Abstract
Background/Aim:
Furazolidone-based therapies are used in developing countries to cure Helicobacter pylori infection due to its low cost. The low bacterial resistance toward furazolidone may render appealing the use of this drug even in developed countries. However, some relevant safety concerns do exist in using furazolidone.
Patients and Methods:
This was a systematic review with pooled-data analysis of data regarding both eradication rate and safety of furazolidone-based therapies for H. pylori infection. Intention-to-treat (ITT) and per-protocol (PP) eradication rates were calculated.
Results:
Following furazolidone-based first-line therapy, H. pylori eradication rates were 75.7% and 79.6% at ITT and PP analysis, respectively (P<0.001). The overall incidence of side effects and severe side effects were 33.2% and 3.8%, respectively. At multivariate analysis, only high-dose furazolidone was associated with increased therapeutic success (OR: 1.5, 95% CI: 1.3-2.7; P<0.001), while occurrence of side effects was relevant following treatment for a long duration (OR: 2.9, 95% CI: 2.2-4.1; P<0.001), high-dose furazolidone (OR: 2.3, 95% CI: 1.7-3.2; P<0.001) and bismuth-containing regimens (OR: 2.1, 95% CI: 1.5-2.8; P<0.001).
Conclusions:
Furazolidone-based regimens usually achieve low eradication rates. Only a high-dose regimen improves the cure rate, but simultaneously increases the incidence of severe side effects. Therefore, we suggest that patients have to be clearly informed about the possible genotoxic and carcinogenetic effects for which furazolidone use is not approved in developed countries.
Keywords: Antibiotic, furazolidone, Helicobacter pylori, side effects, resistance
Therapeutic management of Helicobacter pylori remains an unsolved issue, no therapy regimen being able to cure the infection in all treated patients. Indeed, a recent study demonstrated that H. pylori eradication was achieved in only 89.6% of the 540 patients, even after following three consecutive standard therapies.[1] Therapy failure mainly depends on both primary bacterial resistance towards antibiotics and patient compliance. In addition, the high cost of some drugs such as clarithromycin and quinolones, prevents their use in developing countries, where a high prevalence of primary metronidazole resistance is also present. To overcome these limitations, furazolidone-based treatments have been suggested in developing countries by the World Gastroenterology Organisation and Latin-America guidelines.[2,3] On the other hand, the low rate of primary H. pylori resistance toward furazolidone in developed countries may render appealing the use of this drug also in these geographic areas.[4,5] Furazolidone is a synthetic nitrofuran with a broad spectrum of antimicrobial activities widely used in the treatment of bacterial and protozoal infections in both humans and animals.[6] However, some concerns recently arose in using furazolidone, such as a molecule harboring a potential carcinogenetic effect.[7–13]
The first review on furazolidone-based therapy was published in 1992,[14] while the last study based on generic nitrofurans drugs was in 2007.[15] Because such a drug is still available and used in some Asian and South American countries, we performed a pooled-data analysis to update both efficacy and safety of furazolidone-based treatments for H. pylori eradication.
PATIENTS AND METHODS
Literature search
A computer-assisted search was performed on PubMed. We searched for all English language articles published before August 2011, using the exploded medical subject heading terms Helicobacter pylori and furazolidone. Boolean operators (NOT, AND, OR) also were used in succession to narrow and widen the search. All studies concerning the use of this antibiotic for either first-line or “rescue” therapies were taken into account. Full articles of all relevant studies were retrieved, and manual searches of reference lists from identified relevant articles were performed to find any additional studies that may have been missed. When more than one publication from the same investigator or group was available, only the most updated version, including the entire sample size, was included in this pooled-data analysis, while data published only in abstract form were not considered.
Data extraction
Two investigators (V.D.F and A.Z.) extracted the data from the studies that met the selection criteria. Data were extracted concerning the following items: (1) number of patients included; (2) age (<18 years: Young patients, and >18 years: Adult patients); (3) sex distribution; (4) gastroduodenal pathology (either directly provided or calculated); (5) geographic area involved; (6) the antibiotic association used; (7) furazolidone dose (≤100 mg b.i.d; ≥200 mg b.i.d.); (8) therapy duration (≤7 days; 14 days); (9) side effects incidence; and (10) side effects severity grading as: (a) absent; (b) mild (not interfering with daily activities); (c) moderate (frequently interfering with daily activities); (d) marked (impeding daily activity); and (e) severe (causing treatment interruption).[16] Bacterial eradication rates were calculated at both intention-to-treat (ITT) and per-protocol (PP) analyses.
