Abstract
Pheochromocytomas have been described to be associated with rare vascular abnormalities, most common of them being renal artery stenosis. A 45-year-old woman was admitted to our hospital with complaints of headache, sweating, anxiety, dizziness, nausea, vomiting and severe hypertension. Hypertension was confirmed to result from both excess catecholamine production and hyperreninemia of left kidney. The technical images (abdominal CT and renal arteriography) revealed the presence of a left adrenal pheochromocytoma and stenosis of the renal artery. Surgical removal of pheochromocytoma and correction of renal artery stenosis restored the postoperative plasma catecholamine, renin and blood pressure to normal. To our belief, this is the first such case report from India citing this rare association. We conclude that when the two diseases occur simultaneously, both must be diagnosed accurately and treated in a different manner. We also hereby review the existing literature.
Keywords: Hypertension, pheochromocytoma, renal artery stenosis
INTRODUCTION
Pheochromocytoma, an uncommon cause of hypertension which has been estimated to occur in 0.1–1% of hypertensive patients,[1] may be a potentially lethal disease with protean manifestations. This chromaffin cell tumor may secrete catecholamines and other substances,[2] either continuously or intermittently, causing sustained or paroxysmal symptoms, respectively. Diagnosis is established by measuring metanephrines in the urine or blood.[3] Localization of the tumor is done using computed tomography (CT) or magnetic resonance imaging (MRI) scans.[4] As some patients may have recurrences after removal of the primary tumor,[5] follow-up is essential.
The coexistence of renal artery stenosis and pheochromocytoma has been recognized since 1958 when Harrison’ first reported this unusual occurrence in a 16-year-old girl. Van Way et al,[6] proposed that these two causes of surgically correctable hypertension may be associated through a common pathophysiological mechanism mediated by catecholamine secretion. Stimulated by the case reported here, we have reviewed the literature to identify an inter relationship between these two lesions when they occur concomitantly. To our belief this is the first report of such association from India.
CASE REPORT
A 45-year-old woman was admitted to our hospital complaints of headache, sweating, anxiety, dizziness, nausea and vomiting. The patient was 164-cm tall and weighed 57 kg. On physical examination, there were no café au lait spots or neurofibromas. The patient's blood pressure was 240/150 mm Hg without any variation between limb recordings and her resting pulse was 100 beats/min. Rest of the systemic examination was unremarkable.
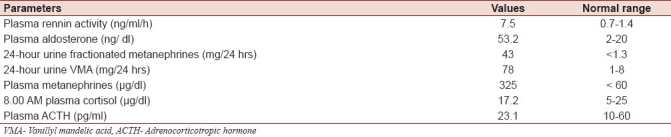
The patient's hemogram and blood biochemistry including potassium, sodium, bicarbonate, calcium, phosphorus, alkaline phosphatase and creatinine levels, and liver function tests were normal. The electrocardiogram revealed left ventricular hypertrophy. No alterations in cardiac as well as renal function were observed, thus the presence of pheochromocytoma was suspected. Endocrinological evaluation revealed that plasma metanephrines, 24-hour fractionated metanephrines and vanillyl mandelic acid (VMA), supine plasma renin activity and plasma aldosterone concentrations were increased [Table 1]. Plasma cortisol and Adrenocorticotropic hormone (ACTH)levels were within normal ranges [Table 1].
Table 1.
Baseline biochemical parameters of the patient
Abdominal computed tomography revealed a large, heterogenous para-aortic mass (5 × 2 cm) between the celiac and the superior mesenteric artery with attenuation score of 35 Hounsfield unit (HU)[Figure 1]. Further workup included aortic/arterial arteriography, which disclosed stenosis of the left renal artery (70%; Figure 2). A diagnosis of left pheochromocytoma was made, and surgical treatment was recommended. α-receptor blocking therapy with prazosin was instituted followed by β-blocker after adequate α-blockade. After 2 weeks, hypertension was well controlled and the remaining symptomatology disappeared. At laparoscopic surgery, the tumor was found in and around the left adrenal gland. Several fibrous brands connected the tumor mass to the mid-portion of the left renal artery causing angulation and kinking of the vessel. Lysis of these adhesive fibrous strands resulted in restoration of patency of the left renal artery. There was no infiltration of the left and right renal arteries, superior mesenteric arteries or the celiac tissue. Gross pathological examination showed that the tumor was a soft mass (diameter 4.8 × 2 × 2 cm; weight 35 g) adhering to the left suprarenal gland. Light microscopy of the specimen revealed characteristic organoid or zellballen nest of cells confirming the diagnosis of pheochromocytoma with no cytoplasmic inclusion, pleomorphism, cytological alterations or necrosis; the mitotic index was low [Figure 3].
