Abstract
Background:
Monosodium glutamate (MSG), a sodium salt of glutamic acid is commonly used as flavor enhancer in Chinese, Japanese and ready to serve foods all over the World, is the inducer of oxidative stress. In the present era, MSG and alcohol is becoming a part of daily food. Concomitantly, there is a tremendous increase in the incidences of cardiovascular diseases. So, the present study was designed to elucidate the effect of MSG by evaluating the changes in oxidative stress markers in cardiac tissue of normal and alcoholic adult male mice.
Materials and Methods:
Animals were divided into six groups of six mice each and MSG at dose levels of 0, 4, and 8 mg/g body weight was given orally for seven consecutive days (that is from 31st day to 37th day of alcohol ingestion) to chronic alcoholic (30% ethanol/100 g body weight) adult male mice. After the dose period (38th day), animals were fasted overnight, sacrificed by decapitation and hearts were removed for the estimation of lipid peroxidation (LPO), xanthine oxidase (XOD), superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) and its metabolizing enzymes like glutathione peroxidase (GPx) and glutathione reductase (GR).
Results:
A significant (P < 0.001) increase was observed in LPO and XOD levels while a significant decrease (P < 0.001) in the levels of SOD, CAT, GSH, GPx and GR was found in cardiac tissue of normal and alcoholic animals.
Conclusion:
These observations suggested that oral ingestion of MSG at dose levels of 4 mg/g body weight and above with and without alcohol increased the oxidative stress and thereby, could act as an additional factor for the initiation of atherosclerosis.
Keywords: Alcohol, atherosclerosis, lipid peroxidation, monosodium glutamate, oxidative stress
INTRODUCTION
Coronary heart diseases (CHD), is a single most important disease entity responsible for both, high mortality and morbidity rates, in the entire World population. It remains a major challenge to the health care managers and scientists. It is predicted that by the year 2020, this disease would persist as the most common and major threat to human life.[1] The underlying cause of this disease is atherosclerosis- a slowly progressive disease which begins in childhood, and manifests in the middle age or later. Atherosclerosis is a multifactorial disease and results from complex interactions among injurious stimuli and healing and reparative responses of the arterial wall occurring, in a hyperlipidemic and dyslipoproteinemic environment. Scientific literature review reflects the opinion that oxidative stress because of formation of free radicals is likely to be involved in the pathogenesis of various diseases like atherosclerosis. Previously, we reported that monosodium glutamate (MSG), a sodium salt of glutamic acid [C5H8NO4NaH2O] and used as flavor enhancer in variety of foods prepared at home/ restaurants and in ready to serve foods like two minute noodles, soup, sauces etc. increased the oxidative stress in various tissues.[2–5] Also, now a day, the younger generation has been found to be more inclined towards Chinese, Japanese and ready to serve foods. Concomitantly, there is a tremendous increase in the incidences of CHD and atherosclerosis in developed and developing countries during the last decade.
In the present era, alcohol, a well known risk factor for atherosclerosis[6–10] and MSG, an inducer of oxidative stress as reported by us,[2–5] are becoming an integral part of daily food, especially in younger generation. There are many reports in literature that the age for the establishment of atherosclerosis in the present times has reduced to 25-30 years, whereas it was more commonly seen in the elderly population few decades back. This is an alarming situation, as the number of premature deaths due to coronary heart disease (CHD) is increasing tremendously. Hence, in our present work, we have studied the effect of oral ingestion of MSG on the cardiac tissue of normal and chronic alcoholic adult male mice. Here, we have tried to ascertain whether MSG could act in synergism with alcohol for the initiation of atherosclerosis by observing its effect on oxidative stress markers like lipid peroxidation (LPO); free radical initiating enzymes like xanthine oxidase (XOD); free radical scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione (GSH); and, metabolizing enzymes such as glutathione peroxidase (GPx) and glutathione reductase (GR).
MATERIALS AND METHODS
Animals
Normal adult male mice (LAKA, US) weighing 25-30 g in body weight were procured from the animal house of Panjab University, Chandigarh - India. Animals were maintained on standard pellet diet (Hindustan Lever Ltd., Bombay) with free access to water.