Statistical analysis
Statistical analysis was performed by using the Chi-squared test and Fisher's exact test, as appropriate. Eradication rates, side effects rates, and their odds ratios with 95% confidence intervals (CIs) were calculated. A model of multivariate logistic regression analysis was performed using the therapeutic outcome and the occurrence of side effects as the dependent variables. As possible candidates for the multivariate model, duration of treatment (≤1 week vs 2 weeks), drug dosage (≤100 mg b.i.d. or ≥200 mg b.i.d.), and bismuth salts inclusion (furazolidone-based therapies with or without bismuth salts), were considered. Variables were kept in the model only if their association with the eradication term improved the fit of the model. The odds ratio (OR) and 95% CI were also calculated. Differences were considered significant at 5% probability level. Analyses were performed by using Statasoft 7.1 program for Windows XP.
RESULTS
First-line therapy: Overall eradication rates
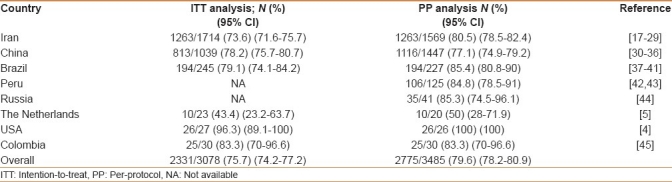
A total of 31 studies meeting the inclusion criteria were identified, reporting data of patients enrolled from 1997 to 2011. In detail, there were 13 studies from Iran,[17–29] 7 from China,[30–36] 5 from Brazil,[37–41] 2 from Peru,[42,43] and 1 each from Russia,[44] The Netherlands,[5] USA,[4] and Colombia.[45] Two studies enrolled pediatric patients.[43,44] In the 26 studies reporting cure rate at both ITT and PP analysis, H. pylori eradication was achieved in 2331 (75.7%, 95% CI=74.2-77.2) out of 3078 patients at ITT analysis, and in 2331 (80.6%, 95% CI=79.1-82) out of 2892 patients at PP analysis, respectively; and the difference being statistically significant (P<0.001; OR: 1.3, 95% CI: 1.1-1.5). In 5 studies,[30,31,42–44] results were exclusively reported at PP analysis, and the infection was cured in 444 (74.8%, 95% CI: 71.3-78.3) out of 593 patients. Therefore, the overall performance at either ITT and PP analysis were 75.7% (95% CI: 74.2-77.2) and 79.6% (95% CI: 78.2-80.9), respectively; and the difference being statistically significant (P<0.001; OR: 1.2, 95% CI: 1.1-1.4). As shown in Table 1, the cure rate substantially varied among different geographic areas, the highest value being reported in the USA (96.3% on 27 patients), whereas the lowest success rate was achieved in the Netherlands (43.4% on 23 patients).
Table 1.
Helicobacter pylori eradication rates following first-line furazolidone-based therapies
First-line therapy: Eradication rates following different therapeutic regimens
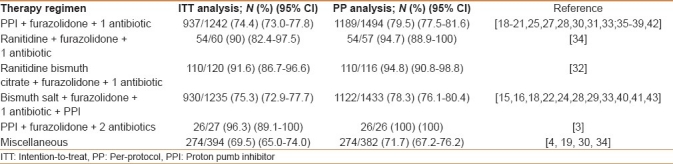
Six different furazolidone-based antibiotic combinations have been used. As shown in Table 2, the highest cure rate were achieved following either a proton pump inhibitor-based quadruple therapy (96.3%) or ranitidine bismuth citrate-based triple therapy (91.6%), whereas lower success was achieved following unusual combinations (ie, monotherapy, one-day therapy, and so on).
Table 2.
Helicobacter pylori eradication rates following different first-line furazolidone-based regimens
According to therapy duration, H. pylori was cured in 1439 out of 1904 patients and in 892 out of 1174 patients following a 7- or 14-day furazolidone-based regimen, respectively. The comparison between 7- and 14-day regimens found a similar efficacy at ITT analysis (1439/1904, 75.5% vs 892/1174, 75.9%; P=0.8), while a significantly higher eradication rate was achieved with the prolonged regimen at PP analysis (892/1068, 83.5% vs 1439/1824, 82.3%; P<0.05; OR: 1.3, 95% CI: 1.1-1.6).
According to the furazolidone dose, comparable eradication rates were achieved following high- (200 mg b.i.d.) and low-dose (100 mg b.i.d.) regimen at ITT (1166/1522, 76.6% vs 962/1284, 74.9%; P=0.3), while significantly different cure rate were found at PP analysis (1166/1373, 84.9% vs 1265/1679, 75.3%; P<0.001; OR: 1.8, 95% CI: 1.5-2.2).
