Abstract
The blood-testis barrier (BTB) is a unique ultrastructure in the mammalian testis. Unlike other blood-tissue barriers, such as the blood-brain barrier and the blood-ocular (or blood-retina) barrier which formed by tight junctions (TJ) between endothelial cells of the microvessels, the BTB is constituted by coexisting TJ, basal ectoplasmic specialization (basal ES), desmosomes and gap junctions between adjacent Sertoli cells near the basement membrane of the seminiferous tubule. The BTB also divides the seminiferous epithelium into the apical (or adluminal) and basal compartments so that meiosis I and II and post-meiotic germ cell development can all take place in a specialized microenvironment in the apical compartment behind the BTB. While the unusual anatomical features of the BTB have been known for decades, the physiological function of the coexisting junctions, in particular the desmosome and gap junction, that constitute the BTB was unknown until recently. Based on recently published findings, we critically evaluate the role of the desmosome and gap junction that serve as a signaling platform to coordinate the “opening” and “closing” of the TJ-permeability barrier conferred by TJ and basal ES during the seminiferous epithelial cycle of spermatogenesis. This is made possible by polarity proteins working in concert with nonreceptor protein tyrosine kinases, such as focal adhesion kinase (FAK) and c-Src, at the site to regulate endosome-mediated protein trafficking events (e.g., endocytosis, transcytosis, recycling or protein degradation). These events not only serve to destabilize the existing “old” BTB above preleptotene spermatocytes in transit in “clones” at the BTB, but also contribute to the assembly of “new” BTB below the transiting spermatocytes. Furthermore, hemidesmosomes at the Sertoli cell-basement membrane interface also contribute to the BTB restructuring events at stage VIII of the epithelial cycle. Additionally, the findings that a gap junction at the BTB provides a possible route for the passage of toxicants [e.g., bisphenol A (BPA)] and potential male contraceptives (e.g., adjudin) across the BTB also illustrate that these coexisting junctions, while helpful to maintain the immunological barrier integrity during the transit of spermatocytes, can be the “gateway” to making the BTB so vulnerable to toxicants and/or chemicals, causing male reproductive dysfunction.
Key words: testis, spermatogenesis, seminiferous epithelial cycle, blood-testis barrier, Sertoli cell, estrogens, bisphenol A, environmental toxicants, adjudin
Introduction
In the mammalian testis, the blood-testis barrier (BTB) forms during puberty in humans (at ∼12–14 years of age) and a functional BTB is established by ∼17–21 day postpartum in rats,1,2 coinciding with the differentiation of Type B spermatogonia to prepare spermatocytes for meiosis I and meiosis II.3 This timing also coincides with terminal differentiation of Sertoli cells which cease to proliferate by ∼15–17 days of age postpartum in rats.4 Thus the Sertoli cell population remains relatively constant in adult mammals with ∼30 million Sertoli cells per testis in adult rats at 150 and 240 days of age post-partum,5 and each Sertoli cell is known to support ∼30–50 developing germ cells in the seminiferous epithelium.6,7 However, recent studies have shown that Sertoli cells are capable of dividing even in adult animals including rats8 and mice9 under experimental and even under physiological conditions in humans.9,10 The BTB anatomically divides the seminiferous epithelium into the basal and apical (or adluminal) compartments (see Fig. 1). This ultrastructure is created by specialized junctions between adjacent Sertoli cells near the basement membrane, and it is crucial to confer cell polarity, to limit paracellular transport of biomolecules, ions, electrolytes and water from the basal to the apical compartment, and to maintain an immunological barrier to sequester post-meiotic germ cell development from the systemic circulation.1,11 Thus, spermiogenesis and spermiation take place in a specialized microenvironment at the apical compartment of the seminiferous epithelium composed of only Sertoli cells and developing germ cells.1,12,13 An earlier study has shown that when the BTB assembly was delayed by ∼4 wk via treatment of neonatal rats with a toxicant diethylstilbestrol, spermatogonial differentiation and the onset of meiosis would be delayed, and their resumption correlate with the establishment of an intact and functional BTB.2 A more recent study has shown that spermatogenesis failed to reinitiate in rats treated with adjudin 1-(2,4-dichorobenzyl)-1H-indazole-3-carbohydrazide] at high doses (e.g., 250 mg/kg b.w.) that irreversibly disrupted the BTB integrity, even though the population of spermatogonial stem cells/spermatogonia did not reduce significantly.14 Interestingly, rats treated with adjudin at a lower dose, such as at 50 mg/kg b.w. which also perturbed the BTB integrity transiently and reversibly, managed to re-initiate spermatogonial differentiation and spermatogenesis when the BTB functionality was restored.14 These findings thus illustrate the significance of the BTB in maintaining spermatogenesis. Unlike other blood-tissue barriers, such as the blood-brain barrier and the blood-ocular barrier which are constituted by TJ-permeability barrier of the endothelial cells in the microvessels in the brain and the eye, respectively,11,15,16 the BTB is constituted by coexisting tight junction (TJ, also known as zonula occludens), basal ectoplasmic specialization [basal ES, a testis-specific adherens junction (AJ, also known as zonula adherens) type], desmosome (also known as macula adherens) and gap junction1 (see Fig. 1). More importantly, in addition to TJ protein complexes (e.g., occludin-ZO-1, JAM-A-ZO-1, claudin-ZO-1), the BTB is composed of adhesion protein complexes found in other junction types, including adherens junction (AJ, e.g., N-cadherin/β-catenin; nectin-afadin), desmosome (e.g., desmoglein-2-desmocollin-2) and gap junction (e.g., connexin 43-plakophilin-2) (see Fig. 1).1,17,18 Yet the roles of desmosome and gap junction at the BTB remain unknown for decades since their first discoveries in the 1970s.19–21 Nevertheless, recent studies have shown that they are crucial to provide the necessary cross-talk between different junction types at the BTB. Thus, the “opening/disruption” and “closing/reassembly” of different junction types can be coordinated temporally and spatially, thereby facilitating the transit of preleptotene spermatocytes across the BTB at stage VIII of the seminiferous epithelial cycle while maintaining the immunological barrier.