Grouping
Animals were divided into six groups of 6 mice each and MSG at dose levels of 0, 4 and 8 mg/g body weight was given orally for 7 consecutive days to normal adult male mice and alcoholic animals (from 31st day to 37th day of alcohol ingestion at dose level of chronic alcoholic 30% ethanol/100 g body weight adult male mice) as follows:
Group-I (Control): 0 mg MSG/g body weight
Group-II: 4 mg MSG/g body weight
Group-III: 8 mg MSG/g body weight
Group-IV: Alcohol for 37 days (30% ethanol/100g body weight).
Group-V: Alcohol for 37 days + 4 mg MSG/g body weight (from 31st to 37th day orally).
Group-VI: Alcohol for 37 days + 8 mg MSG/g body weight (from 31st to 37th day orally).
This experimental design was approved by the Animal Experimental Ethics Committee of Panjab University, Chandigarh and conducted according to Indian National Science Academy Guidelines for the use and care of experimental animals.
Sample preparation
After the dose period (38th day), animals were fasted overnight and sacrificed by decapitation. The hearts were removed, kept on ice and washed with ice-colded saline. 10% homogenate was prepared in potassium phosphate buffer (100 mM, pH 7.5) containing 0.15 M KCl and was centrifuged at 1000 xg for 15 minutes in cold centrifuge (4 ° C). The supernatant was stored at 4 ° C and used for various biochemical assays.
Biochemical assays
Lipid peroxidation (LPO)
The LPO levels were assayed by measuring the pink color chromophore formed by the reaction of thiobarbituric acid with malondialdehyde (MDA) according to the method of Beuge and Aust, 1978.[11]
Xanthine oxidase (EC 1.2.3.2)
The activity XOD was measured by the method of Fried and Fried, 1974[12] using nitro blue tetrazolium (NBT) which formed farmazan. The increase in the intensity of color with time was measured spectrophotometrically at 540 nm for 10 minutes.
Superoxide dismutase (EC 1.5.1.1)
The activity of SOD was assayed by applying the method of Kono, 1978.[13] The activity of SOD was measured by monitoring the rate of inhibition of NBT reduction. One unit is defined as the amount of enzyme, which caused half-maximal inhibition of NBT reduction.
Catalase (EC 1.11.1.6)
The CAT activity was estimated by the method of Luck 1971[14] in which decomposition of H2O2 catalyzed by this enzyme was measured by decrease in absorbance at 240 nm, taking 0.0394 mM-1 cm-1 extinction coefficient and enzyme activity was expressed as nmol of H2O2 degraded/min/ mg protein.
Reduced glutathione (GSH)
GSH level was estimated by the method of Beutler et al., 1963,[15] using 5-5’ dithiobis2-notrobenzoic acid.
Glutathione peroxidase (EC 1.11.1.7)
GPx activity was measured by using H2O2 as a substrate by applying the method of Rotruck et al., 1973.[16]
Glutathione reductase (EC 1.6.4.2)
The activity GR was estimated by measuring the change in absorbance at 340 nm due to NADH utilization and GR activity was expressed as n moles NADPH oxidized/min/ mg proteins using an extinction coefficient of 6.22 mM-1 cm-1 by the method of William and Arscott, 1971.[17]
Protein assay
The protein contents were estimated by Lowry et al., 1951 methods.[18]
Statistical analysis
Results of biochemical analyses are presented as mean value ± standard deviation (S.D.). The difference between control and test groups was analyzed by using Student “t” test (significant difference at p < 0.05 confidence level). Correlation between the investigated groups was performed using test ONE-WAY ANOVA (one-way variance analysis).