Figure 1.
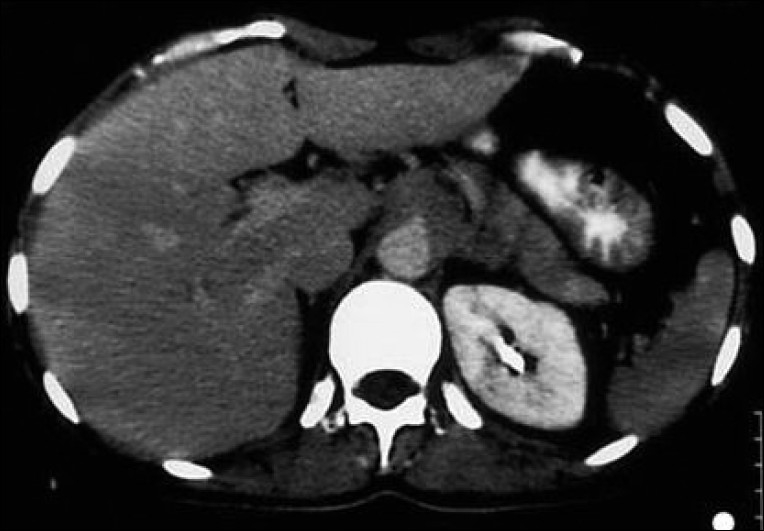
Abdominal CT demonstrating a large, heterogenous para-aortic mass (5×2 cm) with attenuation score of 35 HU between the celiac and superior mesenteric artery
Figure 2.
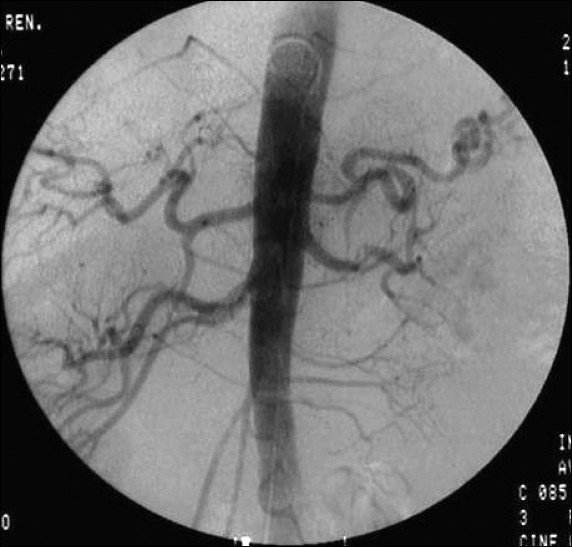
Renal arteriography showed stenosis (> 70%) of the left renal artery
Figure 3.
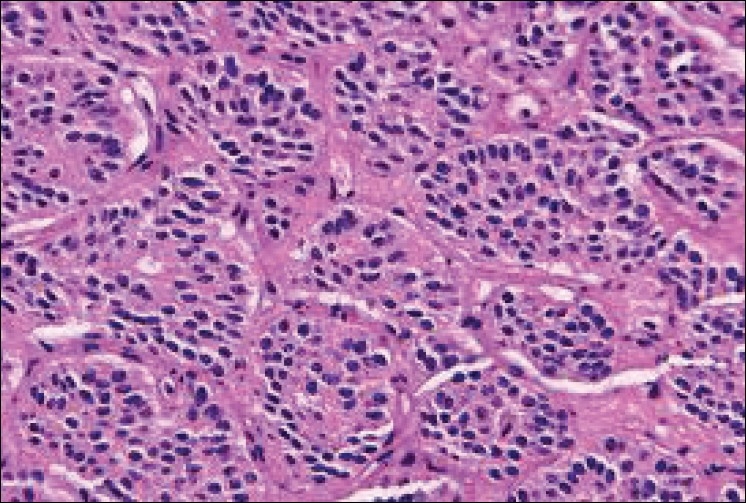
Histology of the biopsied specimen revealing characteristic organoid or zellballen nest of cells
During the postoperative period, the blood pressure was normal, the patient's convalescence uncomplicated, and she was discharged on the 11th postoperative day. During the next 16 months, the patient's blood pressure remained normal. Evaluation at that time revealed blood urea nitrogen of 15 mg/100 ml and serum creatinine of 1.5 mg/100 ml. The estimated renal plasma flow and renal scan were normal although the image of the left kidney appeared smaller than the right kidney. The excretory urogram was also normal. The left kidney measured 11.2 cm in length while the right kidney measured 12.6 cm. Repeat arteriograms showed a normal aorta, right and left renal artery. A peripheral plasma renin level and a 24-hour urine specimen collected for metanephrine, and VMA were within normal limits. At present the patient is asymptomatic, requires no medications, and is employed as a mechanic.