Figure 1.
A schematic illustration of the blood-testis barrier (BTB) in the seminiferous epithelium of adult mammalian testis. This is a schematic representation of the seminiferous epithelium in the adult rat testis of a stage VII tubule. Anatomically, the BTB divides the seminiferous epithelium (composed of only Sertoli and germ cells, lying on top of the basement membrane, which is a modified form of extracellular matrix63,64) into the basal and apical (adluminal) compartments. The BTB is constituted by coexisting TJ, basal ES, desmosome and gap junction. The most obvious feature of the BTB is the tightly packed actin filament bundles which are lying parallel to the plasma membrane and are sandwiched in between cisternae of endoplasmic reticulum and the opposing plasma membranes of the two adjacent Sertoli cells. This tripartite structure is known as the basal ES. This feature is also found at the Sertoli cell-spermatid (step 8–19 spermatids in rats) interface known as the apical ES in the apical compartment, which is the only anchoring device once it appears during spermiogenesis. At the BTB, TJ and gap junction coexist with basal ES, whereas desmosome is an intermediate filament-based anchoring junction type at the BTB, illustrating that the actin- and the intermediate filament-based cytoskeletons are working in concert to regulate BTB dynamics during spermatogenesis. The unusually organized network of actin filament associated with the plasma membrane at the BTB and apical ES are maintained via the intricate interactions of: (1) several actin regulatory proteins (e.g., Eps8, an actin bundling and capping protein) and Arp2/3 protein complex (an actin nucleation protein causing the branching of actin filaments, thereby disrupting the actin filament bundles at the ES, replacing them with branched actin network) and (2) the properly organized cell adhesion protein complexes at the TJ (e.g., occludin-ZO-1) and basal ES (e.g., N-cadherin-β-catenin), which are maintained by the signaling action of the protein complexes at the desmosome (e.g., desmoglein-2/desmocollin-2/c-Src) and the gap junction (e.g., connexin43-plakophilin-2-c-Src) as well as the polarity proteins (e.g., PAR3, PAR6, 14-3-3, Cdc42).
What are the Roles of the Desmosome and Gap Junction at the BTB?
Introduction.
A desmosome (also known as macula adherens) is an intermediate filament-based cell-cell anchoring junction type in which the integral membrane proteins (known as the desmosomal cadherins: desmogleins and desmocollins) are anchored to intermediate filaments (composed of vimentin in adult testes) via the cytolinker desmoplakin.22,23 Desmosomal cadherin-desmoplakin interactions are further reinforced by the armadillo proteins plakoglobin and plakophilin, forming a distinctive electron dense ultrastructure under electron microscopy at the Sertoli-Sertoli cell interface at the BTB.17,24–26 In the testis, besides the BTB, desmosomes can also be found at the Sertoli cell-spermatid (limited to step 1–7 spermatids) or Sertoli-spermatocyte/spermatogonium interface.19,27 A gap junction, on the other hand, is a cell-cell actin-based communicating junction type.21,28–30 A functional gap junction communication channel is composed of two opposing connexons (the functional units of gap junction with each connexon known as a hemichannel) between adjacent Sertoli cells at the BTB [or between Sertoli cells and spermatids (step 1–7 spermatids only), spermatocytes or spermatogonia].21,28 Each connexon is composed of a hexamer of gap junction proteins known as connexins (Cx). At least 20 connexins are found in humans and rodents, such as Cx43, Cx26 and Cx33. The gap junction channels were shown to permit the diffusion of solutes of <1–1.5 kDa in molecular mass.31–33 More recent studies have shown that substances of large molecular sizes, such as siRNA duplexes, polypeptides and cyclic nucleotides can also pass through the gap junction channels with selective permeability.34 Thus, while desmosomes contribute to the cell adhesion function at the BTB, gap junction communication channels provide the means for signaling to coordinate cellular events at the BTB. However, recent findings illustrate that both desmosome and gap junction provide an unusual platform for cell signaling at the BTB, which are discussed in the following sections.
Role of desmosomes.
Recent studies have unequivocally demonstrated that desmosomes are performing some unique and significant roles at the BTB. First, it was shown that while the knockdown of desmoglein-2 or desmocollin-2 alone in the Sertoli cell epithelium by RNAi using specific siRNA duplexes failed to perturb the Sertoli cell TJ-permeability barrier, the knockdown of either desmoglein-2 alone or desmoglein-2 and desmocollin-2 combined caused mis-localization of ZO-1, moving from the Sertoli-Sertoli cell surface to cell cytosol.35 More importantly, a knockdown of both desmoglein-2 and desmocollin-2 by RNAi was found to perturb the Sertoli cell TJ-permeability function in addition to a mis-localization of both c-Src (a nonreceptor protein tyrosine kinase) and coxsackievirus and adenovirus receptor (CAR, an integral membrane protein and a cell adhesion molecule at the BTB,35a,37,58 which likely interacts with the N-cadherin-based adhesion protein complex at the basal ES via β-catenin as its binding partner37), moving from the cell-cell interface to cell cytosol.35 Second, the mis-localization of CAR in the Sertoli cell epithelium induced by the knockdown of desmoglein-2 and desmocollin-2 was shown to be the result of an increase in endocytosis of CAR,35 thereby destabilizing the cell adhesion function at the BTB. Third, studies by co-immunoprecipitation have shown that while desmoglein-2 did not structurally interact with occludin, ZO-1 or Cx43, it was tightly associated c-Src;35 and other studies have shown that c-Src interacted with Cx43,36 CAR,37 occludin, N-cadherin and ZO-1.38 Thus, the desmoglein-2/desmocollin-2/c-Src desmosomal protein complex at the BTB may be an important regulatory complex with c-Src being served as the dominant regulator to confer proper phosphorylation status of proteins at the BTB. The knockdown of desmoglein-2/desmocollin-2 by RNAi in the Sertoli cell epithelium led to improper localization of c-Src which moved away from the cell-cell interface to cytosol,35 thereby “messing up” the homeostasis of c-Src signaling function, leading to improper phosphorylation status of crucial proteins at the Sertoli-Sertoli cell interface (e.g., CAR, ZO-1) and affecting their kinetics of endocytosis. All these contribute to a destabilization of the adhesion function at the site, which in turn, affect the TJ-permeability barrier. Collectively, these findings illustrate that besides conferring adhesion function at the BTB, desmosome serves as a signaling platform at the BTB via its interaction with c-Src.