RESULTS AND DISCUSSION
The activity of XOD, a superoxide initiating enzyme was found to be significantly increased by 8.00% (p < 0.05) in 4 mg MSG/g body weight (Group-II), 19.00% (p < 0.01) in 8 mg MSG/g body weight (Group-III), 12.00% in chronic alcoholic group (Group-IV), 26.00% (p < 0.05) in Alc. + 4 mg MSG/g body weight (Group-V), and 61.00% (P < 0.001) in Alc. + 8 mg MSG/g body weight (Group-VI) with respect to control animals [Figure 1a] and a significant increase by 12.50% and 43.75% (P < 0.001) was observed in group-V and group-VI, respectively, in cardiac tissue with respect to chronic alcoholic animals not receiving MSG [Figure 1b]. XOD, a highly versatile enzyme that is widely distributed from bacteria to human, exists predominantly as NAD+ dependent xanthine dehydrogenase (XDH), that itself has no role in the initiation or potentiation of oxidative damage in cells. However, in many pathological conditions XDH is converted into XOD.[19] XOD, catalyses the oxidation of hypoxanthine/xanthine to uric acid and generates superoxide radical (O2.-). H2O2 formed from O2.- could be converted into highly reactive hydroxyl radical (.OH) leading to high oxidative stress as a result of oxidation of biological molecules. A significant increase in XOD activity in 4 and 8 mg MSG/g body weight orally ingested normal adult male mice (Group-II and Group-III) and alcoholic animals (Group-V and Group-VI) could produce a burst of free radicals. Once O2.- radical is produced, H2O2 and .OH are continuously produced by Haber-Weiss reaction and/or Fenton type reaction.[20] Oxygen radicals might cause the lipid peroxidation of biomembrane through a chain reaction. The first step is the initiation reaction, which begins by taking out hydrogen atoms from polyunsaturated fatty acid (PUFA) by oxygen radical. The second is the propagation and the final step is termination. The extent of LPO has often been determined by the thiobarbituric acid (TBA) test, which has also been considered for the detection of malondialdehyde. A significant increase in LPO levels in all the treated groups [Figure 2a and 2b] was observed in the present study, which might lead to increased susceptibility of the biomembrane, and ultimately cause tissue injury/damage.
Figure 1.
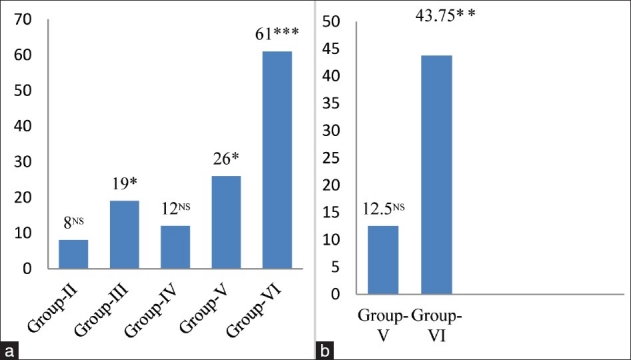
(a) Changes in xanthine oxidase levels in cardiac tissue of different groups. Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to control (Group-I).NS: Non significant, *P < 0.05, ***P < 0.001, (b) Changes in xanthine oxidase levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). NS: Non significant, ***P< 0.001
Figure 2.
(a) Changes in lipid peroxidation levels in cardiac tissue of different groups. Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to control (Group-I). *P < 0.05, **P < 0.01***P < 0.001, (b) Changes in lipid peroxidation levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). *P < 0.05, **P < 0.01
The level of SOD, a superoxide radical scavenging enzyme, was decreased by 8.85% in group-II, 19.42% (P < 0.05) in group-III and 13.75% (P < 0.05) in group-IV with respect to. group-I [Figure 3a] and SOD activity was significantly decreased by 23.99% (P < 0.05) and 45.14% (P < 0.01) in group-V and group-VI as compared to alcoholic animals respectively [Figure 3b]. SOD is considered the first line of defense against the deleterious effect of oxygen radicals in the cells, and it scavenges reactive oxygen radical species by catalyzing the dismutation of O2.- radical to H2O2 and O2. In mammals, three isozyms of SOD, that is CuZn- SOD, Mn-SOD and EC-SOD are seen.[21] CuZn-SOD is located primarily in the cytosol, and consists of two protein sub units, each having an active site, one containing Cu ion and the other containing Zn ion. Cu ion serves as an active redox site, and Zn ion maintain the protein structure. Mn-SOD is mainly located in mitochondrial matrix.[22] It has four subunits, each one with a Mn ion. EC-SOD is present in the plasma, bound to the heparin sulfate ion on the surface of endothelial cells. EC-SOD is a tetrameric glycoprotein, which contains Cu and Zn ion. The presence of SOD in various compartments of our body enables it to dismutate O2. radicals’ immediately, and protect the cells from oxidative damage. A significant inhibition of SOD activity in cardiac tissue of 4 and 8 mg MSG/g body weight ingested normo and alcoholic mice may result in an increased flux of O2.- radicals, and cause the tissue damage/injury as reflected in our study.
