8 to modulate the conversion of F-actin filament bundles to branched actin network, thereby conferring “fluidity” at the apical ES which facilitates changes in spermatid shape and spermatid movement during spermiogenesis.
Acknowledgments
This work was supported by grants from the National Institutes of Health, NICHD, R01 HD056034; R01 HD056034-02-S1; and U54 HD029990 Project 5.
References
- 1.Vigodner M. Roles of small ubiquitin-related modifiers in male reproductive function. Int Rev Cell Mol Biol. 2011;288:227–259. doi: 10.1016/B978-0-12-386041-5.00006-6. [DOI] [PubMed] [Google Scholar]
- 2.Major AT, Whiley PA, Loveland KL. Expression of nucleocytoplasmic transport machinery: Clues to regulation of spermatogenic development. Biochem Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.03.008. In press. [DOI] [PubMed] [Google Scholar]
- 3.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 4.de Kretser D, Kerr J. The cytology of the testis. In: Knobil E,, et al., editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 5.Kierszenbaum AL. Mammalian spermatogenesis in vivo and in vitro: a partnership of spermatogenic and somatic cell lineages. Endocr Rev. 1994;15:116–134. doi: 10.1210/edrv-15-1-116. [DOI] [PubMed] [Google Scholar]
- 6.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Vol. 1. Austin, TX: Landes Bioscience/Springer Science + Business Media LLC; 2008. p. 15. [Google Scholar]
- 7.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–487. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- 10.Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl. 2007;65:33–43. [PubMed] [Google Scholar]
- 11.Chemes HE, Rawe VY. The making of abnormal spermatozoa: cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010;341:349–357. doi: 10.1007/s00441-010-1007-3. [DOI] [PubMed] [Google Scholar]
- 12.Rajender S, Rahul P, Mahdi AA. Mitochondria, spermatogenesis and male infertility. Mitochondrion. 2010;10:419–428. doi: 10.1016/j.mito.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Leblond C, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 14.Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revelaed by the periodic acid-fuchin sulfurous acid technique. Am J Anat. 1952;90:167–210. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- 15.Clermont Y, Leblond CP. Spermiogenesis of man, monkey, ram and other mammals as shown by the “periodic acid-Schiff” technique. Am J Anat. 1955;96:229–253. doi: 10.1002/aja.1000960203. [DOI] [PubMed] [Google Scholar]
- 16.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science + Business Media LLC; 2008. pp. 186–211. [Google Scholar]
- 17.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/S0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 18.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater FL: Cache River Press; 1990. [Google Scholar]
- 19.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/00408166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 21.Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- 22.Russell LD. Form, dimensions and cytology of mammalian Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Vol. 1. Clearwater: Cache River Press; 1993. p. 37. [Google Scholar]
- 23.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoligerm cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 25.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrin S, Mellor H. Actin stress fibers. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 27.Mironova E, Millette CF. Expression of the diaphanous-related formin proteins mDia1 and mDia2 in the rat testis. Dev Dyn. 2008;237:2170–2176. doi: 10.1002/dvdy.21622. [DOI] [PubMed] [Google Scholar]
- 28.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Firat-Karalar EN, Welch MD. New mechanisms and functions of actin nucleation. Curr Opin Cell Biol. 2011;23:4–13. doi: 10.1016/j.ceb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schonichen A, Geyer M. Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–163. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Bach LA, Gallicchio MA, McRobert EA, Tikoo A, Cooper ME. Effects of advanced glycation end products on ezrin-dependent functions in LLC-PK1 proximal tubule cells. Ann NY Acad Sci. 2005;1043:609–616. doi: 10.1196/annals.1338.069. [DOI] [PubMed] [Google Scholar]
- 32.Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/S0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 33.Grove B, Vogl A. Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–323. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- 34.Bartles J. Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol. 2000;12:72–78. doi: 10.1016/S0955-0674(99)00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartles J, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- 36.Yazama F, Sawada H, Hirosawa K, Hayashi Y, Nishida T. Deep-etch visualization of the Sertoli cell (blood-testis) barrier in the boar. Tissue Cell. 1991;23:235–246. doi: 10.1016/00408166(91)90078-8. [DOI] [PubMed] [Google Scholar]