Role of gap junctions.
It is known that gap junctions that lie behind the junctional complexes (composed of TJ, AJ and desmosomes) in multiple epithelia provide the necessary cross-talk and signaling function for the coordination of cellular events to maintain the homeostasis of an epithelium.30,33,39 However, the function of gap junctions at the BTB remains largely unknown until recently because they are found to be intermingled with TJ, basal ES and desmosome instead of being separate entities as in other epithelia (see Fig. 1). It was found that in Sertoli cell epithelium with an established functional TJ-barrier and having ultrastructures that mimicked the Sertoli cell BTB in vivo, a knockdown of Cx43 or its adaptor protein plakophilin-2 (also an adaptor for desmosome, illustrating a tight structural association between actin-based gap junction and intermediate filament-based desmosome at the BTB in the mammalian testis) using specific siRNA duplexes failed to perturbed the Sertoli cell TJ-barrier function.36 However, a simultaneous knockdown of Cx43 and plakophilin-2 by RNAi led to a disruption of the Sertoli cell TJ-permeability barrier, possibly caused by the mis-localization of occludin and ZO-1, with these proteins moving from the Sertoli-Sertoli cell interface to cell cytosol, thereby destabilizing cell adhesion at the TJ and leading to barrier disruption.36 More important, even though the knockdown of Cx43 alone did not perturb the Sertoli cell TJ-barrier function,36 subsequent studies using the [Ca2+]-switch model and the bisphenol A (BPA) model showed that Cx43 knockdown hindered these cells from “re-assembling” a disrupted TJ-barrier.40 These findings thus illustrate that while Cx43 may not be critical for the assembly of a “new” TJ-barrier, it is essential for the maintenance of an established TJ-barrier, such as at the BTB in adult mammalian testes, via its effects on TJ reassembly during spermatogenesis when the BTB is transiently disrupted during the epithelial cycle (e.g., at stage VIII to facilitate the transit of preleptotene spermatocytes), as manifested by a loss of intracellular [Ca2+] or following the exposure of Sertoli cell epithelium to an environmental toxicant BPA.40 Similar to a number of BTB protein complexes (e.g., desmoglein-2-based complex),35 the Cx43-based gap junction protein complex also contains c-Src as an integrated component,36 illustrating that c-Src is likely to modify the phosphorylation status of connexins at the gap junction, thereby regulating the kinetics of their endocytic vesicle-mediated protein trafficking events.
What are the Roles of Polarity Proteins and Hemidesmosome on BTB Dynamics?
Polarity proteins.
Earlier studies in Drosophila melanogaster and Caenorhabditis elegans have shown that cellular asymmetry and apico-basal polarity are established and maintained by polarity proteins.41–44 There are three modules of polarity protein complexes known as (1) the PAR (partitioning defective) protein complex [e.g., PAR3/PAR6/aPKC (atypical protein kinase C, a nonreceptor Ser/Thr protein kinase)], (2) the Crumbs protein complex [e.g., Crumbs-3/PALS1 (protein associated with (LIN-7)-1)/PATJ (PALS1-associated tight-junction protein)] and (3) the Scribble complex [e.g., Scribble/LGL1/2 (Lethal giant larvae 1/2)/DLG1 (Discs large 1)]. Apico-basal polarity is conferred by the differential localization of polarity complexes: the PAR- and the Crumbs-based complexes are localized predominantly to TJ and the Scribble-based protein complex displays basolateral localization. Since each of these protein complexes recruit their own binding partners (including adaptors, kinases, phosphatases and scaffolding proteins), the mutual exclusion between the Scribble-based complex and the TJ localized polarity protein complexes thus confers apico-basal polarity and asymmetrical distribution of proteins and organelles within an epithelial cell. These polarity proteins have at least one homolog found in mammals.41,44 Indeed, recent studies have shown that many of these proteins are expressed by Sertoli and/or germ cells,45 regulating cell polarity in the seminiferous epithelium. For instance, Sertoli cell nuclei are restricted to the basal compartment near the basement membrane, and the heads of developing spermatids are all pointing toward the basement membrane, illustrating cell polarity in the seminiferous epithelium.44
Besides conferring Sertoli cell and spermatid polarity in the testis, polarity proteins (e.g., PAR3, PAR6) were recently shown to be involved in cell adhesion at the Sertoli cell-elongating spermatid interface (known as apical ES) and Sertoli-Sertoli cell interface at the BTB.45 For instance, a knockdown of either PAR3 or PAR6 led to a redistribution of JAM-A and α-catenin at the Sertoli-Sertoli cell interface, moving from cell surface to cell cytosol, thereby destabilizing cell adhesion at the TJ-barrier.45 These findings also implicate polarity proteins may be involved in endocytic vesicle-mediated protein trafficking events, such as endocytosis, transcytosis, recycling and endosome-mediated protein degradation. In fact, subsequent studies have shown that polarity proteins (e.g., 14-3-3 also known as PAR5 and Cdc42 also called cell division control protein 42 and a member of the Rho GTPases are integrated components of the PAR-based polarity protein complex) are involved in protein endocytosis at the Sertoli cell BTB, since a knockdown of 14-3-3 by RNAi led to a significant increase in the kinetics of endocytosis of JAM-A and N-cadherin.46 Additionally, overexpression of a Cdc42 dominant negative mutant in Sertoli cells abolished the TGFβ3-induced acceleration in occludin and CAR endocytosis in the Sertoli cell epithelium, thereby blocking the TGFβ3-induced disruption of the Sertoli cell TJ-barrier.47 These findings thus illustrate that polarity proteins are crucial for the endocytic vesicle-mediated protein trafficking events at the Sertoli BTB. Taking these data collectively, it is likely that the predominant localization of polarity proteins at the BTB (e.g., PAR6 14-3-3) and their stage-specific expression during the seminiferous epithelial cycle are crucial for the desmosome- and gap junction-mediated effects on protein distribution, which require changes in protein endocytosis, transcytosis and/or recycling. The net result of these changes thus regulates cell adhesion at the apical ES, and basal ES at the BTB.