First-line therapy: Eradication rates in different diseases
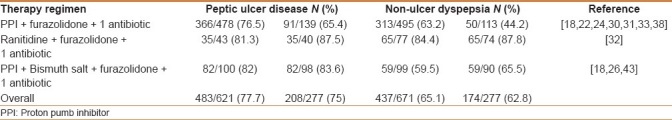
Data on eradication rates in different diseases are available in 10 studies [Table 3].[20,24,26,28,35–38,41,45] Overall, significantly higher H. pylori eradication rates were achieved in peptic ulcer than in non-ulcer dyspeptic patients at both ITT (77.7% vs 65.1%, P<0.001; OR: 1.8, 95% CI: 1.4-2.3) and PP analysis (75% vs 62.8%, P<0.01; OR: 1.7, 95% CI: 1.2-2.5). Higher eradication rates were achieved following bismuth salt-containing as compared with proton pumb inhibitor (PPI)-containing furazolidone-based triple therapies in both peptic ulcer (82.0 vs 76.5%, P=0.2 at ITT, and 83.6 vs 65.4%, P<0.01 at PP analysis) and non-ulcer dyspepsia patients (59.5% vs 63.2% P=0.5 at ITT, and 65.5% vs 44.2%, P<0.01 at PP analysis).
Table 3.
Helicobacter pylori eradication rates in different diseases
Side effects
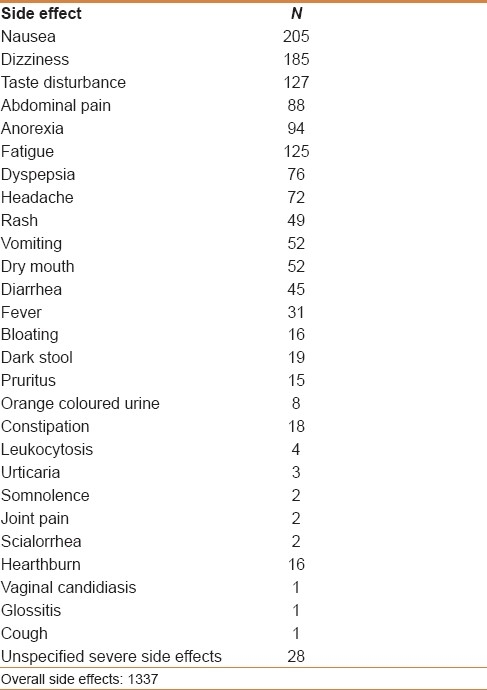
The prevalence of side effects was evaluated according to furazolidone dose, therapy duration, and bismuth/PPI association. Side effects were reported by 805 (32.2%) of the 2420 patients, overall reporting 1337 symptoms [Table 4]. According to the severity, symptoms were graded as severe in 93 (11.5%) of 805 patients, accounting for an overall 3.8% incidence. The rate of both side effects (45.2% vs 23.7%, P<0.001; OR: 2.6, 95% CI: 2.2-3.1) and severe side effects (6.9% vs 1.1%, P<0.001; OR: 6.3, 95% CI: 3.5-11.2) was significantly higher following the 200 mg b.i.d. regimen as compared with the 100 mg b.i.d. schedule. Similarly, the incidence of all side effects (54.4% vs 26.5%, P<0.001; OR: 3.3, 95% CI: 2.7-4) and severe side effects (10.4% vs 1.7%, P<0.001; OR: 6.4, 95% CI: 4.1-10) was higher following the 14- as compared with the 7-day regimen. Finally, as compared with PPI-based therapies, bismuth-based regimens were associated with a higher incidence of all side effects (46.1% vs 25.5%, P<0.001; OR: 2.4, 95% CI: 2-2.9), but with a similar occurrence of severe side effects (4.2% vs. 3.6%, P=0.5; OR: 1.1, 95% CI: 0.7-1.7).
Table 4.
Side effects reported
Multivariate analysis
At multivariate logistic regression, only high-dose furazolidone therapy was significantly associated with a higher therapeutic success (OR: 1.5, 95% CI: 1.3-2.7; P<0.001). Occurrence of side effects was higher following a longer treatment duration (OR: 2.9, 95% CI: 2.2-4.1; P<0.001), high-dose furazolidone (OR: 2.3, 95% CI: 1.7-3.2; P<0.001) and bismuth-containing regimen (OR: 2.1, 95% CI: 1.5-2.8; P<0.001).
“Rescue” therapy: Overall eradication rates
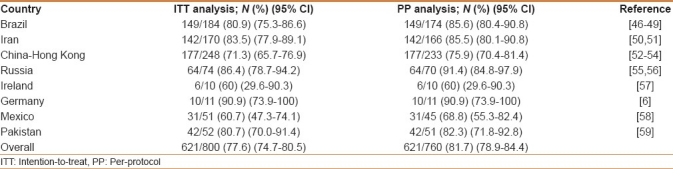
There were 4 studies from Brazil,[46–49] 2 from Iran,[50,51] 3 from China,[52–54] 2 from Russia,[55,56] and 1 each from Ireland,[57] Germany,[6] Mexico,[58] and Pakistan,[59] reporting data of furazolidone-based rescue therapies. In detail, 64 patients failed three consecutive therapies, 114 two treatments, and 622 patients the first-line therapy. A PPI, furazolidone-based triple therapy was used in 6 therapeutic arms, while a PPI, bismuth salts, furazolidone-based quadruple therapy was used in 12 arms. In all but 2 studies a 200 mg b.i.d. furazolidone dose was administered. Overall, H. pylori infection was cured in 621 (77.6%) of 800 patients at ITT analysis and in 621 (81.7%) of 760 patients at PP analysis [Table 5].