Figure 3.
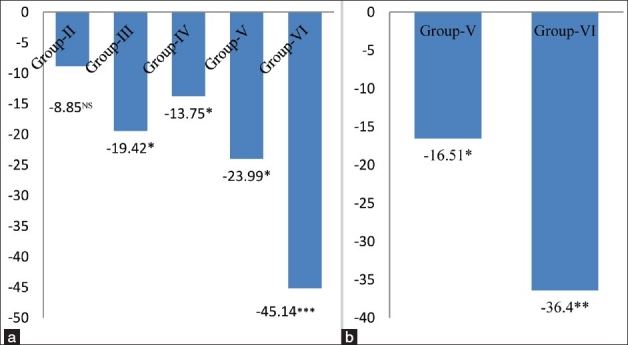
(a) Changes in superoxide dismutase levels in cardiac tissue of different groups. Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to control (Group-I). NS: Non significant, *P < 0.05, ***P < 0.001, (b) Changes in superoxide dismutase levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). *P < 0.05, **P < 0.01
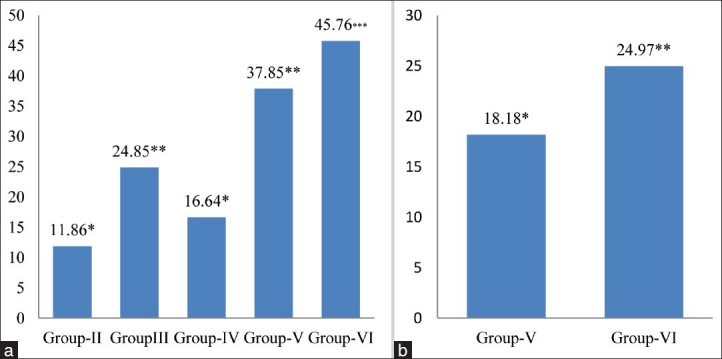
The activity of CAT, another potent antioxidant enzyme, especially against the O2.- radicals and singlet oxygen, was also significantly decreased in cardiac tissue by 14.09% (P < 0.05) in group-II, 21.40% (P < 0.01) in group-III, 14.88% in group-IV, 22.97% (P < 0.01) in group-V and 45.69% (P < 0.001) in group-VI respectively, as compared to the control animals [Figure 4a], and a similar trend in the activity of CAT was also observed in the 4 and 8 mg MSG/g body weight treated alcoholic mice (Group-V and Group-VI) as compared to chronic alcoholic (Group-IV) animals [Figure 4b]. CAT protects cells from the accumulation of H2O2 by dismutating it to form H2O and O2, or by using it as an oxidant, in which it works as a peroxidase. Therefore, the decrease in the activity of CAT observed in the present work could be due to less availability of NADH as MSG favors lipogenesis;[23] and hence, MSG along with alcohol had no beneficial effect on CAT activity to reduce lipogenesis/oxidative stress.
Figure 4.