DISCUSSION
Two aspects render our case unusual 1) the coexistence of pheochromocytoma with renal artery stenosis 2) to our sincere belief ours is the first such report from India.
The simultaneous occurrence of pheochromocytoma and renal artery stenosis is very rare, As per the study by Gill et al, only 87 cases were reported in the literature till then.[7] After that a few sporadic cases citing similar such associations were reported.[8–10] Some pathophysiological mechanisms have been proposed,[11] including (a) an ipsilateral tumor that may compress the renal artery and cause both renovascular and catecholamine hypertension; (b) a prolonged increase in catecholamines that induces an arterial vasospasm that can bring about changes in the renal artery wall, norepinephrine secreted preponderantly by extra adrenal paragangliomas is more potent vasoconstrictor; (c) a periarterial adhesion following the resection of the adrenal tumor; (d) generalized neuroectodermal dysplasia with pheochromocytoma and neurofibromatosis, and (e) a simultaneous but independent occurrence of stenotic lesions of the renal artery (e.g. atherosclerosis, fibroumuscular dysplasia).
Also called “pseudostenosis,” transient renal artery narrowing possesses special features with therapeutic implications. Such stenosis is more evident during a hypertensive pheochromocytoma crisis, which may on occasion be triggered by aortography.[12] Alternatively, stenosis may be mild and hemodynamically insignificant. These stenoses have been noted to regress completely following administration of α-adrenergic blockers for treatment of the concurrent pheochromocytoma.
In our case, the underlying mechanism was the compression of the renal artery by the infiltrating fibrous tissue from the tumor mass. Extrinsic compression of the renal artery by a pheochromocytoma seems to be the most common cause of the association,[7] which can lead to myointimal proliferation over long term further reducing the arterial luminal diameter.
However, even pheochromocytoma without renal artery stenosis can be accompanied by elevated plasma renin activity[13] that may be induced by direct stimulator effect of catecholamines on renin release and can lead to secondary hyperaldosteronism. Other factors contributing to hyperreninemia are decreased plasma volume, salt restriction and diuretic usage for control of hypertension. This suggests that measuring plasma renin activity in a peripheral vein does not reveal an association between renal arterial stenosis and pheochromocytoma. Additional studies such as renal vein renin ratios, radionuclide scanning may be used to lateralize the renal ischemia and establish the functional significance of angiographically demonstrated lesions.[7] Manometric or Doppler flow pressure studies of the involved vessel can also be performed to confirm significant gradient across the stenotic site, which would mandate renal revascularization.[11]
Operative management of patients with coexisting pheochromocytoma and renal artery stenosis may vary according to the anatomical location of the tumor and the pathology of the renal artery. The goals of operation include 1) removal of the tumor, 2) preservation of functioning renal tissue, and 3) correction of physiologically significant renal artery stenosis. Minimally invasive techniques are being increasingly used for resection of adrenal tumors and to treat renal artery lesions. Laparoscopic adrenalectomy is performed by either the transperitoneal or retroperitoneal approach.[14] Similarly, percutaneous balloon angioplasty has come to be the first line of treatment for the majority of cases of renal artery stenosis.[15] The use of endovascular stents has further extended the applicability of percutaneous revascularization techniques. Open surgical revascularization is now reserved for angioplasty failures or some ostial lesions. Our patient was subjected to laparoscopic adrenalectomy with lysis of adhesive fibrous strands, which restored patency of the left renal artery without any need for renal artery specific interventions. The patient recuperated fully without any recurrence of hypertension and normalization of biochemical, angiographic parameters.