Table 5.
Helicobacter pylori eradication rates following furazolidone-based “rescue” therapies
DISCUSSION
In 1990s, the use of furazolidone in combination with different antibiotics and bismuth salts was considered a good therapeutic choice to cure H. pylori infection.[27,60,61] Nonetheless, some relevant alarms arose for the use of this drug during last decades. Indeed, it has been advised that such a molecule is mutagenic, genotoxic, and potentially carcinogenetic.[7–11] Furazolidone significantly increases frequency of sister chromatid exchange in human lymphocytes both in vitro and in vivo.[9] In addition, a dose-related increased incidence of both breast and bronchial adenocarcinomas, as well as of lymphosarcomas, has been observed in animal models.[9,10] The IARC classified furazolidone as a type 3 carcinogen for humans in 1997,[62] and both the European Agency for the Evaluation of Medical Products in 1999 and the US Food and Drug Administration agency in 2002 banned its use in animals, in order to avoid the presence of residues in meat-derived foods.[11,63,64] Furazolidone is not approved by the European Medicines Agency (EMA) either as a human medicine or for animal use,[65] it has been withdrawn in Yemen,[66] and it is not currently commercialized in the USA. Recent findings showing that furazolidone exerts a dose-related cytotoxicty in human HepG2 cells by the induction of intracellular reactive oxygen species (ROS) and a DNA oxidative damage would further support these restrictions.[10] Despite these restrictions, furazolidone use has been proposed in recent guidelines for H. pylori management in developing countries, due to its efficacy, low rate of primary bacterial resistance, and lack of alterative, low-cost therapies.[2,3] In 2007, a meta-analysis evaluated the efficacy of furazolidone-based regimens.[15] However, further 16 studies have been published in the following years, justifying an update of data. The present systematic review found that first-line furazolidone-based regimens achieve <80% eradication rate at ITT. Therefore, in agreement with the “report card” proposed by Graham and Fischbach,[67] these therapies should be considered as not recommendable (grade F) in clinical practice. Moreover, the low eradication rate achieved with these therapy regimens further questions the real economic advantage in using furazolidone, several eradication failure patients requiring a further therapy and a new test. In addition, no standardized rescue therapies have been proposed in those patients who failed the initial furazolidone-based treatment.
Our data failed to confirm the results of previous studies reporting a high efficacy with either a combination with bismuth salts or prolonged therapy,[14] furazolidone dose being identified as the solely independent factor predicting eradication. However, the increased eradication rate achieved by using high-dose furazolidone is to some extent counterbalanced by the high prevalence of side effects. Of note, we computed that the incidence of severe side effects, that is, requiring therapy interruption, was as high as 12% of the overall patients complaining of side effects (4% of all treated cases), the rate being 6-fold higher in those patients receiving either high-dose furazolidone or a 14-day therapy. Such a finding could depend on the observation that furazolidone is a monoamine oxidase (MAO) inhibitor, and it may interact with foods or drugs in inducing side effects. The rate of both the overall and severe side effects appears higher than that observed following furazolidone-free therapies.[68] The clinical relevance of side effects following furazolidone-based therapies is further highlighted by our observation that eradication rates at ITT were significantly lower than those achieved at PP analysis.
Although metronidazole is also listed as a potential carcinogen and it is “black boxed” by the FDA, it continued to be commercialized worldwide for humans. Due to the very high prevalence of primary metronidazole resistance in developing countries, furazolidone, for which bacterial resistance is generally low, was proposed for H. pylori therapy. Therefore, the performance <80% of furazolidone-based treatments in developing areas was unexpected. Nevertheless, the resistance toward this antibiotic was highly variable, ranging from 1% to 25% in Iran,[18,69] and from 0% to 40% in China.[70] Moreover, despite a cross-resistance between furazolidone and metronidazole not being reported, the cure rate with furazolidone-based therapies was significantly lower in metronidazole resistant than in susceptible strains.[71] This finding further undermines the role of furazolidone-based regimens in developing countries with high imidazoles resistance rates.
In conclusion, furazolidone-based regimens achieved low eradication rates, despite antibiotic combinations and treatment duration. High-dose furazolidone increases the cure rate, but it significantly increases incidence of severe side effects. On this basis, our study suggests that the patients have to be clearly informed on the possible genotoxic and carcinogenetic effects for which furazolidone is not currently approved for animal use by the FDA and both human and animal use by EMA. We have to kill H. pylori, but primarily save the patients!