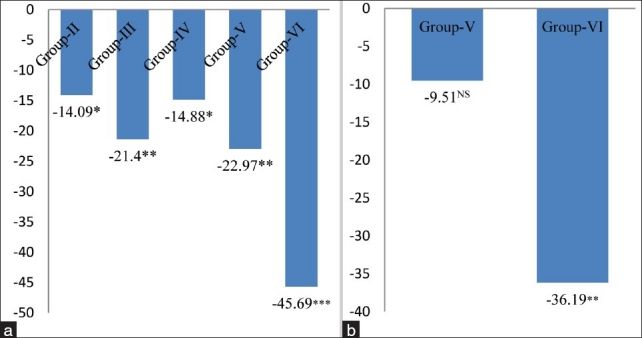
(a) Changes in catalase levels in cardiac tissue of different groups. Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to control (Group-I). *P < 0.05, **P < 0.01***P < 0.001, (b) Changes in catalase levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). NS: Non significant,*P < 0.05, ***P < 0.001
Glutathione (GSH), a tripeptide is maintained in a reduced state by an efficient glutathione peroxidase/glutathione reductase system. Glutathione is a potent endogenous antioxidant that helps to protect the body cells from a number of noxious stimuli including oxygen derived free radicals.[24,25] A significant decrease in the glutathione levels might be accompanied by a significant increase in the LPO levels. In the present work, the level of glutathione significantly decreased by 13.99% (P < 0.05) in 4 mg MSG/g body weight (Group-II), 21.15% (P < 0.05) in 8 mg MSG/g body weight ingested normal adult animals (Group-III), 21.81% (P < 0.05) in chronic alcoholic mice (Group-IV), 33.85% (P < 0.01) in 4 mg MSG/g body weight (Group-V) and 43.88% (P < 0.001) in 8 mg MSG/g body weight (Group-VI) ingested alcoholic with respect to the control group (Group-I) mice [Figure 5a] and a significant decrease was also observed by 15.57% (P < 0.05) and 28.22% (P < 0.01) in 4 and 8 mgMSG /g body weight ingested alcoholic mice as compared to alcoholic mice not receiving MSG (Group-IV), respectively [Figure 5b]. Reduced levels of GSH confirm an increased susceptibility to oxidative damage and this observation is an agreement with the reports that inverse relationship exists between LPO and glutathione status. Glutathione depletion of 20% to 30% can impair the cell defense against the toxic action of xenobiotics and may lead to cell injury/death.[3,26]
Figure 5.
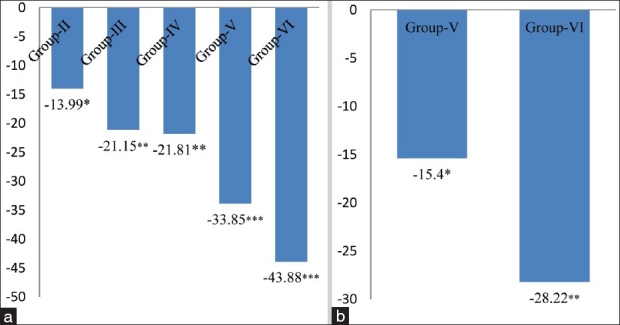
(a) Changes in glutathione levels in cardiac tissue of different groups. Values are expressed as mean ± SD of six observations. The values in figure represent the percent change with respect to control (Group-I). *P < 0.05, **P < 0.01***P < 0.001, (b) Changes in glutathione levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± SD of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). *P < 0.05, **P < 0.01
The activity of GPx, a selenium-containing enzyme was found to be decreased by 12.15% (P < 0.05), 23.13% (P < 0.01), 21.17%% (P < 0.05), 38.82% (P < 0.01) and 55.68% (P < 0.001) in group-II, group-III, group-IV, group-V and group-VI respectively, with respect to group-I [Figure 6a]; and, a significant inhibition in GPx activity was also found by 22.38% (P < 0.01) in group-V, and 43.78% (P < 0.001) in group-VI as compared to alcoholic group not receiving MSG [Figure 6b]. GPx catalyses the reduction of a variety of hydrogen peroxide molecules (ROOH and H2O2) using glutathione as a substrate, thereby protecting mammalian cells against oxidative stress.[27] It is well reported that low activity of this enzyme may render the tissue more susceptible to lipid peroxidation damage. Accordingly, in the present work, we observed a significant decrease in the GPx activity upon increase in the LPO level. This observation is in accordance with the hypothesis that LPO and GPx might play a role in tissue damage.[28–30] A significant decrease was observed in the levels of GR in different groups from 10.25% to 54.31% [Figure 7a and 7b]. The significant inhibition in the activity of GR in cardiac tissue is attributed to an increased oxidation or decreased synthesis of GSH. The less availability of NADPH may also cause a decrease in GR activity.[31] In conclusion, the aforementioned observations suggested that ingestion of MSG at dose levels of 4 mg/g body weight and above in normal and alcoholic animals increased the oxidative stress by altering the levels of oxidative stress markers like LPO, XOD, SOD, CAT, GSH, GPx and GR in cardiac tissue of adult male mice. Hence, MSG might act as an additional factor along with alcohol for the initiation of atherosclerosis.
Figure 6.
