Abstract
Immunoblotting is an analytical technique used by many laboratories to study protein expression. It involves electrophoretic separation of proteins by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), immobilization of these proteins onto a membrane of either nitrocellulose or polyvinylidene difluoride, incubation of the membrane in a monoclonal or polyclonal antibody and detection by a standard method such as enhanced chemiluminescence (ECL). To achieve this, most laboratories opt to use commercially-available chemiluminescence kits which are acceptable but relatively expensive. In this technical report, we show that a self-prepared chemiluminescence reagent is superior to a commercially obtained kit in terms of sensitivity, duration of signal, ease-of-use and shelf-life but at a fraction of the cost of a kit.
Keywords: enhanced chemiluminescence, immunoblotting, protein electrophoresis, antibody, electrophoretic transfer
Introduction
Immunoblotting (i.e., western blotting) is a powerful technique used to detect a specific protein within a given sample or set of samples.1 For example, immunoblotting can be used to effectively assess changes in the protein level of any target gene following a specific cellular treatment, following overexpression or knockout/knockdown of a gene or following co-immunoprecipitation to study different scientific disciplines including spermatogenesis. Thus, it is a technique used by a vast number of laboratories, including ours,2–5 to generate meaningful and interpretable results with relative ease. The most popular method used to visualize a protein at the nanogram level involves enhanced chemiluminescence, and several easy-to-use detection kits are commercially available to accomplish this goal, except that these kits are expensive and reagents have a relatively short shelf-life. Consequently, these pricey kits can put enormous strain on any investigator managing a multi-person lab but with a limited supply budget. After scanning the literature carefully, testing chemicals from different vendors and trying different experimental conditions, we show that there exists an excellent alternative to commercially-available enhanced chemiluminescence kits. In this technical report, we summarize our findings (Fig. 1) and present this protocol which is based on an earlier publication but with minor modifications,6 hoping that it is useful for investigators in the field. We also include some helpful tips on how to avoid high background during immunoblotting.
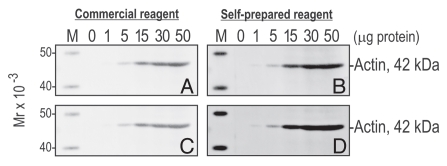
Figure 1.
A comparison between commercial and self-prepared ECL reagents. Increasing concentrations of protein [adult testis lysate prepared in lysis buffer: 50 mM Tris pH 7.4 at 22 °C containing 0.15 M NaCl, 2 mM EDTA, 1% NP-40 (v/v), 10% glycerol (v/v), protease and phosphatases inhibitor cocktails] ranging from 1 to 50 µg total protein (protein estimation was performed by using the Pierce BCA protein assay kit; Thermo Scientific, Rockford, IL) were resolved by SDS-PAGE under reducing conditions and transferred onto a nitrocellulose membrane (BIO-RAD, Hercules, CA) for 4 hr. Thereafter, the nitrocellulose membrane was blocked in 5% non-fat milk (w/v) dissolved in wash buffer [PBS-Tris/0.1% Tween-20 (v/v)] for 1 hr at R.T. with gentle agitation on an orbital shaker, followed by a 10-min washing step in which the wash buffer was changed five times to remove all traces of non-fat milk. The nitrocellulose membrane was incubated in anti-actin IgG [diluted 1:200 in PBS-Tris pH 7.4 at 22 °C containing 0.1% Tween-20 (v/v), 0.1% BSA (w/v) and 0.05% NaN3 (w/v); cat. no. sc-1616; lot no. K0510, Santa Cruz Biotechnology, Santa Cruz, CA] for ∼3 hr at R.T. with agitation. After washing, the membrane was incubated in bovine anti-goat IgG-HRP [diluted 1:2000 in PBS-Tris pH 7.4 at 22 °C containing 0.1% Tween-20 (v/v) and 0.1% BSA (w/v); cat. no. sc-2378; lot. no. D0910], followed by extensive washing as described above. The membrane was cut into two halves, and each half was incubated in ECL reagent. (A and B) Immunoblots incubated in commercial ECL reagent (A) and self-prepared ECL reagent (B) as described above. Both blots were exposed for 1 min in a FujiFilm LAS-4000 mini luminescent image analyzer (GE Life Sciences, Piscataway, NJ). An immunoreactive band corresponding to actin (42 kDa) was detected in both instances. (C and D) Both immunoblots in (A and B) were set-aside for 1 hr at which time they were re-exposed for 10 min. All images were unaltered for brightness and contrast. M, Magic Mark XP western protein standard, 2 µl/lane (Invitrogen, Carlsbad, CA).