Hemidesmosome.
Hemidesmosome is an intermediate filament-based cell-matrix anchoring junction type found at the Sertoli cell-basement membrane interface in the mammalian testis.1 Its composition and function in the testis remain largely unknown, except that β1-integrin and α2-laminin chain appear to be the putative components of the hemidesmosome.48 Most importantly, a knockdown of β1-integrin in Sertoli cell epithelium (without any residual elongating/elongated spermatids and thus no apical ES was present) not only perturbed the hemidesmosome function, but also led to a disruption of the Sertoli cell TJ-barrier function. This effect resulted from the induction of occludin endocytosis and its redistribution from cell surface to cell cytosol, thereby disrupting cell adhesion function at the BTB.48 It remains to be determined the involvement of polarity proteins in this event. These findings thus demonstrate that a disruption of β1-integrin function at the hemidesmosome would affect TJ-barrier function, illustrating a physiological link exists between junctions at the BTB and hemidesmosome.
Does the Gap Junction that Provide the Necessary Cross-Talk between Different Junction Types at the BTB also Make the BTB More Vulnerable to Environmental Toxicants?
As briefly discussed above regarding the role of desmosome and gap junction at the BTB, these two junction types at the BTB likely provide the signaling function and cross-talk between different junction types, maintaining the immunological barrier during the epithelial cycle. Furthermore, molecular modeling studies have shown that toxicants (e.g., BPA), estrogens (e.g., estradiol-17β) and male contraceptives (e.g., adjudin) that can modulate Sertoli cell BTB function can interact with the gap junction communicating channel with putative docking domains (see Figs. 2–4). These interactions can possibly lead to the blockage of Cx43-based gap junction-based communication, as well as the penetration of the BTB by utilizing the gap junction communication junction as a “gateway” and gaining entry into the apical compartment (Figs. 2–4). Indeed, BPA was recently shown to perturb gap junction communication based on a functional dye-transfer assay.40 Based on these findings and those discussed above,1,18,49 we herein propose a hypothetical model (see Fig. 5) summarizing the molecular basis of BTB restructuring during spermatogenesis. In brief, during stage VIII of the epithelial cycle, “new” TJ-fibrils are being established behind the preleptotene spermatocytes in transit at the BTB via testosterone-induced de novo protein synthesis,50–53 as well as testosterone- or cytokine-mediated transcytosis and recycling of integral membrane protein complexes from the “old” BTB site above the transiting spermatocytes.54–56 Once the “new” BTB is established, the “old” BTB site undergoes complete degeneration induced by cytokines (e.g., TNFα, TGFβ3, IL-1α).54–57 Furthermore, TJ, basal ES, desmosome and gap junction proteins expressed by germ cells (e.g., preleptotene spermatocytes) during their transit at the BTB can also form inter-locking interactions with the corresponding proteins in Sertoli cells, even “loosely” associated as recently proposed.58 This thus avoids the formation of a “disrupted” or an “open” configuration at the BTB to elicit an unwanted immunological response in the host immune system. In short, these events, as discussed in above sections and recently published,35,36,40 are regulated and coordinated by gap junctions and desmosomes at the BTB, so that different junction types can be “disrupted” and “re-established” orderly and perhaps in sequence, illustrating the physiological significance of gap junctions and desmosomes at the BTB.
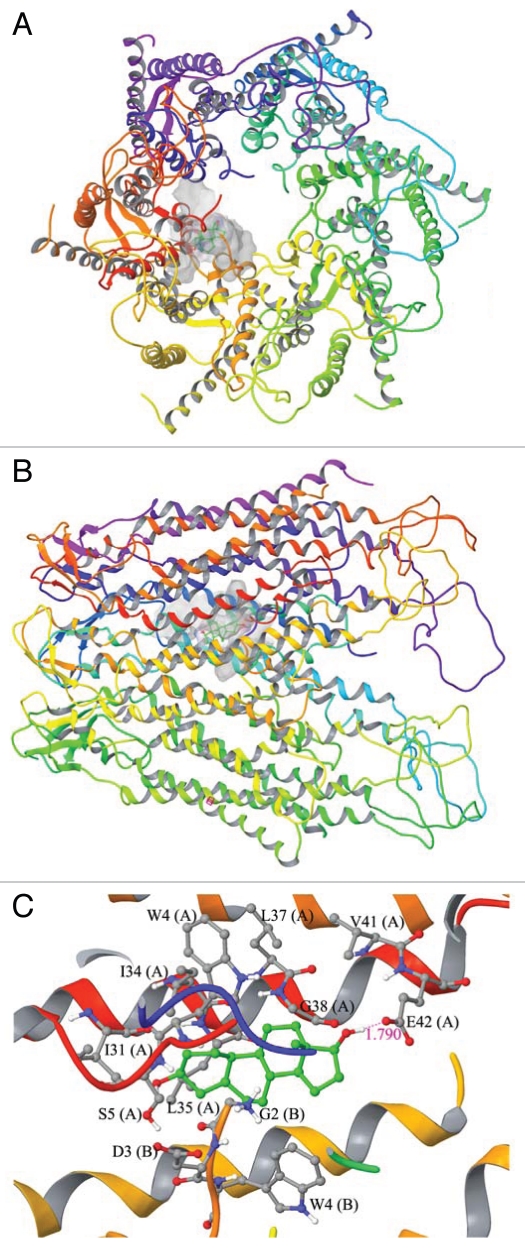
Figure 2.