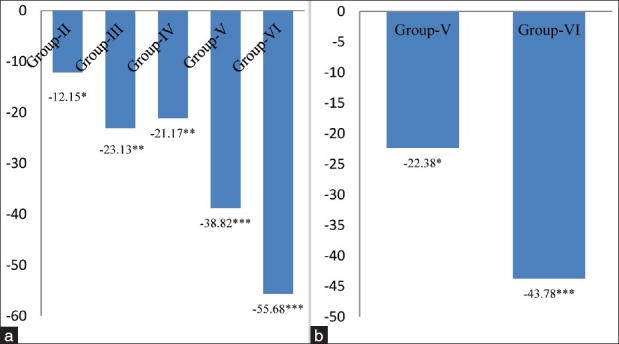
(a) Changes in glutathione peroxidase levels in cardiac tissue of different groups. Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to control (Group-I). *P < 0.05, **P < 0.01,***P < 0.001, (b) Changes in glutathione peroxidase levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± Standard Deviation of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). *P < 0.05, ***P < 0.001
Figure 7.
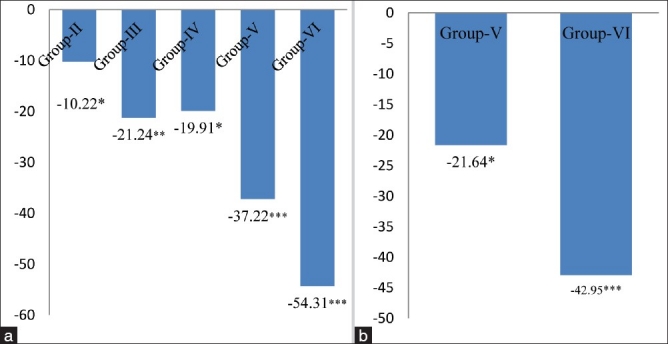
(a) Changes in glutathione reductase levels in cardiac tissue of different groups. Values are expressed as mean ± SD of six observations. The values in figure represent the percent change with respect to control (Group-I). *P < 0.05, **P < 0.01***P < 0.001 (b) Changes in glutathione reductase levels in cardiac tissue of 4 and 8 mg MSG/g body weight ingested alcoholic animals (Group-V and VI). Values are expressed as mean ± SD of six observations. The values in figure represent the percent change with respect to alcoholic animals not receiving monosodium glutamate (Group-IV). *P < 0.05, ***P < 0.001
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Das S, Yadav D, Narang R, Das N. Interrelationship between lipid peroxidation, ascorbic acid and superoxide dismutase in coronary artery disease. Curr Sci. 2002;83:488–91. [Google Scholar]
- 2.Ahluwalia P, Tiwary S, Chaudhary P. Studies on the effect of monosodium glutamate (MSG) administration on oxidative stress in erythrocyte of adult male mice. Toxicol Lett. 1996;84:161–5. doi: 10.1016/0378-4274(95)03612-1. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary P, Malik V BT, Puri S, Ahluwalia P. Studies on the effect of monosodium glutamate (MSG) on hepatic microsomal lipid peroxidation, calcium, ascorbic acid and glutathione and its dependent enzymes in adult male mice. Toxicol Lett. 1996;9:71–6. doi: 10.1016/s0378-4274(96)03786-1. [DOI] [PubMed] [Google Scholar]
- 4.Kuldip S, Ahluwalia P. Studies on the effect of MSG administration on some antioxidant enzymes in arterial tissue of adult male mice. J Nutr Sci Vitaminol. 2003;49:145–8. doi: 10.3177/jnsv.49.145. [DOI] [PubMed] [Google Scholar]
- 5.Kuldip S, Ahluwalia P. Alteration in some antioxidant enzymes in cardiac tissue upon MSG administration to adult male mice. Ind J Clin Biochem. 2005;20:43–6. doi: 10.1007/BF02893040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavolic V, Pavlovic D, Kocic C, Sokolovic D, Jevtovic ST, Cekic S, et al. Effect of monosodium glutamate on oxidative stress and apotosis in rat rhymus. Mol Cell Biochem. 2007;303:161–6. doi: 10.1007/s11010-007-9469-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Cederbaum AI. Alcohol, oxidative stress and free radical damage. Alcohol Res Health. 2004;27:277–82. [PMC free article] [PubMed] [Google Scholar]
- 8.Husain K, Vazquez-Ortiz M, Lalla J. Down regulation of aortic nitric oxide and antioxidant systems in chronic alcohol-induced hypertension in rats. Hum Exp Toxicol. 2007;26:427–34. doi: 10.1177/0960327106072993. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS. Alcoholic fatty liver: Its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Oh SI, Kim CI, Chun HJ, Park SC. Chronic ethanol consumption affects glutathione status in rat liver. J Nutr. 1998;128:758–63. doi: 10.1093/jn/128.4.758. [DOI] [PubMed] [Google Scholar]