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III Consensus and a third-line empirical regimen. Am J Gastroenterol. 2009;104:21–5. doi: 10.1038/ajg.2008.87. [DOI] [PubMed] [Google Scholar]
- 2.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. World Gastroenterology Organisation Global Guideline. Helicobacter pylori in developing countries. J Clin Gastroenterol. 2011;45:383–8. doi: 10.1097/MCG.0b013e31820fb8f6. [DOI] [PubMed] [Google Scholar]
- 3.Coelho LG, Leon-Barua R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection.Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE) Am J Gastroenterol. 2000;95:2688–91. doi: 10.1111/j.1572-0241.2000.03174.x. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, El-Zimaity HM. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther. 2000;14:211–5. doi: 10.1046/j.1365-2036.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 5.van Zwet AA, Thijs JC, van der Wouden EJ, Kooy A. Low cure rate of Helicobacter pylori infection with omeprazole and furazolidone dual therapy for one week. Aliment Pharmacol Ther. 1997;11:533–5. doi: 10.1046/j.1365-2036.1997.00166.x. [DOI] [PubMed] [Google Scholar]
- 6.Treiber G, Ammon S, Malfertheiner P, Klotz U. Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter. 2002;7:225–31. doi: 10.1046/j.1523-5378.2002.00087.x. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed HH, El-Aziem SH, Abdel-Wahhab MA. Potential role of cysteine and methionine in the protection against hormonal imbalance and mutagenicity induced by furazolidone in female rats. Toxicology. 2008;243:31–42. doi: 10.1016/j.tox.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Ali BH. Pharmacological, therapeutic and toxicological properties of furazolidone: Some recent research. Vet Res Commun. 1999;23:343–60. doi: 10.1023/a:1006333608012. [DOI] [PubMed] [Google Scholar]
- 9.Madrigal-Bujaidar E, Ibañez JC, Cassani M, Chamorro G. Effect of furazolidone on sister-chromatid exchanges, cell proliferation kinetics, and mitotic index in vivo and in vitro. J Toxicol Environ Health. 1997;51:89–96. doi: 10.1080/00984109708984013. [DOI] [PubMed] [Google Scholar]
- 10.Jin X, Tang S, Chen Q, Zhang T, Liu F, Zhang S, et al. Furazolidone induced oxidative DNA damage via up-regulating ROS that caused cell cycle arrest in human hepatoma G2 cells. Toxicol Lett. 2011;201:205–12. doi: 10.1016/j.toxlet.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Vincentini O, De Angelis I, Stammati A, Zucco F. Functional alterations induced by the food contaminant furazolidone on the human tumoral intestinal cell line Caco-2. Toxicol in vitro. 1993;7:403–6. doi: 10.1016/0887-2333(93)90036-5. [DOI] [PubMed] [Google Scholar]
- 12.De Francesco V, Ierardi E, Hassan C, Zullo A. Furazolidone therapy for Helicobacter pylori: Is it effective and safe? World J Gastroenterol. 2009;15:1914–5. doi: 10.3748/wjg.15.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Francesco V, Ierardi E, Hassan C, Zullo A. Is furazolidone therapy for Helicobacter pylori effective and safe? Dig Dis Sci. 2009;54:2298–9. doi: 10.1007/s10620-009-0748-x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng ZT, Wang YB. Treatment of peptic ulcer disease with furazolidone. J Gastroenterol Hepatol. 1992;7:533–77. doi: 10.1111/j.1440-1746.1992.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 15.Buzás GM, Józan J. Nitrofuran-based regimens for the eradication of Helicobacter pylori infection. J Gastroenterol Hepatol. 2007;22:1571–81. doi: 10.1111/j.1440-1746.2007.05082.x. [DOI] [PubMed] [Google Scholar]
- 16.De Boer WA, Thys JC, Borody TJ, Grahan DY, O’Morain C, Tytgat GN. Proposal for use of standard side effects scoring system in studies exploring Helicobacter pylori treatment. Eur J Gastroenterol Hepatol. 1996;8:641–3. [PubMed] [Google Scholar]
- 17.Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Dariani N, et al. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of Helicobacter pylori in peptic ulcer disease: A double-blind randomized controlled trial. Helicobacter. 2010;15:497–504. doi: 10.1111/j.1523-5378.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 18.Hasan SR, Vahid V, Reza PM, Roham SR. Short-duration furazolidone therapy in combination with amoxicillin, bismuth subcitrate, and omeprazole for eradication of Helicobacter pylori. Saudi J Gastroenterol. 2010;16:14–8. doi: 10.4103/1319-3767.58762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amini M, Khedmat H, Yari F. Eradication rate of Helicobacter pylori in dyspeptic patients. Med Sci Monit. 2005;11:193–5. [PubMed] [Google Scholar]