A molecular modeling study to assess the docked complex of estradiol-17β with the Cx43-based gap junction communication channel. (A) Topographic view of the Cx43-gap junction protein complex with estradiol-17β. (B) Side view of the Cx43-gap junction protein complex with estradiol-17β. (C) Ball and stick model for the interaction residues and secondary structure representation for protein between estradiol-17β and Cx43. The SOSUI WWW server65 was used to predict transmembrane helices for connexin 43 (Cx43)-based gap junction (GJ) communicating channel. The characteristic feature of the Cx43-GJ channel is the spanning of transmembrane helices in the cell membrane that creates the “pore” which allows passage of small molecules (see “A” and “B”). The crystal structure of human Cx26 at 3.5 Å resolution in humans is known,66 and this information was used for the molecular modeling of Cx43. Initially, the amino acid sequence of Cx43 [UniProtKB ID: P08050] was retrieved from UniProtKB database (www.uniprot.org).67 The crystal structure of Cx26 was retrieved from Protein Data Bank (PDB ID: 2ZW3).68 The MODELLER 9v7 program69 was used for sequence alignment and model building.70 The sequence and structural alignment of Cx43 to human Cx26 was carried out using ‘align2d’ program in MODELLER 9v7. This alignment study illustrated 45% sequence identity in the transmembrane region between the two proteins, which suggested that the most important pore region is conserved between Cx26 and Cx43. The produced alignment was then used to generate hemichannel structure with ‘model-multichain’ program in MODELLER 9v7. The quality of the structure was assessed by submitting the modeled structure into PROCHECK.71 The loop building and energy minimization were carried out with Swiss-PdbViewer. The energy computations were done by GROMOS96 implemented in Swiss-PdbViewer. The modeled three dimensional structure of Cx43 was prepared using Schrödinger Suite 2009 Protein Preparation Wizard. All the hydrogens were added to the backbone of the structure and bond orders was assigned appropriately. Exhaustive sampling method was used for the optimization of the hydrogen bonding network, in which the orientation of hydroxyl groups, amide groups of Asn and Gln, and imidazole ring of His residues were optimized. The RMSD (root mean square deviation) of atom displacement for terminating the minimization was set to be 0.30 Å. The minimization was carried out with OPLS2005 force field. The ligand structure of estradiol-17β (CID: 5757), bisphenol A (CID: 6623) (Fig. 3) and adjudin (CID: 9819086) (Fig. 4) were retrieved from NCBI-PubChem database. LigPrep (Version 2.3, Schrödinger, LLC, New York, NY 2009) was used to generate 3D structure with correct chiralities for each ligand used in this study. Grid files represent the volume of the receptor that can be searched for ligand docking. Grid box was set on the centriod of all the residues in the Cx43 and no constraints were selected. The Standard Precision (SP) mode of Glide (Version 5.5, Schrödinger, LLC, New York, NY 2009) was used for the flexible ligand docking. The best binding conformation was selected based on the one having the lowest docking energy from the generated docking solutions. Molecular docking of estradiol-17β into Cx43 generated 32 solutions, in which the best binding conformation having the docking score of −5.40 kcal/mol. The size of binding site surface area of Cx43 was 864.562 Å, and the size of ligand surface was 271.946 Å. These docking studies revealed that side chain oxygen atom of Glu42 (E42, Chain A) makes a hydrogen bond formation with estradiol-17β in the bond length of 1.790 Å. In addition, Gly2 (G2, Chain B), Asp3 (D3, Chain B), Trp4 (W4, Chain A and B), Ser5 (S5, Chain A), Ile31 (I31, Chain A), Ile34 (I34, Chain A), Leu35 (L35, Chain A), Leu37 (L37, Chain A), Gly38 (G38, Chain A) and Val41 (V41, Chain A) were involved in non-bonded interactions such as van der Waals forces. It is important to note that estradiol-17β binds with amino acid residues of N-terminal helix (NTH) and Trans membrane helix 1 (TMH1) of chain A and B alone and it leaves empty space in the pore region. Thus, it is possible that additional units of estradiol-17β may also bind with remaining chains to alter the Cx43 gating mechanism.
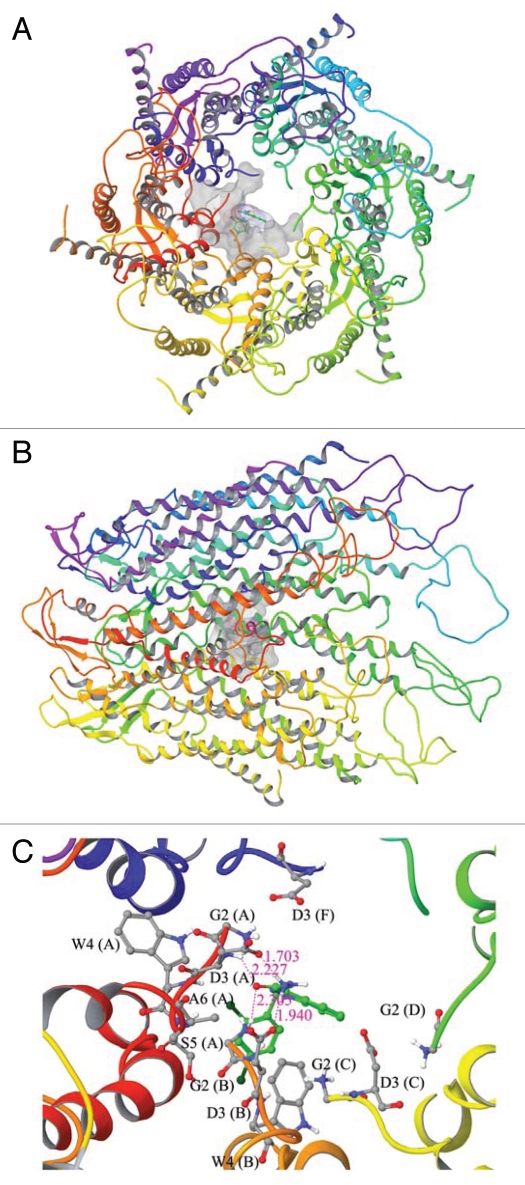
Figure 4.