- 37.Tubb B, et al. Testis fascin (FSCN3): a novel para-log of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. Exp Cell Res. 2002;275:92–109. doi: 10.1006/excr.2002.5486. [DOI] [PubMed] [Google Scholar]
- 38.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL) and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 41.Rawe VY, Ramalho-Santos J, Payne C, Chemes HE, Schatten G. WAVE1, an A-kinase anchoring protein, during mammalian spermatogenesis. Hum Reprod. 2004;19:2594–2604. doi: 10.1093/humrep/deh513. [DOI] [PubMed] [Google Scholar]
- 42.Rottner K, Hanisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–661. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 43.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–1279. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 44.Harris TJC, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 45.Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells. 2010;29:311–325. doi: 10.1007/s10059-0100053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttman JA, Obinata T, Shima J, Griswold MD, Vogl AW. Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol Reprod. 2004;70:805–812. doi: 10.1095/biolreprod.103.022723. [DOI] [PubMed] [Google Scholar]
- 49.Guttman JA, Janmey P, Vogl AW. Gelsolin—evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115:499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- 50.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 51.Chapin R, Wine R, Harris M, Borchers C, Haseman J. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–1052. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 52.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi K, et al. Domain analysis of the actinbinding and actin-remodeling activities of drebrin. Exp Cell Res. 1999;253:673–680. doi: 10.1006/excr.1999.4663. [DOI] [PubMed] [Google Scholar]
- 55.Shirao T. The roles of microfilament-associated proteins, drebrins, in brain morphogenesis: a review. J Biochem. 1995;117:231–236. doi: 10.1093/jb/117.2.231. [DOI] [PubMed] [Google Scholar]
- 56.Dun XP, Chilton JK. Control of cell shape and plasticity during development and disease by actin-binding protein Drebrin. Histol Histopathol. 2010;25:533–540. doi: 10.14670/HH-25.533. [DOI] [PubMed] [Google Scholar]
- 57.Seikino Y, Kojima N, Shirao T. Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int. 2007;51:92–104. doi: 10.1016/j.neuint.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 58.Lappalainen P, Kessels MM, Cope MJ, Drubin DG. The ADF homology (ADF-H) domain: a highly exploited actin-binding module. Mol Biol Cell. 1998;9:1951–1959. doi: 10.1091/mbc.9.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shirao T, et al. Formation of thick, curving bundles of actin by drebrin A expressed in fibroblasts. Exp Cell Res. 1994;215:145–153. doi: 10.1006/excr.1994.1326. [DOI] [PubMed] [Google Scholar]
- 60.Perez-Martinez M, et al. F-actin-binding protein drebrin regulates CXCR4 recruitment to the immune synapse. J Cell Sci. 2010;123:1160–1170. doi: 10.1242/jcs.064238. [DOI] [PubMed] [Google Scholar]
- 61.Majoul I, Shirao T, Seikino Y, Duden R. Many faces of drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes. Histochem Cell Biol. 2007;127:355–361. doi: 10.1007/s00418-0070273-y. [DOI] [PubMed] [Google Scholar]
- 62.Biou V, Brinkhaus H, Malenka RC, Matus A. Interactions between drebrin and Ras regulate dendritic spine plasticity. Eur J Neurosci. 2008;27:2847–2859. doi: 10.1111/j.14609568.2008.06269.x. [DOI] [PubMed] [Google Scholar]
- 63.Peitsch WK, et al. Drebrin particles: components in the ensemble of proteins regulating actin dynamics of lamellipodia and filopodia. Eur J Cell Biol. 2001;80:567–579. doi: 10.1078/01719335-00194. [DOI] [PubMed] [Google Scholar]
- 64.Grintsevich EE, et al. Mapping of drebrin binding site on F-actin. J Mol Biol. 2010;398:542–554. doi: 10.1016/j.jmb.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishikawa R, et al. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem. 1994;269:29928–2933. [PubMed] [Google Scholar]
- 66.Kessels MM, Engqvist-Goldstein AE, Drubin DG. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), an Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol Biol Cell. 2000;11:393–412. doi: 10.1091/mbc.11.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li MWM, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011 doi: 10.4161/spmg.1.2.16393. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peitsch WK, et al. Drebrin is a widespread actinassociating protein enriched at junctional plaques, defining a specific microfilament anchoring system in polar epithelial cells. Eur J Cell Biol. 1999;78:767–778. doi: 10.1016/S0171-9335(99)80027-2. [DOI] [PubMed] [Google Scholar]
- 69.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 70.Li MWM, et al. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 71.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TßR1, TAB1 and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–1813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 72.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGFβ3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]