- 20.Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2004;19:89–93. doi: 10.1046/j.1365-2036.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 21.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2003;18:778–82. doi: 10.1046/j.1440-1746.2003.03058.x. [DOI] [PubMed] [Google Scholar]
- 22.Malekzadeh R, Merat S, Derakhshan MH, Siavoshi F, Yazdanbod A, Mikaeli J, et al. Low Helicobacter pylori eradication rates with 4- and 7-day regimens in an Iranian population. J Gastroenterol Hepatol. 2003;18:13–7. doi: 10.1046/j.1440-1746.2003.02897.x. [DOI] [PubMed] [Google Scholar]
- 23.Mansour-Ghanaei F, Fallah MS, Shafaghi A. Eradication of Helicobacter pylori in duodenal ulcer disease tetracycline and furazolidone vs.metronidazole and amoxicillin in omeprazole based triple therapy. Med Sci Monit. 2002;8:27–30. [PubMed] [Google Scholar]
- 24.Malekzadeh R, Ansari R, Vahedi H, Siavoshi F, Alizadeh BZ, Eshraghian MR, et al. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:299–303. doi: 10.1046/j.1365-2036.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 25.Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori infection in Iran. J Gastroenterol Hepatol. 2007;22:1399–403. doi: 10.1111/j.1440-1746.2007.05029.x. [DOI] [PubMed] [Google Scholar]
- 26.Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ, et al. Clarithromycin vs.furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411–6. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 27.Khatibian M, Ajvadi Y, Nasseri-Moghaddam S, Ebrahimi-Dariani N, Vahedi H, Zendehdel N, et al. Furazolidone-based, metronidazole-based, or a combination regimen for eradication of Helicobacter pylori in peptic ulcer disease. Arch Iran Med. 2007;10:161–7. [PubMed] [Google Scholar]
- 28.Taghavi SA, Jafari A, Eshraghian A. Efficacy of a new therapeutic regimen versus two routinely prescribed treatments for eradication of Helicobacter pylori: A randomized, double-blind study of doxycycline, co-amoxiclav, and omeprazole in Iranian patients. Dig Dis Sci. 2009;54:599–603. doi: 10.1007/s10620-008-0374-z. [DOI] [PubMed] [Google Scholar]
- 29.Ghadir MR, Shafaghi A, Iranikhah A, Pakdin A, Joukar F, Mansour-Ghanaei F. Furazolidone, amoxicillin and omeprazole with or without bismuth for eradication of Helicobacter pylori in peptic ulcer disease. Turk J Gastroenterol. 2011;22:1–5. [PubMed] [Google Scholar]
- 30.Xu C, Xiao L, Zou H. Effect of ibrid triple viable on peptic ulcer patients with Helicobacter pylori infection. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:1000–4. doi: 10.3969/j.issn.1672-7347.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Wang XY, Shen SR. Efficacy of 4 kinds of triple strategy for Helicobacter pylori eradication. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:1129–31. [PubMed] [Google Scholar]
- 32.Huo XH, Chu JK, Yang XF, Wang J, Zhang LJ, Ma JC, et al. Efficacy of one-day quadruple therapy for H.pylori infection in Chinese patients. World J Gastroenterol. 2006;12:3105–7. doi: 10.3748/wjg.v12.i19.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo CY, Wu YB, Liu HL, Wu JY, Zhong MZ. Clinical evaluation of four one-week triple therapy regimens in eradicating Helicobacter pylori infection. World J Gastroenterol. 2004;10:747–9. doi: 10.3748/wjg.v10.i5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H, Zhang DZ, Hu PJ, Li ZS, Lu XH, Fang XC, et al. One-week regimens containing ranitidine bismuth citrate, furazolidone and either amoxicillin or tetracycline effectively eradicate Helicobacter pylori: A multicentre, randomized, double-blind study. Aliment Pharmacol Ther. 2001;15:1975–9. doi: 10.1046/j.1365-2036.2001.01122.x. [DOI] [PubMed] [Google Scholar]