A molecular modeling study to assess the docked complex of adjudin with Cx43-based gap junction communication channel. (A) Topographic view of the Cx43-gap junction protein complex with adjudin. (B) Side view of the Cx43-gap junction protein complex with adjudin. (C) Ball and stick model for the interaction residues and secondary structure representation for protein between adjudin and Cx43. Docking of adjudin into Cx43 shows strong interactions in the pore lining residues of N-terminal helix (NTH). The amino acid residues such as Gly2 (G2, Chain B) and Asp3 (D3, Chain A and B) were involved in multiple hydrogen bond formation with adjudin (C). The backbone nitrogen atom of Gly2 (G2, Chain B) and side chain nitrogen atom of Asp3 (D3, Chain B) were shown to have hydrogen bond interaction with adjudin in the bond distances of 2.305 Å and 1.940 Å, respectively. Whereas the side chain oxygen of Asp3 (D3, Chain A) and backbone nitrogen atom of Asp3 (D3, Chain A) were also involved in hydrogen bond formation in the bond distance of 1.703 Å and 2.227 Å, respectively. In addition to hydrogen bonds, Gly2 (G2, Chain A, C, D and F), Asp3 (D3, Chain C), Trp3 (W3, Chain A and B), Ser5 (S5, Chain A) and Ala6 (A6, Chain A) were involved in non-bonded interaction to further stabilize the interaction. The docking score of this binding complex was −4.47 kcal/mol. The size of surface area for binding site and ligand were 978.172 Å and 290.038 Å, respectively. This docking result has shown that adjudin binds with pore lining N-terminal helix residues of chain (A–D) and (F) except chain (E). Hence, it has the possibility that adjudin can block the gating of this channel protein. From these docking studies, we have observed that large size of both binding site surface in BPA and ligand surface in adjudin makes very strong binding into N-terminal helix of Cx43. On the other hand, docking of estradiol-17β reveals very little docking energy when compared to docking of other two compounds, which indicates estradiol-17β can make very stable binding into Cx43-based GJ communication channel. It can also be noted that both NTH and TMH1 amino residues are involved in the binding pocket for estradiol-17β interactions. Our docking results disclose that each of the compounds is forming at least one hydrogen bond with the binding site residues in the Cx43-based communication channel. As a whole we concluded that all three compounds can regulate the function of Cx43 by binding with pore lining residues of this protein.
Figure 5.
A schematic illustration of a molecular/biochemical model on the regulation of BTB restructuring during the seminiferous epithelial cycle of spermatogenesis in adult mammalian testes. This model was prepared based on recent findings in studies in the rat testis (see text). On the left part, an intact and functional BTB is shown in which the BTB is constituted by actin-based TJ-, basal ES- and gap junction-protein complexes, as well as the intermediate filament-based desmosome. The adhesion function of these protein complexes are maintained by the actin filament bundles sandwiched in between cisternae of endoplasmic reticulum and the apposing plasma membranes of two adjacent Sertoli cells provided by the basal ES, and the integrity of the actin filament bundles is maintained by their intriguing interactions with Eps8 and Arp2/3 protein complex. Intermediate filaments at the desmosome also contribute to the integrity of the BTB, and it is obvious that there are cross-talks between intermediate filaments and actin-based cytoskeleton to maintain the BTB function. Both protein kinases and polarity proteins are also involved in maintaining the proper phosphorylation status and the cellular distribution of the adhesion protein complexes at the BTB. During stage VIII of the epithelial cycle when BTB undergoes restructuring to accommodate the transit of preleptotene spermatocytes (which are interconnected by intercellular bridges as “clones”72–75), “new” TJ-fibrils that create a “new” BTB are being assembled behind the transiting spermatocytes via de novo synthesis of BTB proteins under the influence of androgen and estrogen. Furthermore, proteins at the “old” BTB site also undergo endocytosis facilitated by polarity proteins (e.g., 14-3-3, PAR6). These protein trafficking events are perhaps triggered by c-Src- and/or FAK-mediated phosphorylation under the influence of cytokines (TGFβ3, TNFα, IL-1α) and steroids (e.g., testosterone, estradiol-17β), so that they can be transcytosed and recycled to the “new” BTB site, while some unwanted endocytosed proteins are targeted to degradation via the endosome- or ubiquitin-mediated pathways. Through this mechanism, the integrity of the immunological barrier can be maintained because there is no “actual” “opening” or “degeneration” of the BTB at any time during the transit of preleptotene spermatocytes at the site. Furthermore, TJ, basal ES, desmosome and gap junction integral membrane proteins are also expressed on the cell surface of spermatocytes, so that they can form interlocking interactions with the corresponding proteins on the Sertoli cell plasma membrane to maintain “loosely” associated protein complexes during their transit at the BTB.