ACKNOWLEDGMENT
All the authors would like to express their heartfelt thanks to Dr Jagadeesh Tangudu. M Tech, MS, PhD and Sowmya Jammula, M Tech for their immense and selfless contribution towards manuscript preparation, language editing and final approval of text.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: A multicentric retrospective study. Eur J Endocrinol. 1999;141:619–24. doi: 10.1530/eje.0.1410619. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Yumita S, Kimura S, Miura Y, Yoshinaga K. Immunoreactive corticotropin-releasing hormone, growth hormone-releasing hormone, somatostatin, and peptide histidine methionine are present in adrenal pheochromocytomas, but not in extra-adrenal pheochromocytoma. J Clin Endocrinol Metab. 1990;70:996–9. doi: 10.1210/jcem-70-4-996. [DOI] [PubMed] [Google Scholar]
- 3.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 4.Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H, et al. Adrenocortical carcinomas and adrenal pheochromocytomas: Mass and enhancement loss evaluation at delayed contrast enhanced CT. Radiology. 2005;234:479–85. doi: 10.1148/radiol.2342031876. [DOI] [PubMed] [Google Scholar]
- 5.Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29:1133–9. doi: 10.1161/01.hyp.29.5.1133. [DOI] [PubMed] [Google Scholar]
- 6.van Way CW, 3rd, Michelakis AM, Alper BJ, Hutcheson JK, Rhamy RK, Scott HW., Jr Renal vein renin studies in a patient with renal hilar pheochromocytoma and renal artery stenosis. Ann Surg. 1970;172:212–7. doi: 10.1097/00000658-197008000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill IS, Meraney AM, Bravo EL, Novick AC. Pheochromocytoma coexisting with renal artery lesions. J Urol. 2000;164:296–301. [PubMed] [Google Scholar]
- 8.Kuzmanovska D, Sahpazova E, Kocova M, Damjavnoski M, Popov Z. haeochromocytoma associated with reversible renal artery stenosis. Nephrol Dial Transplant. 2001;16:2092–4. doi: 10.1093/ndt/16.10.2092. [DOI] [PubMed] [Google Scholar]
- 9.Chotsampancharoen T, Patrapinyokul S, Reegkling C, Vachvanichsanong P. Impaired differential renal function in a child with pheochromocytoma. J Hum Hypertens. 2005;19:751–4. doi: 10.1038/sj.jhh.1001894. [DOI] [PubMed] [Google Scholar]
- 10.Camberos A, Bautista N, Rubenzik M, Applebaum H. Renal artery stenosis and pheochromocytoma: Coexistence and treatment. J Pediatr Surg. 2000;35:714–716. doi: 10.1053/jpsu.2000.6032. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JJ. Pheochromocytoma and stenosis of the renal artery. Surg Gynecol Obstet. 1983;156:11–5. [PubMed] [Google Scholar]
- 12.Brewster DC, Jensen SR, Novelline RA. Reversible renal artery stenosis associated with pheochromocytoma. JAMA. 1982;248:1094–6. [PubMed] [Google Scholar]
- 13.Vetter H, Vetter W, Warnholz C, Bayer JM, Kaser H, Vielhaber K, et al. Renin and aldosterone secretion in pheochromocytoma. Am J Med. 1971;60:866–71. doi: 10.1016/0002-9343(76)90906-2. [DOI] [PubMed] [Google Scholar]
- 14.del Pizzo JJ, Schiff JD, Vaughan ED. Laparoscopic adrenalectomy for pheochromocytoma. Curr Urol Rep. 2005;6:78–85. doi: 10.1007/s11934-005-0071-9. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen D, Klow NE, Rogstad B, Leivestad T, Lien B, Vatne K, et al. Preservation of renal function by percutaneous transluminal angioplasty in ischemic renal disease. Nephrol Dial Transplant. 1999;14:1454–61. doi: 10.1093/ndt/14.6.1454. [DOI] [PubMed] [Google Scholar]