- 35.Xiao SD, Liu WZ, Hu PJ, Ouyang Q, Wang JL, Zhou LY, et al. A multicentre study on eradication of Helicobacter pylori using four 1-week triple therapies in China. Aliment Pharmacol Ther. 2001;15:81–6. doi: 10.1046/j.1365-2036.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu WZ, Xiao SD, Hu PJ, Lu H, Cui Y, Tytgat GN. A new quadruple therapy for Helicobacter pylori using tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment Pharmacol Ther. 2000;14:1519–22. doi: 10.1046/j.1365-2036.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 37.Machado RS, Silva MR, Viriato A. Furazolidone, tetracycline and omeprazole: A low-cost alternative for Helicobacter pylori eradication in children. J Pediatr (Rio J) 2008;84:160–5. doi: 10.2223/JPED.1772. [DOI] [PubMed] [Google Scholar]
- 38.Coelho LG, Passos MC, Martins GM, Bueno ML, Gomes BS, Lopes LG, et al. Once-daily Helicobacter pylori treatment to family members of gastric cancer patients. Am J Gastroenterol. 2000;95:832–3. doi: 10.1111/j.1572-0241.2000.01899.x. [DOI] [PubMed] [Google Scholar]
- 39.Dani R, Queiroz DM, Dias MG, Franco JM, Magalhães LC, Mendes GS, et al. Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pylori in patients with duodenal ulcer. Aliment Pharmacol Ther. 1999;13:1647–52. doi: 10.1046/j.1365-2036.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami E, Machado RS, Ogata SK, Langner M, Fukushima E, Carelli AP, et al. Furazolidone-based triple therapy for H pylori gastritis in children. World J Gastroenterol. 2006;12:5544–9. doi: 10.3748/wjg.v12.i34.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frota LC, da Cunha Mdo P, Luz CR, de Araujo-Filho AH, Frota LA, Braga LL. Helicobacter pylori eradication using tetracycline and furazolidone versus amoxicillin and azithromycin in lansoprazole based triple therapy: An open randomized clinical trial. Arq Gastroenterol. 2005;42:111–5. doi: 10.1590/s0004-28032005000200009. [DOI] [PubMed] [Google Scholar]
- 42.De Idiáquez D, Bussalleu A, Rodrigo I, Cabello J, Caviedes G, Cok J, et al. Helicobacter pylori infection eradication in dyspeptic patients with and without peptic ulcer. Rev Gastroenterol Peru. 1999;19:179–94. [PubMed] [Google Scholar]
- 43.Araujo Castillo R, Pinto Valdivia JL, Ramírez D, Cok García J, Bussalleu Rivera A. New ultrashort scheme for Helicobacter pylori infection eradication using tetracyline, furazolidone and colloidal bismuth subcitrate in dyspeptic patients with or without peptic ulceration in the National Hospital Cayetano Heredia. Rev Gastroenterol Peru. 2005;25:23–41. [PubMed] [Google Scholar]
- 44.Nizhevich AA, Iunusbaev BB, Tuiĭgunov MM, Tsiglintseva NP, Nasretdinova EK. Study of gene polymorphism responsible for metabolism of proton pump inhibitors in children with H.pylori infection: Is there a correlation with efficacy of eradication treatment? Eksp Klin Gastroenterol. 2009;3:101–4. [PubMed] [Google Scholar]
- 45.Segura AM, Gutiérrez O, Otero W, Angel A, Genta RM, Graham DY. Furazolidone, amoxycillin, bismuth triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11:529–32. doi: 10.1046/j.1365-2036.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- 46.Coelho LG, Moretzsohn LD, Vieira WL, Gallo MA, Passos MC, Cindr JM, et al. New once-daily, highly effective rescue triple therapy after multiple Helicobacter pylori treatment failures: A pilot study. Aliment Pharmacol Ther. 2005;21:783–7. doi: 10.1111/j.1365-2036.2005.02370.x. [DOI] [PubMed] [Google Scholar]
- 47.Sanches B, Coelho L, Moretzsohn L, Vieira G., Jr Failure of Helicobacter pylori treatment after regimens containing clarithromycin: New practical therapeutic options. Helicobacter. 2008;13:572–6. doi: 10.1111/j.1523-5378.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 48.Eisig JN, Silva FM, Barbuti RC, Rodriguez TN, Malfertheiner P, Moraes Filho JP, et al. Efficacy of a 7-day course of furazolidone, levofloxacin, and lansoprazole after failed Helicobacter pylori eradication. BMC Gastroenterol. 2009;9:38–43. doi: 10.1186/1471-230X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva FM, Eisig JN, Chehter EZ, Silva JJ, Laudanna AA. Omeprazole, furazolidone, and tetracycline: An eradication treatment for resistant H.pylori in Brazilian patients with peptic ulcer disease. Rev Hosp Clin Fac Med Sao Paulo. 2002;57:205–8. doi: 10.1590/s0041-87812002000500003. [DOI] [PubMed] [Google Scholar]
- 50.Ebrahimi-Dariani N, Mirmomen S, Mansour-Ghanaei F, Noormohammadpoor P, Sotodehmanesh R, Haghpanah B, et al. The efficacy of furazolidone-based quadruple therapy for eradication of Helicobacter pylori infection in Iranian patients resistant to metronidazole-based quadruple therapy. Med Sci Monit. 2003;9:105–8. [PubMed] [Google Scholar]