Recent studies using a bisphenol A (BPA) model (note: BPA is an estrogenic environmental toxicant that interacts with estrogen receptor in mammalian cells59–61) have shown that the BPA-induced disruption of the Sertoli cell TJ-barrier, both in vitro and in vivo in immature rats,62 is likely mediated by a disruption of the gap junction communication channel function as demonstrated by a functional dye-transfer assay.40 These findings also provide the first functional proof regarding the role of gap junctions at the BTB. They also prompted us to examine if this BPA-induced Sertoli cell TJ-barrier disruption is mediated by a blockade of the gap junction function due to the physical interaction of BPA with the Cx43-based gap junction channel. Using molecular modeling approach, it was found that similar to estradiol-17β (Fig. 2), BPA (Fig. 3) was capable of interacting with Cx43-based gap junction channel via a putative “docking” domain in the Cx43-based connexon. These findings thus provide a molecular basis that BPA-induced blockage of gap junction channel-mediated dye transfer40 is likely to be the result of BPA “docking” in the functional channel, disrupting the necessary cross-talk maintained by the Cx43-based gap junctions at the BTB. The molecular docking findings (Figs. 2 and 3) also illustrate that BPA can possibly traverse the BTB to enter the apical compartment via the gap junction channels.
Figure 3.
A molecular modeling study to assess the docked complex of bisphenol A (BPA) with Cx43-based gap junction communication channel. (A) Topographic view of the Cx43-gap junction protein complex with BPA. (B) Side view of the Cx43-gap junction protein complex with BPA. (C) Ball and stick model for the interaction residues and secondary structure representation for protein between BPA and Cx43. Molecular docking of bisphenol A into Cx43 shows formation of strong interactions by making two hydrogen bonds in the complex. The side chain oxygen atom of Asp3 (D3, Chain A) and backbone nitrogen atoms of Gly2 (G2, Chain A) were involved in hydrogen bond formation at the bond distance of 1.587 Å and 1.748 Å, respectively. In addition to these residues, Gly2 (G2, Chain B and F), Asp3 (D3, Chain B–F), Trp4 (W4, Chain A and F), Ser5 (S5, Chain F), Ala6 (A6, Chain B, E and F) and Leu7 (L7, Chain B and C) were involved in non-bonded interactions, such as van der Waals forces. Asp3 (D3) and Trp4 (W4) are the major residues involved in the interaction in this docked complex. Glide docking score of the complex was −4.07 kcal/mol. The binding site surface and ligand surface area of the docked complex were 1494.841 Å and 253.687 Å, respectively. It is noted that the residues of N-terminal helix (NTH) in Cx43 alone was involved in the interactions with BPA. The compound BPA appears to occupy largest binding site surface area in the Cx43 and can block the channel based on the molecular modeling, which is consistent with recent findings using a functional GJ communication assay.40
A recent study has shown that rats treated with adjudin at 50 mg/kg b.w. (by gavage) displayed a transient BTB disruption (wherein BTB was disrupted by ∼6–12 wk, but it was fully recovered by 20 wk post adjudin treatment), but when adjudin was used at a higher dose at 250 mg/kg b.w. (by gavage), BTB was disrupted by 2 wk but did not recover up to 30 wk at the end of the experimental period suggesting a permanent BTB damage.14 Interestingly, spermatogenesis failed to “re-initiate” in the high-dose treated group while spermatogenesis re-initiates gradually in the low-dose adjudin treated group. Thus, we sought to examine if adjudin could also interact with the Cx43-based gap junction channel via a “docking” domain. As shown in Figure 4, adjudin was found to interact with the gap junction channel. These observations are significant since gap junctions at the BTB, unlike other blood-tissue barriers, are not sequestered from the junctional complexes behind the TJ and the AJ and desmosome plaques. Instead, they are intermingled with other junction types and are immediately accessible to toxicants (e.g., BPA) or chemicals (e.g., adjudin) when the host, including rodents and humans, is exposed to harmful substances that exert their effects via the gap junction. This thus renders the BTB (and the testis) more vulnerable to environmental toxicants than the TJ-permeability barrier of the microvessels in the interstitium. This apparently new concept also opens a possible new window of research to “manage” toxicant-induced male reproductive dysfunction by protecting or sequestering gap junctions and desmosomes from these toxicants.
Concluding Remarks and Future Perspectives
Herein, we review some unexpected findings based on recent reports regarding the role of gap junctions and desmosomes that coexist with TJ and basal ES at the BTB, illustrating these junctions provide the necessary cross-talks and serve as the signaling platform to maintain the homeostasis of different junction types in the seminiferous epithelium during the cyclic restructuring of the BTB throughout the seminiferous epithelial cycle of spermatogenesis. A loss of the function of these proteins, such as Cx43, plakophillin-2, desmoglein-2, desmocollin-2, PAR3, PAR6, 14-3-3 and Cdc42, even transiently, leads to a disruption of the Sertoli cell TJ-permeability barrier by causing mislocalization of proteins at the TJ (e.g., occludin, ZO-1) and the basal ES (e.g., N-cadherin, β-catenin). These effects apparently are mediated by an alteration of the endocytic vesicle-mediated protein trafficking events at the site (e.g., endocytosis, recycling), which in turn are regulated by the PAR-based polarity proteins (e.g., PAR6, 14-3-3, Cdc42). These findings thus prompt us to propose a likely molecular regulatory pathway that maintains the immunological barrier during the transit of preleptotene spermatocytes at the BTB at stage VIII of the epithelial cycle as depicted in Figure 5, in which desmosomes, gap junctions and polarity proteins are working in concert with protein kinases (e.g., FAK, c-Src) to establish a “new” BTB behind the transiting spermatocytes while an orderly disassembly of the “old” BTB can take place to avoid a disruption or an unwanted “fall through” of the immunological barrier.
Acknowledgments
Studies in the authors' laboratories were supported by grants from the National Institutes of Health (R01 HD056034 to C.Y.C.; R01 HD056034-02-S1 to C.Y.C.; U54 HD029990, Project 5 to C.Y.C.; R03 HD061401 to D.D.M.); Hong Kong Research Grants Council (HKU772009 to W.Y.L.; HKU7693/07M to W.M.L.); and the Government of India, Department of Biotechnology (BT/BI/03/015/2002 to P.P.M.) and Department of Information Technology (DIT/R&D/BIO/15(9)/2007 to P.P.M.).