Materials and Reagents
Luminol (C8H7N3O2, 5-amino-2,3-dihydro-1,4-phthalazinedione, also known as 3-aminophthalhydrazide), ≥97% HPLC (cat. no. A8511, Sigma-Aldrich, St. Louis, MO); 250 mM luminol prepared in dimethyl sulfoxide (DMSO), stored in ∼55 µl aliquots at −20°C
p-Coumaric acid, ≥98% HPLC (cat. no. C9008, Sigma-Aldrich); 90 mM p-coumaric prepared in DMSO, stored in ∼25 µl aliquots at −20°C
H2O2 solution, 30% (w/v) (cat. no. 216763, Sigma-Aldrich)
DMSO, ≥99.5% (cat. no. D5879, Sigma-Aldrich)
ECL buffer; 0.1 M Tris pH 8.6 at 22°C, stored at 4°C
Methods
After SDS-PAGE7 and electrophoretic transfer,8 block the membrane in 5% non-fat dry milk (w/v) (Nestle USA Division Beverage, Freehold, NJ) dissolved in a suitable wash buffer [i.e., PBS-Tris/Tween-20: 10 mM Tris pH 7.4 at 22°C containing 0.15 M NaCl, 10 mM NaH2PO4 and 0.1% Tween-20 (v/v)] for 1 hr at room temperature (R.T.) with gentle agitation on an orbital laboratory shaker (e.g., Barnstead/Lab Line Lab Rotator, Model 1309). Other blocking solutions such as BSA (bovine serum albumin) may also be used, if needed.
Wash the membrane for a total of 10 min with several changes of wash buffer.
Incubate the membrane in primary antibody.
Wash the membrane for a total of 10 min with two changes of wash buffer.
Incubate the membrane in the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody.
Wash the membrane with several changes of wash buffer. We routinely wash our membranes nine times at 5 min per wash.
Drain the membrane of wash buffer. Position the membrane on a piece of Saran Wrap and quickly combine the following in chronological order while stirring: 10 ml ECL buffer (see Materials and Reagents), 22 µl p-coumaric acid, 50 µl luminol and 3 µl H2O2. Pour onto membrane and allow to stand undisturbed for 90 sec. Drain the membrane of ECL detection reagent, wrap the membrane in Saran Wrap or a sheet protector and expose.
Additional Notes
H2O2 should be fresh (i.e., purchased within the past 6 months).
A common problem associated with immunoblotting is high background, which can affect data quality.
Tween-20 can cause high background as it can interfere with the ECL reagent. Rinsing the membrane briefly with Milli-Q or double-distilled water just prior to ECL detection may alleviate high background. It should be noted that a membrane rinsed with water tends to air-dry more quickly than one that was rinsed with wash buffer so that the ECL detection reagent should be prepared promptly.
High background can also be alleviated by briefly re-blocking the membrane with non-fat milk prior to incubation in secondary antibody. Milk concentration and blocking time should be determined empirically, but 5–10% milk (w/v) for 5–10 min at R.T. with gentle agitation usually works well for many of the primary antibodies we had studied. This should be followed by a brief washing step prior to incubation in secondary antibody.
Finally, washing the membrane with high salt wash buffer [10 mM Tris pH 7.4 at 22 °C containing 0.5 M NaCl, 1 mM NaH2PO4, 0.2% SDS (w/v) and 0.1% Tween-20 (v/v)] for 30 min at R.T. with gentle agitation, followed by a brief rinse with Milli-Q or double-distilled water and ECL detection, can also remove persistent background in some cases.
References
- 1.Burnette WN. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 2.Lie PP, Cheng CY, Mruk DD. The biology of the desmosome-like junction: a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol. 2011;286:223–269. doi: 10.1016/B978-0-12-385859-7.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su L, Cheng CY, Mruk DD. Drug transporter, P-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Int J Biochem Cell Biol. 2009;41:2578–2587. doi: 10.1016/j.biocel.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 5.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1459. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haan C, Behrmann I. A cost effective non-commercial ECL-solution for Western blot detections yielding strong signals and low background. J Immunol Methods. 2007;318:11–19. doi: 10.1016/j.jim.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitro-cellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]