- 51.Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion. 2001;64:222–5. doi: 10.1159/000048865. [DOI] [PubMed] [Google Scholar]
- 52.Wong WM, Wong BC, Lu H, Gu Q, Yin Y, Wang WH, et al. One-week omeprazole, furazolidone and amoxicillin rescue therapy after failure of Helicobacter pylori eradication with standard triple therapies. Aliment Pharmacol Ther. 2002;16:793–8. doi: 10.1046/j.1365-2036.2002.01223.x. [DOI] [PubMed] [Google Scholar]
- 53.Cheng H, Hu FL. Furazolidone, amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World J Gastroenterol. 2009;15:860–4. doi: 10.3748/wjg.15.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu LY, Lin WW, Lu H, Chen XY, Ge ZZ, Li XB. Quadruple therapy with medications containing either rufloxacin or furazolidone as a rescue regimen in the treatment of Helicobacter pylori-infected dyspepsia patients: A randomized pilot study. Helicobacter. 2011;16:284–8. doi: 10.1111/j.1523-5378.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 55.Isakov V, Domareva I, Koudryavtseva L, Maev I, Ganskaya Z. Furazolidone-based triple ‘rescue therapy’ vs.quadruple ‘rescue therapy’ for the eradication of Helicobacter pylori resistant to metronidazole. Aliment Pharmacol Ther. 2002;16:1277–82. doi: 10.1046/j.1365-2036.2002.01299.x. [DOI] [PubMed] [Google Scholar]
- 56.Nijevitch AA, Shcherbakov PL, Sataev VU, Khasanov RS, Al Khashash R, Tuygunov MM. Helicobacter pylori eradication in childhood after failure of initial treatment: Advantage of quadruple therapy with nifuratel to furazolidone. Aliment Pharmacol Ther. 2005;22:881–7. doi: 10.1111/j.1365-2036.2005.02656.x. [DOI] [PubMed] [Google Scholar]
- 57.Qasim A, Sebastian S, Thornton O, Dobson M, McLoughlin R, Buckley M, et al. Rifabutin- and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment Pharmacol Ther. 2005;21:91–6. doi: 10.1111/j.1365-2036.2004.02210.x. [DOI] [PubMed] [Google Scholar]
- 58.Felga GE, Silva FM, Barbuti RC, Navarro-Rodriguez T, Zaterka S, Eisig JN. Quadruple therapy with furazolidone for retreatment in patients with peptic ulcer disease. World J Gastroenterol. 2008;14:6224–7. doi: 10.3748/wjg.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abbas Z, Yakoob J, Abid S, Jafri W, Islam M, Azam Z, et al. Furazolidone, co-amoxiclav, colloidal bismuth subcitrate, and esomeprazole for patients who failed to eradicate Helicobacter pylori with triple therapy. Dig Dis Sci. 2009;54:1953–7. doi: 10.1007/s10620-008-0582-6. [DOI] [PubMed] [Google Scholar]
- 60.Mégraud F, Marshall BJ. How to treat Helicobacter pylori. First-line, second-line, and future therapies. Gastroenterol Clin North Am. 2000;29:759–73. doi: 10.1016/s0889-8553(05)70145-x. [DOI] [PubMed] [Google Scholar]
- 61.Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H.pylori isolates to metronidazole and tetracycline. Comparison of three 3-year studies. Arch Iran Med. 2010;13:177–87. [PubMed] [Google Scholar]
- 62.IARC Monographs on the evaluation of carcinogenic risks to humans. Furazolidone. 1998;31 [PMC free article] [PubMed] [Google Scholar]
- 63.The European Agency for the Evaluation of Medicinal Products. Veterinary Medicines Evaluation Unit. Annex IV. 1999 Oct;:14. [Google Scholar]
- 64.FDA Prohibits Nitrofuran Drug Use. Veterinarian Newsletter. 2002;17(2) [Google Scholar]
- 65. [Last accessed on 2011 Oct 12]. Available from: http://www.ema.europa.eu .
- 66.Furazolidone: Withdrawn: Yemen: WHO Pharmaceuticals Newsletter; 1999. No 01and02 (1999) [Google Scholar]
- 67.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 68.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: A pooled-data analysis. Gut. 2007;56:1353–7. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abadi AT, Taghvaei T, Vaira D. Considerable use of furazolidone in Iran. Saudi J Gastroenterol. 2010;16:308–9. doi: 10.4103/1319-3767.70631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su Z, Xu H, Zhang C, Li L, Wang H, Wang H, et al. Mutations in Helicobacter pylori porD and oorD genes may contribute to furazolidone resistance. Croat Med J. 2006;47:410–5. [PMC free article] [PubMed] [Google Scholar]
- 71.Leung WK, Graham DY. Rescue therapy for Helicobacter pylori. Curr Treat Options Gastroenterol. 2002;5:133–8. doi: 10.1007/s11938-002-0060-8. [DOI] [PubMed] [Google Scholar]