- 11.Beuge JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 12.Fried R, Fried LW. Bergmeyers HU, editor. Xanthine oxidase (Xanthine dehydrogenase) Method of enzymatic analysis. 1974;2:644–9. [Google Scholar]
- 13.Kono Y. Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 14.Luck H. Catalase, method of enzymatic analysis. In: Bermeyer HO, editor. London: New York Academic Press; 1971. pp. 855–93. [Google Scholar]
- 15.Beutler E, Duron O, Kally BM. Improved method of determination of blood glutathione. J Lab Clin Methd. 1963;61:351–8. [PubMed] [Google Scholar]
- 16.Rotruk JT, Pope AL, Ganther HC, Hafeman DG, Hoekstro WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 17.Williams CH, Arscott ID. Tabor H., editor. Glutathione reductase. Methods in Enzymology. 1971;XVIIB:503–509. [Google Scholar]
- 18.Lowry OH, Rosenbrough NS, Farr AL, Randall RJ. Protein measurement with Folin-Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Xu P, Hurerison R, Hoidal JR. Molecular cloning, tissue expression of human xanthine dehydrogenase. Biochem Biophys Res Commum. 1994;199:998–1002. doi: 10.1006/bbrc.1994.1328. [DOI] [PubMed] [Google Scholar]
- 20.Gill P, Fernando F, Angela C. Encarnation and malondialdehyde: A possible marker of aging. Gerontol. 2002;48:209–14. [Google Scholar]
- 21.Marklund SL. Properties of extra cellular superoxide dismutase form human lung. Biochem J. 1984;220:269–72. doi: 10.1042/bj2200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bast A, Haenen GR. Cytochrome P450 and glutathione: What is the Significance of their interrelationship in lipid peroxidation? Trends Bio Sci. 1984;9:510–3. [Google Scholar]
- 23.Malik VB, Ahluwalia P. Studies on effect of MSG on various fractions of lipids and certain carbohydrate metabolizing enzymes in liver and blood of adult male mice. Toxicol Lett. 1994;74:69–77. doi: 10.1016/0378-4274(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, et al. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250–60. doi: 10.1002/pmic.200500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–4. [PubMed] [Google Scholar]
- 26.Padmini E, Sundari BT. Erythrocyte Glutathione depletion impairs resistance to haemolysis in women consuming alcohol. J Clin Biochem Nutr. 2008;42:14–20. doi: 10.3164/jcbn.2008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakazawa H, Genka C, Fujishima M. Pathological aspects of active oxygen/ free radicals. Jpn Pathol. 1996;46:15–32. doi: 10.2170/jjphysiol.46.15. [DOI] [PubMed] [Google Scholar]
- 28.Blum J, Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240:500–8. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- 29.Raes M, Mihiels MC, Remacle J. Comparative study of the enzymatic defence systes against oxygen derived free radicals the key role of glutathione peroxidase. Free Radic Biol Med. 1987;3:3–20. doi: 10.1016/0891-5849(87)90032-3. [DOI] [PubMed] [Google Scholar]
- 30.Lapennna D, Gioia S, Ciotani G, Mezzetti TA, Ucchino S, Calafiore AM, et al. Glutathione related antioxidants defense in human atherosclerotic plaques. Circulation. 1998;97:1930–4. doi: 10.1161/01.cir.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 31.Gul M, Kutay FZ, Temocin S, Hanninen O. Cellular and clinical implications of glutathione. Ind J Exp Biol. 2000;38:625–34. [PubMed] [Google Scholar]