References
- 1.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyama Y, Ohkawa M, Oku R, Maekawa M, Yuasa S. Neonatally administered diethylstilbestrol retards the development of the blood-testis barrier in the rat. J Androl. 2001;22:413–423. [PubMed] [Google Scholar]
- 3.Clermont Y, Perry B. Quantitative study of the cell population of the seminiferous tubules in immature rats. J Anat. 1957;100:241–267. doi: 10.1002/aja.1001000205. [DOI] [PubMed] [Google Scholar]
- 4.Orth JM. Proliferation of Sertoli cells in fetal and post-natal rats: A quantitative autoradiographic study. Anat Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 5.Berndtson WE, Thompson TL. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl. 1990;11:429–435. [PubMed] [Google Scholar]
- 6.Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- 7.Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration and dimensions. Am J Anat. 1983;167:143–161. doi: 10.1002/aja.1001670202. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol Reprod. 2005;72:1205–1217. doi: 10.1095/biolreprod.104.035717. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–1091. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- 10.Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, et al. Characterization and functionality of proliferative human Sertoli cells. Cell Transplant. 2011 doi: 10.3727/096368910X536563. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr Topics Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2011 doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easton AS. Regulation of permeability across the blood-brain barrier. In: Cheng CY, editor. Biology and Regulation of Blood-Tissue Barriers. Austin, TX: Landes Bioscience and Springer Science Business Media LLC; 2011. In press. [Google Scholar]
- 16.Hosoya KI, Tachikawa M. The inner blood-retinal barrier: Molecular structure and transport biology. In: Cheng CY, editor. Biology and Regulation of Blood-Tissue Barriers. Austin, TX: Landes Bioscience and Springer Science+Business Media LLC; 2011. In press. [Google Scholar]
- 17.Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction: A versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell LD. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- 20.Vogl A, Vaid K, Guttman J. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience and Springer Science + Business Media LLC; 2008. pp. 186–211. [Google Scholar]
- 21.Enders G. Sertoli-Sertoli and Sertoli-germ cell communications. In: Russell L, Griswold M, editors. The Sertoli Cell. St. Louis, MO: Cache River Press; 1990. pp. 447–460. [Google Scholar]
- 22.Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- 23.Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Pespect Biol. 2009;1:2543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mruk DD, Cheng CY. Desmosomes in the testis: Moving into an unchartered territory. Spermatogenesis. 2011;1:47–51. doi: 10.4161/spmg.1.1.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCrea PD, Gu D. The catenin family at a glance. J Cell Sci. 2010;123:637–642. doi: 10.1242/jcs.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 28.Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. In: Cheng CY, editor. Biology and Regulation of Blood-Tissue Barriers. Austin, TX: Landes Bioscience and Springer Science + Business Media LLC; 2011. In press. [Google Scholar]
- 29.Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D'hondt C, Bultynck G, et al. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815:13–25. doi: 10.1016/j.bbcan.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeaeting the junctional membrane channels. Science. 1977;195:294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- 32.Loewenstein WR. Junctional intercellular communciation: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 33.Maeda S, Tsukihara T. Structure of the gap junction channel and its implications for its biological functions. Cell Mol Life Sci. 2011;68:1115–1129. doi: 10.1007/s00018-010-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein-complexes regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Mirza M, Petersen C, Nordqvist K, Sollerbrandt K. Coxsackievirus and adenovirus receptor is up-regulated in migratory germ cells during passage of the blood-testis barrier. Endocrinology. 2007;148:5459–5469. doi: 10.1210/en.2007-0359. [DOI] [PubMed] [Google Scholar]
- 36.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee NPY, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- 39.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harbor Perspect Biol. 2009;1:2576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein B, Macara IG. The PAR proteins: Fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGFβ3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng CY, Wong EWP, Yan HHN, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang RS, Decrock E, De Vuyst E, Ponsaerts R, D'hondt C, Bultynck G, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 51.Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 52.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16670. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaitu'u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 54.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia W, Wong EWP, Mruk DD, Cheng CY. TGFβ3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGFβ3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lie PPY, Cheng CY, Mruk DD. Interleukin-1α is a regulator of the blood-testis barrier. FASEB J. 2011;25:1244–1253. doi: 10.1096/fj.10-169995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang CQF, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol. 2007;178:549–556. doi: 10.1083/jcb.200704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong EWP, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng CY, Wong EWP, Lie PPY, Li MWM, Su L, Siu ER, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 64.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 65.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–379. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 66.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the Cx26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 67.Consortium U. The Universal Protein Resource (UniProt) Nucleic Acid Res. 2009;37:169–174. doi: 10.1093/nar/gkn664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berman HM, et al. The Protein Data Bank. Nucleic Acid Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol. 2003;374:461–491. doi: 10.1016/S0076-6879(03)74020-8. [DOI] [PubMed] [Google Scholar]
- 70.Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, et al. Comparative protein structure modeling with MODELLER. Curr Protoc Bioinformatics. 2006;15:1–30. doi: 10.1002/0471250953.bi0506s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roman A, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 72.Miething A. Local desynchronization of cellular development within mammalian male germ cell clones. Ann Anat. 2010;192:247–250. doi: 10.1016/j.aanat.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Hamer G, Roepers-Gajadien HL, Gademan IS, Kal HB, De Rooij DG. Intercellular bridges and apoptosis in clones of male germ cells. Int J Androl. 2003;26:348–353. doi: 10.1111/j.1365-2605.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 74.Ren HP, Russell LD. Clonal development of interconnected germ cells in the rat and its relationship to the segmental and subsegmental organization of spermatogenesis. Am J Anat. 1991;192:121–128. doi: 10.1002/aja.1001920203. [DOI] [PubMed] [Google Scholar]
- 75.Russell LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell. 1981;13:349–367. doi: 10.1016/0040-8166(81)90010-0. [DOI] [PubMed] [Google Scholar]